The publishers would like to apologise for an error that occurred in a recent issue of the Journal of Enzyme Inhibition and Medicinal Chemistry, 2009; 24(4): 972–985.
Synthesis of imidazole-containing analogues of farnesyl pyrophosphate and evaluation of their biological activity on protein farnesyltransferase
Laëtitia Coudray, Renata Marcia de Figueiredo, Stéphanie Duez, Sylvie Cortial, Joëlle Dubois
Scheme 3 & 4 should have been displayed as they are below:
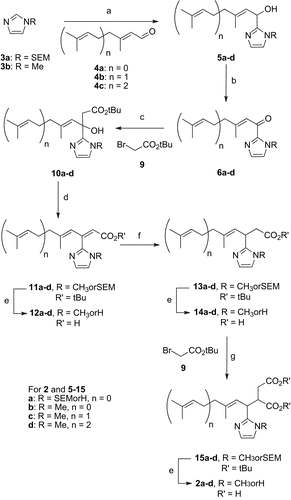
Scheme 3. Second synthetic pathway. a) nBuLi, THF, − 78°C, 45 min then addition of 4a-c, − 78°C, 20 min to 2h30 to RT 1h (68-100%); b) MnO2, THF, 0°C, 2h (80-100%); c) 9, THF, Zn, ultrasounds, 40°C, 5h (51-79%); d) POCl3, Pyridine, 0°C to RT 14h (75-85%); e) for a (R = SEM, n = 0) TFA, CH2Cl2, RT, 5h, for b (R = CH3, n = 0) HCO2H RT, 19h and for c (R = CH3, n = 1) and d (R = CH3, n = 2) SiO2, toluene, reflux, 14h; f) Mg, MeOH, RT, 3h (62-80%); g) LDA, THF, − 78°C, 35 min then addition of 9, − 78°C, 4h (42-72%).
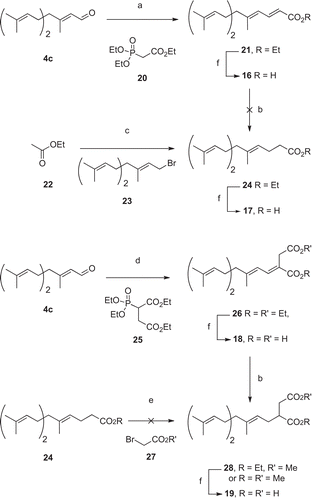
Scheme 4. Synthesis of farnesyl acids. a) NaH and 20, THF, 0°C, 10 min then 30 min RT, then addition of 4c, RT, 4h30 (74%); b) Mg, MeOH, RT, 4h (38%); c) CuI, LDA, THF, 2h, -110°C, then addition of 23, -110°C, 2h (69%); d) NaH and 25, THF, 0°C, 10 min then RT, 40 min, then addition of 4c, RT, 2h15 (66%); e) LDA, THF, − 78 °C, 35 min then addition of 27, − 78°C; f) NaOH 2M, EtOH, 70 °C, 15h (77-100%).