Abstract
A collection of 4-(4-morpholinophenyl)-6-aryl-6H-1,3-thiazin-2-amines (20–28) were synthesized and their in vitro antimicrobial activity was investigated. Compound 21 against P. aeruginosa, 23 against B. subtilis, 24 against V. cholerae and P. aeruginosa, 26 against S. aureus and B. subtilis, 27 against B. subtilis and E. coli, and 28 against all tested bacterial strains exerted excellent antibacterial activity. Compound 20 against A. flavus and Rhizopus, 21, 26 against Rhizopus, 22, 27 against Mucor, 23 against A. flavus, 24 against both A. flavus and Mucor, 25 against all tested strains, and 28 against Rhizopus and M. gypseum exerted excellent antifungal activity.
Introduction
2-Amino-1,3-thiazines and their derivatives are chemically and pharmaceutically interesting entities, and have been used as antimicrobial agentsCitation1, as cannabinoid receptor agonistsCitation2, in the discovery of CB2 receptorsCitation3, in the phagocytic activity of human neutrophilsCitation4, as vasopressin receptor antagonistsCitation5, and have central nervous system (CNS) and antioxidant propertiesCitation6, analgesic and anti-inflammatory activitiesCitation7, antifilarial propertiesCitation8, metalloprotease inhibition activityCitation9, and antihypertensive activityCitation10. The presence of the N–C–S linkage is believed to account for the extensive biological activities of the thiazine nucleus.
Promising diverse pharmacological activities are shown by various N-fuctionalized morpholines. They are reported to exert a number of important physiological activities such as antidiabeticCitation11, antiemeticCitation12, platelet aggregation inhibition, antihyperlipoproteinemicCitation13, bronchodilatation, growth stimulantCitation14, and antidepressantCitation15. They are also used in the treatment of inflammatory diseases, pain, migraine, and asthmaCitation16. 4-Phenyl morpholine derivatives are reported to possess antimicrobialCitation17, anti-inflammatoryCitation18, and CNSCitation19 activities.
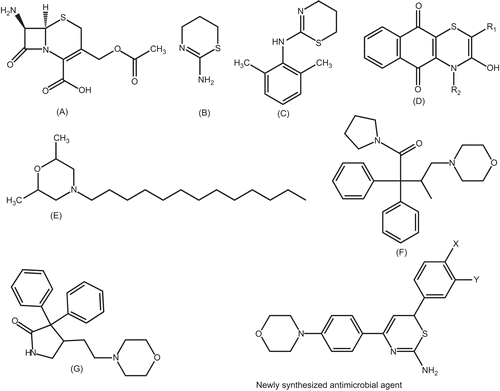
It is known () that the antibiotic activities of cephalosporins (A) are due to the presence of the 1,3-thiazine partCitation19. Inhibition studies with 2-amino-5,6-dihydro-4H-1, 3-thiazine (B) have indicated its use in the expression and immunoaffinity purification of human inducible nitric-oxide synthaseCitation20. Xylazine (C) is used for sedation, anesthesia, muscle relaxation, and analgesia in animalsCitation21. Naphtho[2,3-b][1,4]-thiazine-5,10-diones and 3-substituted-1,4-dioxo-1,4-dihydronaphthalen-2-yl-thioalkanoate derivatives (D) have been screened for their biological evaluation as potential antibacterial and antifungal agentsCitation22. Tridemorph, a morpholine derivative (E), is used as an antifungal agentCitation23. Drugs derived from morpholine-incorporated compounds include dextromoramide (F), a narcotic analgesic, and doxapram·HCl (G), a respiratory stimulant; Dopram® is used in the treatment of respiratory depression following anesthesia.
Scheme 1. Novel synthesized compounds having core thiazine and morpholine nuclei of therapeutic importance.
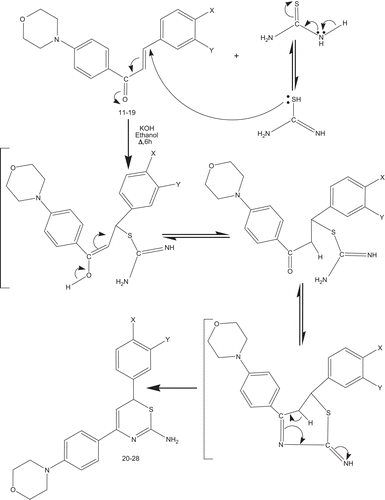
The synthesis of molecules that are novel but still resemble known biologically active molecules by virtue of the presence of some critical structural features is an essential component of the search for new leads in drug design programs. As part of our research program aimed at the synthesis of structurally diverse nitrogen/sulfur and selenium containing heterocyclesCitation24–29 comprising piperidino 1,2,3-selenadiazoles, 1,2,3-thiadiazoles, 1,2,4-triazolidine-3-thiones, diazepans, 1,2,4,5-tetrazinan-3-thiones, and indazoles, we performed the synthesis of 4-(4-morpholinophenyl)-6-aryl-2H-1,3-thiazine-2-amines (20–28) from (E)-1-(4-morpholinophenyl)-3-aryl-prop-2-en-1-ones (11–19) and evaluated their in vitro antibacterial and antifungal activities.
Experimental
Chemistry
The progress of the reaction was monitored by thin layer chromatography (TLC) analysis. All the reported melting points were taken in open capillaries and are uncorrected. Infrared (IR) spectra were recorded in KBr (pellet form) on a Nicolet Avatar-330 Fourier transform (FT)-IR spectrophotometer, and noteworthy absorption values (cm−1) alone are listed. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded at 400 MHz and 100 MHz, respectively, on a Bruker AMX 400 NMR spectrometer using dimethylsulfoxide (DMSO)-d6 as solvent. Electrospray ionization mass spectrometry (ESI +ve MS) spectra were recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalyses were obtained on a Carlo Erba 1106 CHN analyzer.
General procedure for the synthesis of (E)-1-4-morpholinophenyl)-3-aryl-prop-2-en-1-ones 11–19
To 50 mL ethanolic solution of 1-(4-morpholinophenyl) ethanone 1 (0.001 mol) and substituted benzaldehyde 2–10 (0.001 mol), aqueous sodium hydroxide (0.005 mol) was added drop-wise with stirring on a mechanical stirrer for 10 min, and stirring was continued for 1 h. After completion of the reaction, the crude product isolated by suction was washed with water, dried, and recrystallized from ethanol.
(E)-1-(4-Morpholinophenyl)-3-phenyl-prop-2-en-1-one 11 IR (KBr) ν (cm−1): 3007, 2962, 2924, 2852, 1646, 1606, 1190, 769; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2, J = 4.7 Hz), 3.87–3.89 (t, 4H, O(CH2)2, J = 4.7 Hz), 6.93–6.95 (d, 1H, H2, J = 8.9 Hz), 7.38–7.82 (m, 9H, Harom), 8.01–8.03 (d, 1H, H3, J = 8.9 Hz); 13C NMR (δ ppm): 47.7 N(CH2)2, 66.5 O(CH2)2, 122.3 C-2, 143.2 C-3, 113.6, 128.8–130.3 -Carom, 128.2 ipso-C to C=O, 135.4 Ar-ring ipso-C, 154.1 morpholine ipso-C, 188.1 C-1.
(E)-3-(4-Methylphenyl)-1-(4-morpholinophenyl)prop-2-en-1-one 12 IR (KBr) ν (cm−1): 3012, 2923, 2924, 2851, 1645, 1600, 1194, 810; 1H NMR (δ ppm): 1.57 (s, 3H, CH3 at phenyl ring), 3.32–3.35 (t, 4H, N(CH2)2, J = 4.8 Hz), 3.86–3.89 (t, 4H, O(CH2)2, J = 4.8 Hz), 6.92–6.94 (d, 1H, H2, J = 8.8 Hz), 7.21–7.80 (m, 8H, Harom), 8.00–8.02 (d, 1H, H3, J = 8.8 Hz); 13C NMR (δ ppm): 21.0 CH3 at phenyl ring, 47.7 N(CH2)2, 66.5 O(CH2)2, 121.2 C-2, 143.3 C-3, 113.5, 129.3–130.5 -Carom, 128.2 ipso-C to C=O, 132.7 Ar-ring ipso-C, 140.5 Ar-substitutent ipso-C, 154.1 morpholine ipso-C, 188.2 C-1.
(E)-3-(4-Chlorophenyl)-1-(4-morpholinophenyl)prop-2-en-1-one 13 IR (KBr) ν (cm−1): 3087, 2967, 2920, 2859, 1597, 1654, 1202, 817; 1H NMR (δ ppm): 3.34–3.37 (t, 4H, N(CH2)2, J = 4.7 Hz), 3.89–3.91 (t, 4H, O(CH2)2, J = 4.8 Hz), 6.97–6.99 (d, 1H, H2, J = 8.8 Hz), 7.35–7.76 (m, 8H, Harom), 8.00–8.02 (d, 1H, H3, J = 8.9 Hz); 13C NMR (δ ppm): 47.6 N(CH2)2, 66.5 O(CH2)2, 121.2 C-2, 141.8 C-3, 113.5, 129.4–130.6 -Carom, 129.1 ipso-C to C=O, 133.8 Ar-ring ipso-C, 135.0 Ar-substitutent ipso-C, 154.0 morpholine ipso-C, 192.2 C-1.
(E)-3-(4-Methoxyphenyl)-1-(4-morpholinophenyl)prop-2-en-1-one 14 IR (KBr) ν (cm−1): 3010, 2961, 2918, 2841, 1645, 1601, 1225; 1H NMR (δ ppm): 3.32–3.35 (t, 4H, N(CH2)2, J = 4.8 Hz), 3.87–3.90 (t, 4H, O(CH2)2, J = 4.8 Hz), 3.86 (s, 3H, OCH3 at phenyl ring), 7.59–7.61 (d, 1H, H2, J = 8.6 Hz), 6.92–7.46 & 7.75–7.79 (m, 8H, Harom), 8.00–8.02 (d, 1H, H3, J = 8.7 Hz); 13C NMR (δ ppm): 47.6 N(CH2)2, 55.3 OCH3 at phenyl ring, 66.5 O(CH2)2, 119.6 C-2, 143.1 C-3, 113.5, 129.2–130.4 -Carom, 127.9 ipso-C to C=O, 129.9 Ar-ring ipso-C, 153.9 Ar-substitutent ipso-C, 161.3 morpholine ipso-C, 187.8 C-1.
(E)-3-(4-Fluorophenyl)-1-(4-morpholinophenyl)prop-2-en-1-one 15 IR (KBr) ν (cm−1): 3009, 2969, 2919, 2849, 1650, 1602, 1227; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2, J = 4.7 Hz), 3.87–3.89 (t, 4H, O(CH2)2, J = 4.8 Hz), 6.93–6.95 (d, 1H, H2, J = 8.9 Hz), 7.08–7.78 (m, 8H, Harom), 8.00–8.02 (d, 1H, H3, J = 8.9 Hz); 13C NMR (δ ppm): 47.5 N(CH2)2, 66.5 O(CH2)2, 121.6 C-2, 141.9 C-3, 113.4, 115.8, 130.1, 131.5 -Carom, 128.8 ipso-C to C=O, 130.6 Ar-ring ipso-C, 154.1 Ar-substitutent ipso-C, 162.5 morpholine ipso-C, 187.8 C-1.
(E)-3-(4-Bromophenyl)-1-(4-morpholinophenyl)prop-2-en-1-one 16 IR (KBr) ν (cm−1): 3001, 2960, 2923, 2845, 1657, 1612, 1227; 1H NMR (δ ppm): 3.32–3.35 (t, 4H, N(CH2)2, J = 4.5 Hz), 3.86–3.87 (t, 4H, O(CH2)2, J = 4.6 Hz), 6.94–6.96 (d, 1H, H2, J = 8.8 Hz); 7.18–7.82 (m, 8H, Harom), 8.01–8.03 (d, 1H, H3, J = 8.6 Hz); 13C NMR (δ ppm): 47.9 N(CH2)2, 65.6 O(CH2)2, 121.8 C-2, 142.3 C-3, 113.8, 115.1, 130.7, 131.7 -Carom, 128.5 ipso-C to C=O, 131.2 Ar-ring ipso-C, 144.7 Ar-substitutent ipso-C, 162.7 morpholine ipso-C, 188.8 C-1.
(E)-1-(4-Morpholinophenyl)-3-(3-nitrophenyl)prop-2-en-1-one 17 IR (KBr) ν (cm−1): 3087, 2966, 2923, 2862, 1651, 1608, 1224; 1H NMR (δ ppm): 3.36–3.38 (t, 4H, N(CH2)2, J = 4.5 Hz), 3.88–3.90 (t, 4H, O(CH2)2, J = 4.6 Hz), 6.95–6.97 (d, 1H, H2, J = 8.9 Hz), 7.27–7.91 & 8.23–8.25 (m, 8H, Harom), 8.03–8.05 (d, 1H, H3, J = 8.9 Hz); 13C NMR (δ ppm): 47.3 N(CH2)2, 66.9 O(CH2)2, 122.0 C-2, 140.1 C-3, 113.3, 124.2–134.2 -Carom, 128.9 ipso-C to C=O, 137.1 Ar-ring ipso-C, 148.7 Ar-substitutent ipso-C, 154.3 morpholine ipso-C, 187.8 C-1.
(E)-1-(4-Morpholinophenyl)-3-(3-chlorophenyl)prop-2-en-1-one 18 IR (KBr) ν (cm−1): 3093, 2969, 2928, 2857, 1593, 1652, 1212, 830; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2, J = 4.6 Hz), 3.89–3.91 (t, 4H, O(CH2)2, J = 4.6 Hz), 6.96–6.98 (d, 1H, H2, J = 8.9 Hz), 7.33–7.81 (m, 8H, Harom), 7.98–8.00 (d, 1H, H3, J = 8.7 Hz); 13C NMR (δ ppm): 47.8 N(CH2)2, 66.4 O(CH2)2, 121.2 C-2, 141.6 C-3, 113.3, 128.8–130.1 -Carom, 129.3 ipso-C to C=O, 133.7 Ar-ring ipso-C, 145.2 Ar-substitutent ipso-C, 154.1 morpholine ipso-C, 192.3 C-1.
(E)-1-(4-Morpholinophenyl)-3-(3-fluorophenyl)prop-2-en-1-one 19 IR (KBr) ν (cm−1): 3018, 2974, 2924, 2843, 1649, 1605, 1226; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2, J = 4.8 Hz), 3.86–3.88 (t, 4H, O(CH2)2, J = 4.7 Hz), 6.92–6.94 (d, 1H, H2, J = 8.8 Hz), 7.18–7.68 (m, 8H, Harom), 7.92–7.94 (d, 1H, H3, J = 8.7 Hz); 13C NMR (δ ppm): 47.5 N(CH2)2, 66.6 O(CH2)2, 121.3 C-2, 141.8 C-3, 113.4, 125.3–130.1 -Carom, 128.6 ipso-C to C=O, 130.5 Ar-ring ipso-C, 154.3 morpholine ipso-C, 162.7 Ar-substitutent ipso-C, 187.5 C-1.
General method for the synthesis of 4-(4-morpholinophenyl)-6-aryl-6H-1,3-thiazin-2-amines 20–28
A mixture of (E)-1-(4-morpholinophenyl)-3-aryl-prop-2-en-1-ones 11–19 (0.001 mol) and thiourea (0.001 mol) in ethanol (50 mL) was refluxed, while a solution of potassium hydroxide (0.005 mol) in water (10 mL) was added portion-wise for 2 h. Refluxing was continued for a further 4 h and the mixture was poured into ice-cold water. The formed solid was separated by filtration, and purified by column chromatography using silica gel (100–200 mesh), with ethyl acetate–petroleum ether (b.p. 40–60°C) in the ratio 1:9 as eluent.
4-(4-Morpholinophenyl)-6-phenyl-6H-1,3-thiazin-2-amine 20 IR (KBr) (cm−1): 3399, 3289, 3000, 2965, 2921, 2852, 1660, 1600, 1240, 929, 821, 766, 700, 605; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2, J = 4.6 Hz), 3.87–3.89 (t, 4H, O(CH2)2, J = 4.9 Hz), 5.23–5.25 (d, 1H, H6, J = 12.2 Hz), 5.54–5.57 (d, 1H, H5, J = 11.9 Hz), 7.11–7.82 (m, 9H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 46.7 N(CH2)2, 55.0 C-6, 65.6 O(CH2)2, 112.8 C-5, 140.6 C-4, 126.8–134.3 -Carom, 142.1 153.8 ipso-C, 170.6 C-2.
6-(4-Methylphenyl)-4-(4-morpholinophenyl)-6H-1,3-thiazin-2-amine 21 IR (KBr) (cm−1): 3429, 3300, 2960, 2922, 2852, 1648, 1604, 1227, 928, 820, 768, 662; 1H NMR (δ ppm): 2.33 (s, 3H, CH3), 3.33–3.37 (t, 4H, N(CH2)2, J = 4.5 Hz ), 3.86–3.89 (t, 4H, O(CH2)2, J = 4.8 Hz), 5.22–5.24 (d, 1H, H6, J = 12.4 Hz), 5.55–5.56 (d, 1H, H5, J = 11.1 Hz), 7.26–7.89 (m, 8H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 24.4 CH3, 46.7 N(CH2)2, 55.0 C-6, 65.7 O(CH2)2, 113.0 C-5, 141.3 C-4, 125.9–134.3 -Carom, 154.0, 161.0 ipso-C, 171.6 C-2.
6-(4-Chlorophenyl)-4-(4-morpholinophenyl)-6H-1,3-thiazin-2-amine 22 IR (KBr) (cm−1): 3428, 3202, 2965, 2922, 2852, 1648, 1603, 1226, 928, 814, 744, 634; 1H NMR (δ ppm): 3.35–3.38 (t, 4H, N(CH2)2, J = 4.6 Hz ), 3.88–3.91 (t, 4H, O(CH2)2, J = 4.7 Hz), 5.24–5.27 (d, 1H, H6, J = 12.7 Hz), 5.57–5.60 (d, 1H, H5, J = 11.8 Hz), 7.30–7.93 (m, 8H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 46.8 N(CH2)2, 55.6 C-6, 66.5 O(CH2)2, 113.5 C-5, 140.5 C-4, 126.6–134.9 -Carom, 143.9, 154.1 ipso-C, 172.8 C-2.
6-(4-Methoxyphenyl)-4-(4-morpholinophenyl)-6H-1,3-thiazin-2-amine 23 IR (KBr) (cm−1): 3425, 3300, 2967, 2922, 2853, 1644, 1601, 1226, 819, 669; 1H NMR (δ ppm): 3.33–3.38 (t, 4H, N(CH2)2, J = 4.8 Hz ), 3.86 (s, 3H, OCH3), 3.87–3.92 (t, 4H, O(CH2)2, J = 4.6 Hz), 5.23–5.26 (d, 1H, H6, J = 11.9 Hz), 5.57–5.60 (d, 1H, H5, J = 11.3 Hz), 7.20–8.03 (m, 8H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 46.7 N(CH2)2, 55.1 OCH3, 55.9 C-6, 65.8 O(CH2)2, 112.9 C-5, 141.3 C-4, 127.9–134.6 -Carom, 152.5, 153.9 ipso-C, 170.8 C-2.
6-(4-Fluorophenyl)-4-(4-morpholinophenyl)-6H-1,3-thiazin-2-amine 24 IR (KBr) (cm−1): 3429, 3200, 2972, 2922, 2853, 1649, 1602, 1227, 929, 817, 669; 1H NMR (δ ppm): 3.33–3.36 (t, 4H, N(CH2)2, J = 4.8 Hz ), 3.87–3.90 (t, 4H, O(CH2)2, J = 5.1 Hz), 5.23–5.25 (d, 1H, H6, J = 12.9 Hz), 5.58–5.61 (d, 1H, H5, J = 11.1 Hz), 7.27–8.09 (m, 8H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 46.8 N(CH2)2, 55.2 C-6, 65.9 O(CH2)2, 113.8 C-5, 140.5 C-4, 126.6–132.1 -Carom, 153.9, 152.1 ipso-C, 171.3 C-2.
6-(4-Bromophenyl)-4-(4-morpholinophenyl)-6H-1,3-thiazin-2-amine 25 IR (KBr) (cm−1): 3429, 3200, 2967, 2922, 2855, 1646, 1602, 1226, 928, 817, 743, 631; 1H NMR (δ ppm): 3.34–3.39 (t, 4H, N(CH2)2, J = 4.7 Hz ), 3.86–3.89 (t, 4H, O(CH2)2, J = 4.4 Hz), 5.24–5.26(d, 1H, H6, J = 12.5 Hz), 5.57–5.61 (d, 1H, H5, J = 11.6 Hz), 7.32–7.95 (m, 8H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 46.9 N(CH2)2, 55.8 C-6, 66.7 O(CH2)2, 112.9 C-5, 140.7 C-4, 126.7–133.8 -Carom, 153.4, 154.5 ipso-C, 172.3 C-2.
4-(4-Morpholinophenyl)-6-(3-nitrophenyl)-6H-1,3-thiazin-2-amine 26 IR (KBr) (cm−1): 3419, 3200, 2965, 2920, 2848, 1660, 1599, 1241, 929, 820, 737, 606; 1H NMR (δ ppm): 3.36–3.39 (t, 4H, N(CH2)2, J = 4.9 Hz ), 3.88–3.90 (t, 4H, O(CH2)2, J = 4.8 Hz), 5.23–5.25 (d, 1H, H6, J = 12.8 Hz), 5.57–5.60 (d, 1H, H5, J = 10.9 Hz), 7.67–8.33 (m, 8H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 46.7 N(CH2)2, 54.9 C-6, 65.8 O(CH2)2, 113.6 C-5, 142.1 C-4, 126.8–136.6 -Carom, 147.2, 154.5 ipso-C, 170.1 C-2.
6-(3-Chlorophenyl)-4-(4-morpholinophenyl)-6H-1,3-thiazin-2-amine 27 IR (KBr) (cm−1): 3427, 3201, 2967, 2926, 2850, 1647, 1602, 1229, 921, 817, 744, 637; 1H NMR (δ ppm): 3.36–3.39 (t, 4H, N(CH2)2, J = 4.7 Hz ), 3.86–3.88 (t, 4H, O(CH2)2, J = 4.6 Hz), 5.23–5.26 (d, 1H, H6, J = 12.4 Hz), 5.57–5.60 (d, 1H, H5, J = 11.4 Hz), 7.33–7.89 (m, 8H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 46.6 N(CH2)2, 55.2 C-6, 66.9 O(CH2)2, 113.1 C-5, 140.8 C-4, 126.1–134.2 -Carom, 148.2, 154.5 ipso-C, 171.8 C-2.
6-(3-Fluorophenyl)-4-(4-morpholinophenyl)-6H-1,3-thiazin-2-amine 28 IR (KBr) (cm−1): 3434, 3209, 2969, 2927, 28513, 1648, 1604, 1225, 927, 814; 1H NMR (δ ppm): 3.32–3.35 (t, 4H, N(CH2)2, J = 4.8 Hz ), 3.86–3.89 (t, 4H, O(CH2)2, J = 5.1 Hz), 5.23–5.25 (d, 1H, H6, J = 12.7 Hz), 5.57–5.60 (d, 1H, H5, J = 11.3 Hz), 7.24–8.03 (m, 8H, Harom), the signal for NH2 protons was exchanged with H2O in the solvent (d6-DMSO); 13C NMR (δ ppm): 46.7 N(CH2)2, 55.4 C-6, 66.1 O(CH2)2, 113.5 C-5, 140.7 C-4, 126.1–132.4 -Carom, 148.3, 152.8 ipso-C, 171.8 C-2.
Microbiology
Materials
All the clinically isolated bacterial strains, namely Staphylococcus aureus, Bacillus subtilis, Vibreo cholerae, Escherichia coli, and Pseudomonas aeruginosa and fungal strains, namely Aspergillus flavus, Mucor, Rhizopus, and Microsporum gypseum were obtained from the Faculty of Medicine, Annamalai University, Annamalainagar, Tamil Nadu, India.
In vitro antibacterial and antifungal activity
The minimum inhibitory concentration (MIC) in μg/mL was determined by the two-fold serial dilution methodCitation30. The respective test compounds (20–28) were dissolved in DMSO to obtain 1 mg/mL stock solution. Seeded broth (broth containing microbial spores) was prepared in nutrient broth (NB) from 24-h-old bacterial cultures on nutrient agar (HiMedia, Mumbai) at 37 ± 1°C, while fungal spores from 1- to 7-day-old Sabouraud agar (HiMedia, Mumbai) slant cultures were suspended in Sabouraud dextrose broth (SDB). The number of colony forming units (cfu) of the seeded broth were determined by the plating technique, and adjusted in the range of 104–105 cfu/mL. The final inoculum size was 105 cfu/mL for the antibacterial assay and 1.1–1.5 × 102 cfu/mL for the antifungal assay. Testing was performed at pH 7.4 ± 0.2 for bacteria (NB) and at pH 5.6 for fungi (SDB). Exactly 0.4 mL of the solution of the test compound was added to 1.6 mL of seeded broth to form the first dilution. One milliliter of this was diluted with a further 1 mL of seeded broth to give the second dilution, and so on, till six such dilutions were obtained. A set of assay tubes containing only seeded broth were kept as control. The tubes were incubated in BOD (biochemical oxygen demand) incubators at 37 ± 1°C for bacteria and 28 ± 1°C for fungi. The MICs were recorded by visual observation after 24 h (for bacteria) and 72–96 h (for fungi) of incubation. Ciprofloxacin was used as the standard for bacterial studies and fluconazole as the standard for fungal studies.
Results and discussion
Chemistry
The classical approach for the synthesis of 4-(4-morpholinophenyl)-6-aryl-2H-1,3-thiazine-2-amines (20–28) was used as follows. (E)-1-(4-Morpholinophenyl)-3-aryl-prop-2-en-1-ones (11–19) were synthesized by the Claisen–Schmidt condensation of 1-(4-morpholinophenyl)ethanone and substituted benzaldehydes in the presence of alcoholic sodium hydroxide. Treatment of (E)-1-(4-morpholinophenyl)-3-aryl-prop-2-en-1-ones (11–19) with thiourea in the presence of potassium hydroxide in refluxing ethanol (, ) afforded the respective target molecules (20–28). The structures of these compounds were confirmed by melting points, FT-IR, MS, 1H NMR, and 13C NMR spectral studies, and elemental analysis. The mechanism involves the formation of a Michael adduct and its subsequent heterocyclization () with a tautomeric change to afford the title compounds.
Scheme 2. Synthesis reaction pathway for formation of 4-(4-morphlinophenyl)-6-aryl-6H-1,3-thiazin-2-amines.
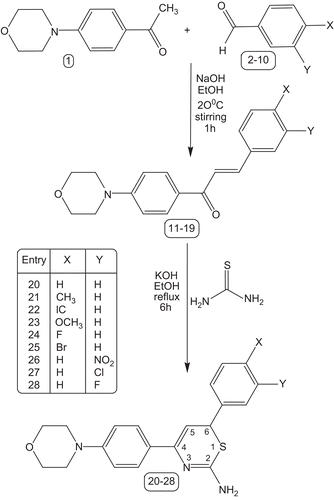
Table 1. Physical and analytical data of (E)-1-4-morpholinophenyl)-3-aryl-prop-2-en-1-ones (11–19) and 4-(4-morpholinophenyl)-6-aryl-6H-1,3-thiazin-2-amines (20–28).
The FT-IR spectrum of 4-(4-morpholinophenyl)-6-phenyl-6H-1,3-thiazin-2-amine 20 showed characteristic absorptions at 3342–3446 cm−1 due to N-H asymmetric and symmetric stretching vibrations of the primary amino group. The band at 1661 cm−1 is due to the presence of the C=N stretching frequency. The absorption frequency at 2961–3002 cm−1 is assigned to the aromatic C-H stretching vibration. The absorption band at 1229 cm−1 is consistent with the C-N stretching vibration. The observed NH2, C=N, C-H, and C-N stretching vibrational bands are supporting evidence for the formation of synthesized compound 20. Two doublets were obtained in the 1H NMR spectrum of 4-(4-morpholinophenyl)-6-phenyl-6H-1,3-thiazin-2-amine 20, due to H-5 and H-6 protons. The doublet at 5.14 ppm is assigned to the H-6 proton. The doublet observed in the downfield region at 5.55 ppm is due to the H-5 proton. The amino protons signal was exchanged with water in the solvent. Two triplets were observed, due to the methylene protons of the morpholine ring. The triplet observed in the region of 3.33–3.36 ppm corresponds to two protons and this signal is due to methylene protons N(CH2)2 of the morpholine ring. There was a another triplet in the region of 3.87–3.89 ppm, corresponding to two protons, which can be assigned to methylene protons O(CH2)2 of the morpholine ring. The aromatic protons appeared as a multiplet in the range 7.11–7.82 ppm. The 13C resonance at 170.6 ppm is assigned to the amino group bearing carbon C-2 for 4-(4-morpholinophenyl)-6-phenyl-6H-1,3-thiazin-2-amine 20. The 13C resonances at 140.6 and 112.8 ppm are due to the C-4 and C-5 carbons, respectively. The 13C resonance observed at 55.0 ppm is assigned to the C-6 carbon. Two signals were observed at 46.7 and 65.6 ppm. Of the two signals, one 13C resonance at 46.7 ppm is due to the methylene carbon N(CH2)2 of the morpholine ring, and the 13C resonance at 65.6 ppm is unambiguously assigned to the methylene carbon O(CH2)2 of the morpholine ring. The remaining 13C signal at 153.8ppm is due to ipso carbon. Aromatic carbons were observed in the range of 126.8–134.3 ppm.
Antibacterial activity
Novel 4-(4-morpholinophenyl)-6-aryl-2H-1,3-thiazine-2-amines (20–28) were tested for their antibacterial activity in vitro against S. aureus, B. subtilis, V. cholerae, E. coli, and P. aeruginosa. Ciprofloxacin was used as the standard drug. The minimum inhibitory concentration (MIC) values in μg/mL are reproduced in . A close survey of the MIC values indicates that all the compounds (20–28) exhibited a varied range (6.25–200 μg/mL) of antibacterial activity against all the tested bacterial strains, except compound 23 against P. aeruginosa and compound 26 against V. cholerae, which did not have activity at a maximum concentration of 200 μg/mL. Compound 20, which has no substitution at the para position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, exerted two-fold increased activity at a MIC value of 12.5 μg/mL against E. coli, and showed moderate activity against all other tested bacterial strains. Compound 21, with electron-donating methyl substitution at the para position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, showed four-fold increased activity against P. aeruginosa at a MIC value of 6.25 μg/mL, and showed moderate activity against all other tested bacterial strains with a varied range of 100–25 μg/mL. Compound 22, which has an electron-withdrawing chloro substitution at the para position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, showed potent activity against all the tested bacterial strains. Compound 23, with electron-donating methoxy substitution at the para position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, showed two-fold increased activity against B. subtilis and V. cholerae. Compound 24, with electron-withdrawing fluoro substitution at the para position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, showed excellent activity against all the tested bacterial strains except S. aureus, with a MIC value of 100 μg/mL. Compound 24 showed four-fold increased activity against V. cholerae and P. aeruginosa. Bromo-substituted compound 25 exerted moderate activity against all the tested strains, whereas meta-substituted nitro compound 26 possessed excellent antibacterial activity against all the tested strains except V. cholerae and E. coli. Against S. aureus, compound 26 exerted four-fold increased activity, and against B. subtilis, it exerted two-fold increased activity. Compound 27, which has electron-withdrawing chloro substitution at the meta position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, showed four-fold increased activity against S. aureus and two-fold increased activity against B. subtilis. Compound 28, which has electron-withdrawing fluoro substitution at the meta position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, showed four-fold increased activity against V. cholerae, E. coli, and P. aeruginosa, and exerted two-fold increased activity against S. aureus.
Table 2. In vitro antibacterial activity (MIC) values for compounds 20–28.
Antifungal activity
The in vitro antifungal activity of 4-(4-morpholinophenyl)-6-aryl-2H-1,3-thiazine-2-amines (20–28) was studied against the fungal strains, viz. Aspergillus flavus, Mucor, Rhizopus, and Microsporum gypseum. Fluconazole was used as the standard drug. The minimum inhibitory concentration (MIC) values in μg/mL are reproduced in . A close survey of the MIC values indicates that all the compounds (20–28) exhibited a varied range (6.25–200 μg/mL) of antifungal activity against all the tested bacterial strains except compounds 22, 24, and 28, which had no antifungal activity against A. flavus, M. gypseum, and Mucor, respectively, even at a high concentration of 200 μg/mL. Compound 20, having no substitution at the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, exerted four-fold increased activity against A. flavus and two-fold increased activity against Rhizopus, and showed moderate activity against Mucor and M. gypseum. Two-fold increased activity was noted against A. flavus and Rhizopus for compound 21, which had electron-donating methyl substitution at the para position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety. Four-fold increased activity was noted against Mucor for compound 22, with electron-withdrawing chloro substitution at the para position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety. Methoxy-substituted compound 23 showed four-fold increased activity against A. flavus, whereas fluoro-substituted compound 24 exerted four-fold increased activity against A. flavus and Mucor. Compound 25, with electron-withdrawing bromo substitution at the para position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, showed excellent activity against all the tested fungal strains. Against Rhizopus, compound 26, which had an electron-withdrawing nitro functional group, exerted four-fold increased activity. Two-fold increased activity was noted against Mucor for compound 27, with electron-withdrawing chloro substitution at the meta position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety. Compound 28, with electron-withdrawing fluoro substitution at the meta position of the phenyl rings attached to C-4 and C-6 carbons of the pyrimidine moiety, showed four-fold increased activity against Rhizopus and M. gypseum.
Table 3. In vitro antifungal activity (MIC) values for compounds 20–28.
Conclusion
Results of microbiological screening studies carried out to evaluate the antibacterial and antifungal potencies of the newly synthesized 4-(4-morpholinophenyl)-6-aryl-6H-1,3-thiazin-2-amines (20–28) are clearly known from and . Close inspection of the in vitro antibacterial and antifungal activity profiles in the differently electron-donating (CH3 and OCH3) and electron-withdrawing (-F, -Cl, Br, and -NO2) functional group-substituted phenyl rings of the novel 4-(4-morpholinophenyl)-6-aryl-2H-1,3-thiazine-2-amines (20–28) shows that they exerted strong antibacterial activity against all the tested bacterial strains. Electron-donating methyl-substituted compound 21 against P. aeruginosa, electron-donating methoxy-substituted compound 23 against B. subtilis, and strong, small-size electron-withdrawing fluoro-substituted compound 24 against V. cholerae and P. aeruginosa exerted excellent antibacterial activity. Compound 26, with an electron-withdrawing nitro group, exerted good activity against S. aureus and B. subtilis. Electron-withdrawing m-chloro-substituted compound 27 exerted excellent antibacterial activity against B. subtilis and E. coli, whereas electron-withdrawing fluoro-substituted compound 28 possessed good activity against all the tested bacterial strains. Results of the antifungal activity study show that the nature of the substituents on the phenyl ring, viz. chloro, fluoro, bromo, methyl, and methoxy functions at the para/meta positions of the aryl moieties, are determinants of the nature and extent of the antifungal activity of all the synthesized compounds 20–28 on the fungal strains, namely A. flavus, Mucor, Rhizopus, and M. gypseum. Compound 20 against A. flavus and Rhizopus, compound 21 against Rhizopus, compound 22 against Mucor, 23 against A. flavus, 24 against both A. flavus and Mucor, possessed excellent antifungal activity. Bulky bromo-substituted compound 25 exerted good activities against all the tested strains, whereas electron-withdrawing nitro-substituted compound 26 exerted good activity against Rhizopus. Electron-withdrawing chloro-substituted compound 27 against Mucor and strong electron-withdrawing fluoro-substituted compound 28 against Rhizopus and M. gypseum exerted excellent antifungal activity. The method of action of these compounds is unknown. These observations may promote further development of our research in this field. Further development of this group of morpholino 1,3-thiazin-2-amines may lead to compounds with better pharmacological profiles than standard antibacterial and antifungal drugs.
Acknowledgements
The authors are thankful to the NMR Research Center, Indian Institute of Science, Bangalore for recording spectra. One of the authors (V.K.) is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, Republic of India for providing financial support in the form of a CSIR-Senior Research Fellowship (SRF) in Organic Chemistry. Another author (J.T.) wishes to thank Annamalai University authorities for providing financial support in the form of a Research Fellowship.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Koketsu M, Tanaka K, Takenaka Y, Kwong CD, Ishihara H. Synthesis of 1,3-thiazine derivatives and their evaluation as potential antimycobacterial agents. Eur J Pharm Sci 2002;15:307–10.
- Kai H, Morioka Y, Tomida M, Takahashi T, Hattori M, Hanasaki K, et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 2: Orally bioavailable compounds. Bioorg Med Chem Lett 2007;17:3925–9.
- Kai H, Morioka Y, Murashi T, Morita K, Shinonome S, Nakazato H, et al. 2-Arylimino-5,6-dihydro-4H-1,3-thiazines as a new class of cannabinoid receptor agonists. Part 1: Discovery of CB2 receptor selective compounds. Bioorg Med Chem Lett 2007;17:4030–4.
- Barros-García FJ, Bernalte-García A, Lozano-Vila AM, Luna-Giles F, Pariente JA, Pedrero-Marín R, et al. Synthesis, structural characterization and influence on the phagocytic activity of human neutrophils of thiazoline and thiazine derivative ligands and their zinc(II) complexes. J Inorg Biochem 2006; 100:1861–1870
- Galanski ME, Erker T, Handler N, Lemmens-Gruber R, Kamyar M, Studenik CR. Studies on the chemistry of thienoanellated O,N- and S,N-containing heterocycles. Part 30: Synthesis and pharmacological properties of thieno[2,3-b][1,4]thiazines with potential vasopressin receptor antagonistic activity. Bioorg Med Chem 2006;14:826–36.
- Malinka W, Kaczmarz M, Filipek B, Sapa J, Glod B. Preparation of novel derivatives of pyridothiazine-1,1-dioxide and their CNS and antioxidant properties. Farmaco 2002;57:737–46.
- Tozkoparan B, Aktay G, Yesilada E. Synthesis of some 1,2,4-triazolo[3,2-b]-1,3-thiazine-7-ones with potential analgesic and antiinflammatory activities. Farmaco 2002; 57:145–52.
- Loiseau PM, Mettey Y, Vierfond JM. Antifilarial and trypanocidal properties of phenothiazines and related polycyclics as new lead structures. Int J Parasitol 1996;26:1115–17.
- Das J, Robl JA, Reid JA, Chong-Qing Sun, Misra RN, Brown BR, et al. Dual metalloprotease inhibitors. IV. Utilization of thiazepines and thiazines as constrained peptidomimetic surrogates in mercaptoacyl dipeptides. Bioorg Med Chem Lett 1994;4:2193–8.
- Skiles JW, Suh JT, Williams BE, Menard PR, Barton JN, Love B, et al. Angiotensin-converting enzyme inhibitors: new orally active 1,4-thiazepine-2,5-diones, 1,4-thiazine-2,5-diones, and 1,4-benzothiazepine-2,5-diones possessing antihypertensive activity. J Med Chem 1986;29:784–96.
- Manfred R, Michael M, Robert S, Wolfgang G. 1H and 13C NMR spectra of N-substituted morpholines. Eur Pat Appl (1989); EP 334146:28. Chem Abstr 1989;112:178999.
- Hale JJ, Mills SG, MacCross M, Dorn CP, Finke PE, Budhu RJ, et al. Phosphorylated morpholine acetal human neurokinin-1 receptor antagonists as water-soluble prodrugs. J Med Chem 2000;43:1234–7.
- Fisher MH, Wyvratt MJ. Morpholine derivatives compositions and uses. US Patent (1991); 5077290:10. Chem Abstr 1991;116:214513.
- Avramova P, Danchev N, Buyukliev R, Bogoslovova T. Synthesis, toxicological, and pharmacological assessment of derivatives of 2-aryl-4-(3-arylpropyl)morpholines. Arch Pharm 1998;331:342–6.
- Dorn CP, Hale JJ, MacCross M, Mills SG. Morpholine compounds are prodrugs useful as tachykinin receptor antagonists. US Patent (1997); 5691336:82. Chem Abstr 1997;128:48231.
- Varma RS, Prakash R, Khan MM, Ali A. 4-Phenyl morpholines as potent antimicrobial agents. Indian Drugs 1986;23:345–9.
- Misra VS, Singh S, Agarwal R, Chaudhary KC. Substituted morpholines derivatives as potent anti-inflammators. J Chem Soc Pak 1981;3:209–11
- Agarwal R, Chaudhary KC, Misra VS. Substituted morpholines compounds possessing central nervous system activities. J Chem Soc Pak 1984;6:308–10.
- Barret GC, Kane VV, Lowe G. Studies related to cephalosporin C. Part I. 3-Hydroxy- and 3-amino-furan-2(5H)-ones. J Chem Soc 1964:783–7.
- Calaycay JR, Kelly TM, MacNaul KL, McCauley ED, Qi H, Grant SK, et al.Expression and immunoaffinity purification of human inducible nitric-oxide synthase. Inhibition studies with 2-amino-5,6-dihydro-4H-1,3-thiazine. J Biol Chem 1996;271:28212–19.
- McCurnin DM, Bassert JM. Clinical Textbook for Veterinary Technicians, 5th ed. Philadelphia, PA: Saunders, 2002.
- Tandon VK, Maurya HK, Yadav DB, Tripathi A, Kumar M, Shukla PK. Naphtho[2,3-b][1,4]-thiazine-5,10-diones and 3-substituted-1,4-dioxo-1,4-dihydronaphthalen-2-yl-thioalkanoate derivatives: synthesis and biological evaluation as potential antibacterial and antifungal agents. Bioorg Med Chem Lett 2006;16:5883–7.
- Parks LW. Metabolism of sterols in yeast. CRC Crit Rev Microbiol 1978;6:301–41.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Design, synthesis, characterization, antibacterial and antifungal activities of novel class of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-selenadiazoles. J Enzyme Inhib Med Chem 2008;23:347–51.
- Gopalakrishnan M, Thanusu J, Kanagarajan V, Govindaraju R. Design, synthesis and in vitro microbiological evaluation of 6,6-dimethyl-7,9-diaryl-1,2,4,8-tetraazaspiro[4.5]decan-3-thiones – a new series of “tailor-made” compounds. J Enzyme Inhib Med Chem 2009;24:406–12.
- Gopalakrishnan M, Thanusu J, Kanagarajan V. Synthesis and biological evaluation of 5,7-diaryl-4,4-dimethyl-4,5,6,7-tetrahydropyridino[3,4-d]-1,2,3-thiadiazoles. Med Chem Res 2007;16:392–401.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V, Govindaraju R, Jayasri G. A convenient “one-pot” synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J Enzyme Inhib Med Chem 2007;22:709–15.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V. Synthesis, spectral analysis, antibacterial and antifungal activities of some 4,6-diaryl-4,5-dihydro-3-hydroxy-2[H]-indazole – a novel fused indazole derivative. J Enzyme Inhib Med Chem 2008;23:974–9.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Design, “one-pot” synthesis, characterization, antibacterial and antifungal activities of novel 6-aryl-1,2,4,5-tetrazinan-3-thiones in dry media. J Sulfur Chem 2007;28:383–92.
- Dhar MH, Dhar MM, Dhawan BN, Mehrotra BN, Ray C. Screening of Indian plants biological activity. Part I. Indian J Exp Biol 1968;6:232–47.