Abstract
The synthesis of a new series of 1-[(benzazole-2-yl)thioacetyl]-3,5-diaryl-2-pyrazoline derivatives was obtained by reacting 1-(chloroacetyl)-3,5-diaryl-pyrazolines with 2-mercaptobenzimidazole/benzoxazole/benzothiazole. The chemical structures of the compounds were elucidated by 1H-NMR, 13C-NMR, and FAB+-MS spectral data. Their antifungal activities against Candida albicans, Candida glabrata, Candida utilis, Candida tropicalis, Candida krusei, and Candida parapsilosis were investigated. A significant level of activity was observed.
Keywords::
Introduction
Over the last three decades there has been a dramatic increase in the incidence of fungal infections. The discovery of new drugs for the treatment of systemic mycoses is a major challenge in infectious disease research. There is an urgent need for new antifungal remedies with novel modes of action, due to a decreased antifungal susceptibility of newly emerging fungi in the growing setting of the immunocompromised patient (e.g. human immunodeficiency virus (HIV)-positive and neutropenic patients). As is known, not only is the biochemical similarity of the human cell and fungi forms a handicap for selective activity, but also, easily gained resistance is the main problem encountered in developing safe and efficient antifungalsCitation1–3.
In order to overcome this handicap, new agents should preferably have chemical characteristics that clearly differ from those of existing agents. In drug design programs, an essential component of the search for new leads is the synthesis of molecules that are novel yet resemble known biologically active molecules by virtue of the presence of critical structural features. Certain small heterocyclic molecules act as highly functionalized scaffolds and are known pharmacophores of a number of biologically active and medicinally useful moleculesCitation4,Citation5.
The benzimidazoles and their bioisosters the benzoxazoles have been proved to be the most important group of fungicides with systemic activity, and are well known for their pronounced ability to control a large number of fungal diseasesCitation6,Citation7. Thiabendazole, benomyl, carbendazim, chlorfenazole, cypendazole, debacarb, fuberidazole, mecarbinzid, and rabenzazole, which include the benzimidazole moiety, are the main examples of this fungicide classCitation8,Citation9. In this group, the well-known fungicide thiabendazole inhibits fungal microtubular function, resulting in nondisjunction of chromosomes at cell divisionCitation10,Citation11.
On the other hand, a literature survey showed that there have been many studies on the pyrazoline moiety and its antifungal activityCitation12–16.
In the interest of the above, we planned to synthesize a system that combines these two biolabile components, i.e. benzimidazoles/benzoxazoles/benzothiazoles and pyrazolines, to give compact-structure title compounds.
Experimental
Chemistry
All melting points (m.p.) were determined in open capillaries on a Gallenkamp apparatus (Weiss-Gallenkamp, Loughborough, UK) and are uncorrected. The purity of the compounds was routinely checked by thin layer chromatography (TLC) using silica gel 60G (Merck, Darmstadt, Germany). Spectroscopic data were recorded with the following instruments: 1H-nuclear magnetic resonance (NMR), Bruker 400 MHz spectrometer and 13C-NMR, Bruker 100 MHz spectrometer (Bruker, Billerica, MA, USA) in dimethylsulfoxide (DMSO)-d6 using tetramethylsilane (TMS) as internal standard; and fast atom bombardment-mass spectrometry (FAB-MS), VG Quattro spectrometer (Agilent, Minnesota, USA).
General procedure for synthesis of the compounds
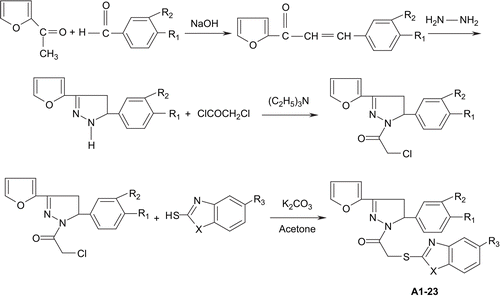
1-(2-Furanyl)-3-aryl-2-propen-1-ones A mixture of 2-acetylfuran (0.06 mol), aromatic aldehyde (0.06 mol), and 10% aqueous sodium hydroxide (10 mL) in ethanol (30 mL) was stirred at room temperature for about 3 h. The resulting solid was washed, dried, and crystallized from ethanol.
5-Aryl-3-(2-furanyl)-2-pyrazolines A solution of the appropriate furanyl chalcone (0.03 mol) and hydrazine hydrate (80%) (0.06 mol) in ethanol (30 mL) was refluxed for 3 h. The reaction mixture was cooled and kept at 0°C overnight. The resulting solid was recrystallized from ethanol.
1-(Chloroacetyl)-3-(2-furanyl)-5-aryl-2-pyrazolines The 5-aryl-3-(2-furanyl)-2-pyrazolines (0.02 mol) and triethylamine (0.02 mol) were dissolved in dry toluene (30 mL) with constant stirring. Later, the mixture was cooled in an ice bath, and chloroacetyl chloride (0.02 mol) was added dropwise with stirring. The reaction mixture thus obtained was further agitated for 1 h at room temperature. The precipitate was filtered, the solvent was evaporated to dryness under reduced pressure, and the products were recrystallized from ethanol.
1-[(Benzazole-2-yl)thioacetyl]-3-(2-furanyl)-5-aryl-2-pyrazoline derivatives (A1–23) A mixture of 1-(chloroacetyl)-3-(2-furanyl)-5-aryl-2-pyrazoline (0.01 mol), benzazol-2-thiole (0.01 mol), and K2CO3 (0.01 mol) in acetone (50 mL) was refluxed for 8 h. After cooling, the solution was evaporated until dryness. The residue was washed with water and recrystallized from ethanolCitation17.
Some characteristics of the synthesized compounds are shown in . Analytical and spectral data (1H-NMR, 13C-NMR, FAB+-MS) confirmed the structures of the new compounds.
Table 1. Some characteristics of the compounds.
1-[(Benzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(4-chlorophenyl)-2-pyrazoline (A1) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.07 (1H, dd, J = 18.0, 4.6 Hz), 3.84 (1H, dd, J = 18.0, 11.8 Hz), 4.57 (1H, d, J = 16.0 Hz), 4.82 (1H, d, J = 16.0 Hz), 5.59 (1H, dd, J = 11.7, 4.6 Hz), 6.67 (1H, dd, J = 3.4, 1.8 Hz), 7.02 (1H, d, J = 3.4 Hz), 7.10–7.17 (2H, m), 7.25–7.35 (4H, m), 7.47 (2H, m), 7.92 (1H, d, J = 1.2 Hz), 12.63 (1H, b). 13C-NMR (100 MHz, δ, DMSO-d6): 34.86 (CH2), 41.83 (CH2), 58.86 (CH), 109.45 (CH), 112.27 (CH), 114.77 (CH), 121.37 (CH), 122.26 (CH), 127.45 (2CH), 128.54 (2CH), 131.86 (C), 132.26 (CH), 140.37 (C), 145.84 (C), 145.88 (CH), 146.40 (C), 146.42 (C), 149.63 (C), 164.68 (C), 164.67 (C). FAB+-MS: m/z: 436 (M+), 437 (M+ + 1).
1-[(5-Chlorobenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(4-chlorophenyl)-2-pyrazoline (A2) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.09 (1H, dd, J = 18.0, 4.6 Hz), 3.82 (1H, dd, J = 18.0, 11.8 Hz), 4.56 (1H, d, J = 16.0 Hz), 4.80 (1H, d, J = 16.0 Hz), 5.58 (1H, dd, J = 11.7, 4.7 Hz), 6.67 (1H, dd, J = 3.5, 1.8 Hz), 7.01 (1H, d, J = 3.5 Hz), 7.11 (1H, d, J = 8.5), 7.25–7.35 (4H, m), 7.45 (1H, d, J = 8.5 Hz), 7.51 (1H, d, J = 2.0 Hz), 7.91 (1H, dd, J = 3.5, 2.3 Hz ), 12.64 (1H, b). 13C-NMR (100 MHz, δ, DMSO-d6): 34.86 (CH2), 41.83 (CH2), 58.85 (CH), 112.27 (CH), 113.72 (CH), 114.67 (CH), 114.78 (CH), 121.05 (CH), 125.42 (C), 127.46 (2CH), 128.54 (2CH), 131.87 (C), 138.56 (C), 140.38 (C), 141.24 (C), 145.84 (C), 145.82 (C), 145.86 (CH), 146.41 (C), 152.12 (C), 164.55 (C). FAB+-MS: m/z: 470 (M+), 471 (M+ + 1), 472 (M+ + 2).
1-[(5-Methylbenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(4-chlorophenyl)-2-pyrazoline (A3) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 2.39 (3H, s), 3.06 (1H, dd, J = 18.0, 4.6 Hz), 3.83 (1H, dd, J = 18.0, 11.8 Hz), 4.57 (1H, d, J = 15.9 Hz), 4.80 (1H, d, J = 15.9 Hz), 5.58 (1H, dd, J = 11.7, 4.6 Hz), 6.67 (1H, dd, J = 3.5, 1.8 Hz), 6.95 (1H, d, J = 8.2 Hz), 7.01 (1H, d, J = 3.4 Hz), 7.29 (6H, m), 7.91 (1H, d, J = 1.2 Hz), 12.46 (1H, s). 13C-NMR (100 MHz, δ, DMSO-d6): 30.62 (CH3), 34.88 (CH2), 41.82 (CH2), 58.85 (CH), 112.25 (CH), 114.71 (CH), 122.65 (CH), 123.15 (CH), 127.44 (2CH), 128.54 (2CH), 130.46 (C), 131.85 (CH), 131.87 (C), 140.37 (C), 143.43 (C), 145.85 (CH), 145.81 (C), 146.35 (C), 148.95 (C), 149.63 (C), 164.73 (C), 164.77 (C). FAB+-MS: m/z: 450 (M+), 451 (M+ + 1).
1-[(5-Nitrobenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(4-chlorophenyl)-2-pyrazoline (A4) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.08 (1H, dd, J = 18.0, 4.7 Hz), 3.85 (1H, dd, J = 18.0, 11.8 Hz), 4.61 (1H, d, J = 16.0 Hz), 4.84 (1H, d, J = 16.0 Hz), 5.59 (1H, dd, J = 11.8, 4.7 Hz), 6.67 (1H, dd, J = 3.5, 1.8 Hz), 7.03 (1H, dd, J = 3.5, 0.7 Hz), 7.31 (6H, m), 7.57 (1H, d, J = 8.8 Hz), 7.91 (1H, d, J = 1.8 Hz), 8.01 (1H, dd, J = 8.8, 2.3 Hz), 8.31 (1H, d, J = 2.0 Hz), 12.52 (1H, b). 13C-NMR (100 MHz, δ, DMSO-d6): 34.92 (CH2), 41.83 (CH2), 58.88 (CH), 110.22 (CH), 112.27 (CH), 113.28 (CH), 114.82 (CH), 116.89 (CH), 127.50 (2CH), 128.53 (2CH), 131.88 (C), 140.38 (C), 141.51 (C), 145.15 (C), 145.82 (C), 145.89 (CH), 146.48 (C), 150.26 (C), 157.21 (C), 164.50 (C). FAB+-MS: m/z: 483 (M+), 484 (M+ + 1).
1-[(Benzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(3,4-methylenedioxyphenyl)-2-pyrazoline (A5) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.08 (1H, dd, J = 17.9, 4.5 Hz), 3.79 (1H, dd, J = 17.9, 11.6 Hz), 4.60 (1H, d, J = 15.9 Hz), 4.79 (1H, d, J = 15.9 Hz), 5.50 (1H, dd, J = 11.6, 4.5 Hz), 5.97 (2H, s), 6.67 (1H, dd, J = 3.5, 1.8 Hz), 6.73 (1H, dd, J = 8.1, 1.7 Hz), 6.78–6.82 (2H, m), 7.02 (1H, d, J = 3.5 Hz), 7.13 (2H, m), 7.38 (1H, m), 7.55 (1H,m), 7.91 (1H, d, J = 1.8 Hz), 12.59 (1H, s). 13C-NMR (100 MHz, δ, DMSO-d6): 34.99 (CH2), 42.03 (CH2), 59.21 (CH), 100.97 (CH2), 106.01 (CH), 108.19 (CH), 110.27 (CH), 112.24 (CH), 114.65 (CH), 117.34 (CH), 118.69 (CH), 121.07 (CH), 121.56 (CH), 135.39 (C), 135.51 (C), 143.58 (C), 145.80 (CH), 145.95 (C), 146.42 (C), 146.47 (C), 147.50 (C), 149.69 (C), 164.55 (C). FAB+-MS: m/z: 446 (M+).
1-[(5-Chlorobenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(3,4-methylenedioxyphenyl)-2-pyrazoline (A6) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.07 (1H, dd, J = 18.0, 4.5 Hz), 3.80 (1H, dd, J = 18.0, 11.8 Hz), 4.64 (1H, d, J = 16.0 Hz), 4.81 (1H, d, J = 16.0 Hz), 5.52 (1H, dd, J = 11.8, 4.5 Hz), 5.97 (2H, s), 6.68 (1H, dd, J = 3.5, 1.8 Hz), 6.73 (1H, dd, J = 8.1, 1.8 Hz), 6.79–6.82 (2H, m), 7.04 (1H, d, J = 3.5 Hz), 7.14 (2H, m), 7.36 (1H, m), 7.56 (1H, m), 7.92 (1H, d, J = 1.8 Hz), 12.60 (1H, s). FAB+-MS: m/z: 480 (M+), 481 (M+ + 1), 482 (M+ + 2).
1-[(5-Methylbenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(3,4-methylenedioxyphenyl)-2-pyrazoline (A7) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 2.38 (3H, s), 3.06 (1H, dd, J = 17.9, 4.5 Hz), 3.79 (1H, dd, J = 17.9, 11.6 Hz), 4.56 (1H, d, J = 15.8 Hz), 4.74 (1H, d, J = 15.8 Hz), 5.50 (1H, dd, J = 11.6, 4.5 Hz), 5.97 (2H, s), 6.67 (1H, dd, J = 3.5, 1.8 Hz), 6.73 (1H, dd, J = 8.2, 1.7 Hz), 6.77–6.82 (2H, m), 6.94 (1H, dd, J = 8.2, 1.2 Hz), 7.01 (1H, d, J = 3.5 Hz), 7.10–7.43 (2H, m), 7.91 (1H, d, J = 1.8 Hz), 12.44 (1H, s). 13C-NMR (100 MHz, δ, DMSO-d6): 21.16 (CH3), 34.97 (CH2), 42.02 (CH2), 59.18 (CH), 100.96 (CH2), 106.01 (CH), 108.20 (CH), 110.18 (CH), 112.24 (CH), 114.65 (CH), 117.32 (CH), 118.69 (CH), 122.42 (CH), 135.40 (C), 141.73 (C), 143.98 (C), 145.80 (CH), 145.95 (C), 146.40 (C), 146.43 (C), 147.49 (C), 148.83 (C), 149.25 (C), 164.59 (C). FAB+-MS: m/z: 461 (M+ + 1).
1-[(5-Nitrobenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(3,4-methylenedioxyphenyl)-2-pyrazoline (A8) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.06 (1H, dd, J = 17.9, 4.4 Hz), 3.80 (1H, dd, J = 17.9, 11.6 Hz), 4.57 (1H, d, J = 15.9 Hz), 4.78 (1H, d, J = 15.9 Hz), 5.51 (1H, dd, J = 11.6, 4.4 Hz), 5.97 (2H, s), 6.66–7.02 (6H, m), 7.46 (1H, d, J = 8.8 Hz), 7.88–7.95 (3H, m), 8.25 (1H, d, J = 2.3 Hz), 12.42 (1H, s). 13C-NMR (100 MHz, δ, DMSO-d6): 34.81 (CH2), 42.03 (CH2), 59.14 (CH), 100.95 (CH2), 105.97 (CH), 108.19 (CH), 110.18 (CH), 112.23 (CH), 113.03 (CH), 114.60 (CH), 115.86 (CH), 118.71 (CH), 135.48 (C), 140.32 (C), 142.36 (C), 145.78 (CH), 145.99 (C), 146.33 (C), 146.37 (C), 147.46 (C), 150.71 (C), 159.74 (C), 164.82 (C). FAB+-MS: m/z: 493 (M+), 494 (M+ + 1).
1-[(Benzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-phenyl-2-pyrazoline (A9) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.10 (1H, dd, J = 18.0, 4.5 Hz), 3.82 (1H, dd, J = 18.0, 11.8 Hz), 4.61 (1H, d, J = 15.8 Hz), 4.82 (1H, d, J = 15.8 Hz), 5.55 (1H, dd, J = 11.8, 4.5 Hz), 6.68 (1H, dd, J = 3.3, 1.6 Hz), 7.01 (1H, dd, J = 3.3, 0.7 Hz), 7.29 (6H, m), 7.56 (1H, d, J = 8.7 Hz), 7.89 (1H, d, J = 1.6 Hz), 8.01 (1H, dd, J = 8.7, 2.3 Hz), 8.32 (1H, d, J = 2.1 Hz), 12.54 (1H, s) FAB+-MS: m/z: 403 (M+ + 1).
1-[(5-Chlorobenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-phenyl-2-pyrazoline (A10) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.08 (1H, dd, J = 18.0, 4.6 Hz), 3.83 (1H, dd, J = 18.0, 11.8 Hz), 4.56 (1H, d, J = 16.0 Hz), 4.81 (1H, d, J = 16.0 Hz), 5.58 (1H, dd, J = 11.7, 4.6 Hz), 6.67 (1H, dd, J = 3.4, 1.8 Hz), 7.03 (1H, d, J = 3.4 Hz), 7.10–7.18 (2H, m), 7.25–7.35 (4H, m), 7.48 (2H, m), 7.93 (1H, d, J = 1.2 Hz), 12.62 (1H, b). FAB+-MS: m/z: 436 (M+), 437 (M+ + 1).
1-[(5-Methylbenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-phenyl-2-pyrazoline (A11) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 2.38 (3H, s), 3.07 (1H, dd, J = 18.0, 4.6 Hz), 3.85 (1H, dd, J = 18.0, 11.8 Hz), 4.56 (1H, d, J = 15.9 Hz), 4.79 (1H, d, J = 15.9 Hz), 5.57 (1H, dd, J = 11.7, 4.6 Hz), 6.68 (1H, dd, J = 3.5, 1.8 Hz), 6.92 (1H, d, J = 8.2 Hz), 7.02 (1H, d, J = 3.4 Hz), 7.26 (7H, m), 7.89 (1H, d, J = 1.2 Hz), 12.48 (1H, s). FAB+-MS: m/z: 416 (M+), 417 (M+ + 1).
1-[(5-Nitrobenzimidazole-2-yl)thioacetyl]-3-(2-furanyl)-5-phenyl-2-pyrazoline (A12) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.06 (1H, dd, J = 17.9, 4.7 Hz), 3.80 (1H, dd, J = 17.9, 11.8 Hz), 4.63 (1H, d, J = 15.9 Hz), 4.87 (1H, d, J = 15.9 Hz), 5.56 (1H, dd, J = 11.8, 4.7 Hz), 6.69 (1H, dd, J = 3.5, 1.8 Hz), 7.01 (1H, dd, J = 3.5, 0.7 Hz), 7.28 (7H, m), 7.54 (1H, d, J = 8.8 Hz), 7.89 (1H, d, J = 1.8 Hz), 8.00 (1H, dd, J = 8.8, 2.3 Hz), 8.29 (1H, d, J = 2.0 Hz), 12.50 (1H, b). FAB+-MS: m/z: 450 (M+ + 1).
1-[(Benzoxazole-2-yl)thioacetyl]-3-(2-furanyl)-5-phenyl-2-pyrazoline (A13) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.08 (1H, dd, J = 17.9, 4.5 Hz), 3.81 (1H, dd, J = 17.9, 11.8 Hz), 4.62 (1H, d, J = 16.0 Hz), 4.83 (1H, d, J = 15.9 Hz), 5.53 (1H, dd, J = 11.7, 4.6 Hz), 6.64 (1H, dd, J = 3.6, 1.7 Hz), 7.02 (1H, dd, J = 3.5, 0.6 Hz), 7.28–7.36 (6H, m), 7.58 (1H, m), 7.69 (1H, m), 7.89 (1H, dd, J = 8.6, 2.2 Hz), 8.27 (1H, d, J = 2.1 Hz). FAB+-MS: m/z: 404 (M+ + 1).
1-[(5-Chlorobenzoxazole-2-yl)thioacetyl]-3-(2-furanyl)-5-phenyl-2-pyrazoline (A14) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.10 (1H, dd, J = 18.0, 4.7 Hz), 3.86 (1H, dd, J = 18.0, 11.8 Hz), 4.65 (1H, d, J = 16.3 Hz), 4.89 (1H, d, J = 16.3 Hz), 5.61 (1H, dd, J = 11.7, 4.7 Hz), 6.67 (1H, dd, J = 3.5, 1.8 Hz), 7.03 (1H, d, J = 3.5, 0.7 Hz), 7.27–7.36 (6H, m), 7.61 (1H, m), 7.66 (1H, m), 7.92 (1H, dd, J = 1.8, 0.7 Hz). 13C-NMR (100 MHz, δ, DMSO-d6): 35.73 (CH2), 41.88 (CH2), 59.00 (CH), 110.12 (CH), 112.29 (CH), 114.96 (CH), 118.20 (CH), 124.19 (CH), 124.56 (CH), 127.47 (2CH), 128.57 (2CH), 131.99 (C), 140.21 (C), 141.26 (C), 145.75 (C), 145.96 (CH), 146.79 (C), 151.31 (C), 163.70 (C), 163.82 (C). FAB+-MS: m/z: 437 (M+), 438 (M+ + 1), 439 (M+ + 2).
1-[(5-Methylbenzoxazole-2-yl)thioacetyl]-3-(2-furanyl)-5-phenyl-2-pyrazoline (A15) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 2.38 (3H, s), 3.07 (1H, dd, J = 18.0, 4.5 Hz), 3.84 (1H, dd, J = 18.0, 11.6 Hz), 4.56 (1H, d, J = 15.9 Hz), 4.82 (1H, d, J = 15.9 Hz), 5.55 (1H, dd, J = 11.7, 4.5 Hz), 6.66 (1H, dd, J = 3.5, 1.7 Hz), 6.94 (1H, d, J = 8.3 Hz), 7.03 (1H, d, J = 3.5 Hz), 7.27 (7H, m), 7.90 (1H, d, J = 1.3 Hz). FAB+-MS: m/z: 418 (M+ + 1).
1-[(5-Chlorobenzoxazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(4-chlorophenyl)-2-pyrazoline (A16) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.11 (1H, dd, J = 18.0, 4.6 Hz), 3.87 (1H, dd, J = 18.0, 11.7 Hz), 4.66 (1H, d, J = 16.3 Hz), 4.87 (1H, d, J = 16.3 Hz), 5.60 (1H, dd, J = 11.7, 4.6 Hz), 6.68 (1H, dd, J = 3.4, 1.7 Hz), 7.05 (1H, d, J = 3.4 Hz), 7.25–7.38 (5H, m), 7.65 (1H, d, J = 8.7 Hz), 7.77 (1H, d, J = 2.0 Hz), 7.92 (1H, s). 13C-NMR (100 MHz, δ, DMSO-d6): 35.90 (CH2), 41.86 (CH2), 58.99 (CH), 111.39 (CH), 112.31 (CH), 115.08 (CH), 118.03 (CH), 124.14 (CH), 127.52 (2CH), 128.59 (2CH), 128.92 (C), 131.98 (C), 140.20 (C), 142.50 (C), 145.71 (C), 146.01 (C), 146.88 (CH), 150.06 (C), 163.50 (C), 165.87 (C). FAB+-MS: m/z: 471 (M+), 472 (M+ + 1), 473 (M+ + 2).
1-[(5-Methylbenzoxazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(4-chlorophenyl)-2-pyrazoline (A17) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 2.4 (3H, s), 3.10 (1H, dd, J = 18.0, 4.7 Hz), 3.86 (1H, dd, J = 18.0, 11.8 Hz), 4.63 (1H, d, J = 16.3 Hz), 4.85 (1H, d, J = 16.3 Hz), 5.60 (1H, dd, J = 11.8, 4.7 Hz), 6.68 (1H, dd, J = 3.5, 1.8 Hz), 7.04 (1H, d, J = 3.0 Hz), 7.10 (1H, dd, J = 8.4, 1.1 Hz), 7.25–7.38 (4H, m), 7.44 (1H, m), 7.48 (1H, d, J = 8.3 Hz), 7.92 (1H, d, J = 1.2 Hz). 13C-NMR (100 MHz, δ, DMSO-d6): 20.89 (CH3), 35.66 (CH2), 41.85 (CH2), 58.97 (CH), 109.49 (CH), 112.29 (CH), 114.96 (CH), 118.17 (CH), 125.00 (CH), 127.49 (2CH), 128.58 (2CH), 131.97 (C), 133.95 (C), 140.23 (C), 141.46 (C), 145.76 (C), 145.97 (CH), 146.76 (C), 149.56 (C), 163.66 (C), 163.71 (C). FAB+-MS: m/z: 451 (M+), 452 (M+ + 1).
1-[(Benzoxazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(3,4-methylenedioxyphenyl)-2-pyrazoline (A18) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.09 (1H, dd, J = 17.9, 4.5 Hz), 3.82 (1H, dd, J = 17.9, 11.6 Hz), 4.64 (1H, d, J = 16.3 Hz), 4.86 (1H, d, J = 16.3 Hz), 5.52 (1H, dd, J = 11.6, 4.5 Hz), 5.97 (2H, s), 6.68 (1H, dd, J = 3.5, 1.8 Hz), 6.74 (1H, dd, J = 8.0, 1.8 Hz), 6.77–6.84 (2H, m), 7.05 (1H, d, J = 3.5 Hz), 7.32 (2H, m), 7.64 (2H, m), 7.92 (1H, d, J = 1.8 Hz). 13C-NMR (100 MHz, δ, DMSO-d6): 35.81 (CH2), 42.08 (CH2), 59.31 (CH), 100.98 (CH2), 105.99 (CH), 108.21 (CH), 110.11 (CH), 112.27 (CH), 114.89 (CH), 118.23 (CH), 118.74 (CH), 124.19 (CH), 124.56 (CH), 135.24 (C), 141.25 (C), 145.85 (C), 145.92 (CH), 146.45 (C), 146.82 (C), 147.49 (C), 151.28 (C), 163.55 (C), 163.84 (C). FAB+-MS: m/z: 447 (M+), 448 (M+ + 1).
1-[(5-Chlorobenzoxazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(3,4-methylenedioxyphenyl)-2-pyrazoline (A19) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.08 (1H, dd, J = 18.0, 4.5 Hz), 3.82 (1H, dd, J = 18.0, 11.8 Hz), 4.68 (1H, d, J = 16.3 Hz), 4.87 (1H, d, J = 16.3 Hz), 5.52 (1H, dd, J = 11.6, 4.5 Hz), 5.97 (2H, dd, J = 7.8, 0.9 Hz), 6.69 (1H, dd, J = 3.5, 1.8 Hz), 6.73 (1H, dd, J = 8.1, 1.8 Hz), 6.80 (1H, d, J = 1.7 Hz), 6.83 (1H, d, J = 8.0 Hz), 7.05 (1H, d, J = 3.5 Hz), 7.35 (1H, dd, J = 8.7, 2.2 Hz), 7.67 (1H, d, J = 8.7 Hz), 7.76 (1H, d, J = 2.0 Hz), 7.92 (1H, d, J = 1.8 Hz). 13C-NMR (100 MHz, δ, DMSO-d6): 35.96 (CH2), 42.10 (CH2), 59.33 (CH), 100.98 (CH2), 105.94 (CH), 108.23 (CH), 111.38 (CH), 112.29 (CH), 114.97 (CH), 118.04 (CH), 118.83 (CH), 124.12 (CH), 128.85 (C), 135.23 (C), 142.50 (C), 145.82 (C), 145.94 (CH), 146.43 (C), 146.88 (C), 147.46 (C), 150.07 (C), 163.37 (C), 165.94 (C). FAB+-MS: m/z: 481 (M+), 482 (M+ + 1).
1-[(5-Methylbenzoxazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(3,4-methylenedioxyphenyl)-2-pyrazoline (A20) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 2.37 (3H, s), 3.09 (1H, dd, J = 18.0, 4.5 Hz), 3.83 (1H, dd, J = 18.0, 11.6 Hz), 4.57 (1H, d, J = 15.7 Hz), 4.76 (1H, d, J = 15.7 Hz), 5.54 (1H, dd, J = 11.6, 4.5 Hz), 5.99 (2H, s), 6.66 (1H, dd, J = 3.5, 1.8 Hz), 6.75 (1H, dd, J = 8.1, 1.8 Hz), 6.76–6.83 (2H, m), 6.95 (1H, dd, J = 8.1, 1.3 Hz), 7.03 (1H, d, J = 3.5 Hz), 7.12–7.42 (2H, m), 7.89 (1H, d, J = 1.8 Hz). FAB+-MS: m/z: 462 (M+ + 1).
1-[(Benzothiazole-2-yl)thioacetyl]-3-(2-furanyl)-5-phenyl-2-pyrazoline (A21) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.07 (1H, dd, J = 18.0, 4.5 Hz), 3.84 (1H, dd, J = 18.0, 11.7 Hz), 4.68 (1H, d, J = 15.8 Hz), 4.83 (1H, d, J = 15.8 Hz), 5.58 (1H, dd, J = 11.6, 4.5 Hz), 6.67 (1H, dd, J = 3.3, 1.6 Hz), 7.05 (1H, dd, J = 3.2, 0.6 Hz), 7.22–7.34 (5H, m), 7.43 (2H, m), 7.81 (1H, m), 7.88 (1H, dd, J = 1.7, 0.7 Hz), 7.99 (1H, d, J = 8.0 Hz). FAB+-MS: m/z: 420 (M+ + 1).
1-[(Benzothiazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(4-chlorophenyl)-2-pyrazoline (A22) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.10 (1H, dd, J = 18.0, 4.7 Hz), 3.87 (1H, dd, J = 18.0, 11.8 Hz), 4.65 (1H, d, J = 16.0 Hz), 4.88 (1H, d, J = 16.0 Hz), 5.60 (1H, dd, J = 11.7, 4.7 Hz), 6.69 (1H, dd, J = 3.5, 1.8 Hz), 7.05 (1H, dd, J = 3.5, 0.6 Hz), 7.26–7.39 (5H, m), 7.47 (1H, dd, J = 12.3, 6.2 Hz), 7.87 (1H, m), 7.93 (1H, dd, J = 1.8, 0.6 Hz), 8.00 (1H, d, J = 8.1 Hz). 13C-NMR (100 MHz, δ, DMSO-d6): 36.21 (CH2), 41.85 (CH2), 58.92 (CH), 112.31 (CH), 114.97 (CH), 121.10 (CH), 121.79 (CH), 124.47 (CH), 126.32 (CH), 127.50 (2CH), 128.57 (2CH), 131.93 (C), 134.77 (C), 140.33 (C), 145.79 (C), 145.98 (CH), 146.66 (C), 152.51 (C), 163.87 (C), 165.93 (C). FAB+-MS: m/z: 453 (M+), 454 (M+ + 1), 455 (M+ + 2).
1-[(Benzothiazole-2-yl)thioacetyl]-3-(2-furanyl)-5-(3,4-methylenedioxyphenyl)-2-pyrazoline (A23) 1H-NMR (400 MHz, δ ppm, DMSO-d6): 3.08 (1H, dd, J = 17.9, 4.5 Hz), 3.81 (1H, dd, J = 17.9, 11.6 Hz), 4.65 (1H, d, J = 15.9 Hz), 4.86 (1H, d, J = 15.9 Hz), 5.51 (1H, dd, J = 11.6, 4.5 Hz), 5.96 (2H, s), 6.68 (1H, dd, J = 3.5, 1.8 Hz), 6.73 (1H, dd, J = 8.2, 1.7 Hz), 6.77–6.82 (2H, m), 7.04 (1H, dd, J = 3.5, 0.7 Hz), 7.46 (1H, dd, J = 8.2, 7.3 Hz), 7.86 (1H, d, J = 7.6 Hz), 7.92 (1H, d, J = 1.8 Hz), 7.99 (1H, d, J = 8.0 Hz). 13C-NMR (100 MHz, δ, DMSO-d6): 36.34 (CH2), 42.05 (CH2), 59.27 (CH), 100.98 (CH2), 106.01 (CH), 108.20 (CH), 112.27 (CH), 114.83 (CH), 118.72 (CH), 121.11 (CH), 121.72 (CH), 124.43 (CH), 126.27 (CH), 134.74 (C), 135.32 (C), 145.89 (CH), 145.80 (C), 146.44 (C), 146.67 (C), 147.49 (C), 152.54 (C), 163.75 (C), 165.97 (C). FAB+-MS: m/z: 463 (M+), 464 (M+ + 1).
Microbiology
Antifungal activity
The antifungal properties of compounds A1–23 were evaluated by the broth microdilution method according to the National Committee on Clinical Laboratory Standards (NCCLS) reference document M27-A2Citation18 against Candida albicans (isolate, obtained from the Department of Microbiology, Faculty of Medicine, Osmangazi University, Eskişehir, Turkey), Candida albicans (ATCC 90028), Candida glabrata (isolate, obtained from the Department of Microbiology, Faculty of Medicine, Osmangazi University, Eskişehir, Turkey), Candida utilis (NRRL Y-900), Candida tropicalis (NRRL Y-12968), Candida krusei (NRRL Y-7179), Candida parapsilosis (NRRL Y-12696), and Candida albicans s(NRRL Y-12983). Ketoconazole was used as positive control.
Broth microdilution assay
The test compounds and the antimicrobial standards were first dissolved in DMSO, which was used to prepare the stock solutions at an initial concentration of 2 mg/mL. Serial dilution series were prepared in 100 µL Müller–Hinton broth (MHB) with equal amounts of the test samples. The last row was filled only with water as a growth control for the microorganism. Overnight-grown microorganism suspensions were first diluted in double strength MHB and standardized to 108 CFU/mL (using McFarland No: 0.5) under sterile conditions. Then each microorganism suspension was pipetted into each well and incubated at 37°C for 24 h. Ketoconazole was used as a standard antifungal agent against Candida spp. Sterile distilled water and medium served as a positive growth control. The first well without turbidity was assigned as the minimum inhibitory concentration (MIC, in mg/mL). Average results of three separately performed experiments are given in .
Table 2. Anticandidal evaluation of 1-[(benzazole-2-yl)thioacetyl]-3,5-diaryl-2-pyrazoline derivatives (MIC values in mg/mL).
Results and discussion
In the present work, 23 new compounds were synthesized. The reaction of 1-(chloroacetyl)-3-(2-furanyl)-5-aryl-2-pyrazolines with appropriate benzazol-2-thiole gave 1-[(benzazole-2-yl)thioacetyl]-3,5-diaryl-2-pyrazoline derivatives (, ).
The structure of the compounds was elucidated by 1H-NMR, 13C-NMR, and FAB+-MS spectra. In the 400 MHz 1H-NMR spectra of the compounds, the CH2 protons of the pyrazoline ring resonated as a pair of doublets at δ 3.06–3.11 ppm (Ha), 3.79–3.87 ppm (Hb). The CH proton appeared as a doublet of doublets at δ 5.50–5.61 ppm (HX) due to vicinal coupling with the two magnetically non-equivalent protons of the methylene group at position 4 of the pyrazoline ring (JAB = 17.9–18.0 Hz, JAX = 4.4–4.7 Hz, JBX = 11.6–11.8 Hz). The CH2 protons of the acetyl at position 1 of the pyrazoline derivatives A1–23 was observed at 4.56–4.87 ppm as a doublet (J = 15.7–16.3 Hz). The benzimidazole derivatives showed a specific NH proton at 12.42–12.64 ppm as a broad singlet. All the other aromatic and aliphatic protons were observed at the expected regions. 13C-NMR chemical shift values of the carbon atoms at 41.82–42.85 ppm (C-4), 58.85–59.33 ppm (C-5), and about 149.25–152.54 ppm (C-3) corroborate the 2-pyrazoline character deduced from the 1H-NMR data. Mass spectra (FAB) of compounds showed M + 1 peaks, in agreement with their molecular formulae.
The antifungal activity of the compounds was studied using eight pathogenic fungi. Ketoconazole was used as the reference agent for inhibitory activity against the tested fungi. Minimal inhibitory concentration (MIC) was recorded as the minimum concentration of a compound that inhibited the growth of a tested microorganism. All of the compounds tested illustrated medium to very good anticandidal inhibitory activity when compared with the reference agent. The MIC values were found to be within the range 0.003–2 mg/mL against all evaluated strains. The results are summarized in .
In comparing their MIC values with ketoconazole, all of the compounds were effective against Candida parapsilosis; A1, A2, A5, A6, A7, A8, A10, A11, A13, A14, A18, A19, A20, A21, A22, and A23 especially showed strong activity; A4, A9, A12, and A15 showed a similar level of activity to ketoconazole.
Compounds A5, A6, A7, A18, A19, A20, A21, A22, and A23 were also effective against C. krusei. Compounds A5, A6, A7, A19, A20, A22, and A23 especially showed strong inhibitory activity against the tested microorganisms. Compounds A18 and A21 showed a similar level of activity to the reference agent, whereas A1 and A8 showed moderate activity.
All of the compounds were effective against Candida glabrata (clinical isolate). The compounds A1, A5, A6, A18, A20, and A23 especially showed strong activity. A19 showed the same level of activity when compared with the reference agent.
On the other hand, the compounds exhibited comparable activities against Candida albicans (ATCC 90028). Strong inhibitory activity with a MIC value of 0.003 mg/mL was observed for compound A18. The compounds A10, A21, A22, and A23 showed equal activity to, and the other compounds were found to be less active than, the reference agent.
When compared with ketoconazole, compound A8 showed similar activity against Candida albicans (clinical isolate), whereas all other compounds showed less activity.
Compounds A1–23 were found to be inactive against Candida utilis, Candida tropicalis, and Candida albicans (NRRL Y-12983) when compared with ketoconazole.
Considering all the results obtained from the antifungal screen, in comparison with the reference agent, it can be concluded that compounds A5, A6, A18, A20, and A23 were more active than the others in the screen, including the reference agent.
Based on the 23 compounds evaluated, it appears that 3,4-methylenedioxyl substitution on the phenyl ring made a good contribution to the antifungal activity in this series of benzazolyl–pyrazoline combinations. Additionally, substitutional changes in benzimidazole/benzoxazole rings on the basic structure did not affect the activity.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Rees JR, Pinner RW, Hajjeh RA. The epidemiological features of invasive mycotic infections in the San Francisco Bay Area, 1992-1993: results of population-based laboratory active surveillance. Clin Infect Dis 1998;27:1138–47.
- Polak A. The past, present and future of antimycotic combination therapy. Mycoses 1999;42:355–70.
- Fostel JM, Lartey PA. Emerging novel antifungal agents. Drug Discov Today 2000;5:25–32.
- Silverman RB. Organic Chemistry of Drug Design and Drug Action. San Diego: Academic Press, 1992.
- Thompson LA, Ellman JA. Synthesis and applications of small molecule libraries. Chem Rev 1996;96:555–600.
- Foye WO, Thomas LL, David A, Williams BI. Principles of Medicinal Chemistry, 4th ed. New Delhi, Waverly Pvt. Ltd., 1995.
- Ramalingan C, Balasubramanian S, Kabilan S, Vasudevan M. Synthesis and study of antibacterial and antifungal activities of novel 1-[2-(benzoxazol-2-yl)ethoxy]-2,6-diarylpiperidin-4-ones. Eur J Med Chem 2004;39:527–33.
- http://www.alanwood.net/pesticides/class_fungicides.html#benzimidazole_fungicides.
- Goodman-Gilman A. The Pharmacological Basis of Therapeutics. New York: McGraw-Hill, 2001.
- Watanabe-Akanuma M, Ohta T, Sasaki YF. A novel genotoxic aspect of thiabendazole as a photomutagen in bacteria and cultured human cells. Toxicol Lett 2005;158:213–19.
- Allen P, Gootlied B. Mechanism of action of the fungicide thiabendazole, 2-(4′-thiazolyl)benzimidazole. Appl Microbiol 1970;20:919–26.
- Showa DKK. Jpn Kokai Tokkyo Koho JP 60 08. 211 (1985); Chem Abstr 102, 216885q (1985).
- Safak C, Tayhan A, Sarac S. Synthesis of some 1-acetyl-3,5-diaryl-2-pyrazoline derivatives and their antimicrobial activities. J Indian Chem Soc 1990;67:571–4.
- Grant N, Mishriky N, Asaad FM, Fawzy NG. Pyridines and pyrazolines from salicylic acid derivatives with propenone residue and their antimicrobial properties. Pharmazie 1998;53:543–7.
- Turan-Zitouni G, Özdemir A, Güven K. Synthesis of some 1-[(N,N-disubstituted thiocarbamoylthio)acetyl]-3-(2-thienyl)-5-aryl-2-pyrazoline derivatives and investigation of their antibacterial and antifungal activities. Arch Pharm Pharm Med Chem 2005;338:96–104.
- Turan-Zitouni G, Özdemir A, Kaplancıklı ZA. Synthesis and antimicrobial activities of some 1-[(N,N-disubstitutedthiocarbamoylthio)acetyl]-3,5-diaryl-2-pyrazolines. Phosphorus Sulfur Silicon Relat Elem 2005;180:2717–24.
- Kaplancıklı ZA, Turan-Zitouni G, Özdemir A, Can ÖD, Chevallet P. Synthesis and antinociceptive activities of some pyrazoline derivatives. Eur J Med Chem 2009;44:2606–10.
- NCCLS. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, Approved Standard, 2nd ed. NCCLS document M27-A2. Wayne, PA: NCCLS, 2002.