Abstract
Phytochemical investigations were performed on the EtOAc-soluble fraction of the whole plant of the sky flower (Duranta repens) which led to the isolation of the iridoid glycosides 1–6. Their structures were elucidated by both 1D and 2D NMR spectroscopic analysis. All the compounds showed potent antioxidative scavenging activity in four different tests, with half maximal inhibitory concentration (IC50) values in the range 0.481–0.719 mM against DPPH radicals, 4.07–17.21 µM for the hydroxyl radical (•OH) inhibitory activity test, 43.3–97.37 µM in the total reactive oxygen species (ROS) inhibitory activity test, and 3.39–18.94 µM in the peroxynitrite (ONOO−) scavenging activity test. Duranterectoside A (1) displayed the strongest scavenging potential with IC50 values of (0.481 ± 0.06 mM, 4.07 ± 0.03, 43.30 ± 0.05, 3.39 ± 0.02 µM) for the DPPH radicals, •OH inhibitory activity test, total ROS inhibitory activity test and the ONOO− scavenging activity test, respectively.
Introduction
Antioxidants, which scavenge active oxygen species (free radicals), have been found in a variety of foodstuffs and are commonly referred to as scavengers [Citation1,Citation2]. Many oxidants are plant based, they play an important role in protecting plants that are exposed to sunlight and live under severe oxygen stress. Antioxidants also play an important role in human health because the biologic defense mechanism cannot operate under severe oxygen stress. According to recent research, activated oxygen is thought to be a major factor in aging, hardening of the arteries, diabetes, cancer and in skin tissue injury [Citation3,Citation4]. Indeed approximately 90% of age related diseases have been linked to activated oxygen.
The genus Duranta (Verbenaceae) comprises about 35 species which mainly occur in the West Indies, tropical and South America. It is represented in Pakistan by the two species namely Duranta repens and Duranta stenostachya [Citation5].
Duranta repens Linn (syn D. erecta, D. microphylla, D. plumieri common names: sky flower, golden dew drop, pigeon berry) is a large sub-tropical plant varying from a shrub to a small tree and can reach up to 18 feet in height. It is commonly grown as a hedge plant and when trimmed forms a strong compact hedge, which is almost impenetrable to cattle [Citation6]. Duranta repens is widely distributed in the northern area of Pakistan. Medicinally, the fruit of this plant is used for the treatment of malaria [Citation7]. The MeOH extract of the plant also shows insecticidal and antifeedant properties against Aedes aegypti and Attagenus piceus, respectively [Citation8].
Previous studies on the genus Duranta have resulted in the isolation of various compounds including coumarinolignoids [Citation9], (E)-cinnamic acid, (E)-p-methoxycinnamic acid [Citation10], diterpenoids [Citation11,Citation12], flavonoids [Citation11,Citation12], steroids [Citation13], glycosides of phenylpropanoids [Citation14,Citation15], triterpenes [Citation16], and iridoids [Citation10,Citation15,Citation17,Citation18].
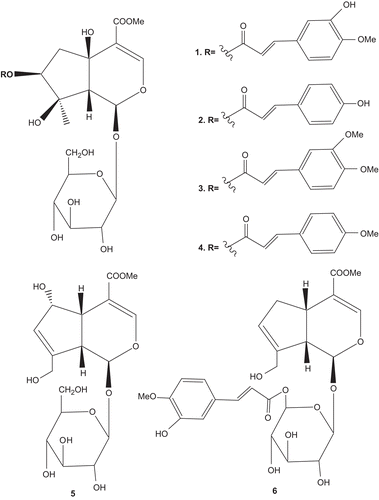
As part of our ongoing search for antioxidative constituents from the genus Buddleja [Citation19,Citation20], we investigated the antioxidative activities of the solvent-partitioned fractions from the MeOH extract of the whole plant of Duranta repens (). The EtOAc-soluble fraction of the plant showed stronger antioxidative activity than that of the other fractions (IC50 of 16.21 ± 0.054 μg/mL, in the hydroxyl radical inhibitory activity test) which prompted us to carry phytochemical studies on this plant. As a result, we isolated and characterised six known iridoid glycosides from the EtOAc soluble fraction. Duraterectoside A (1) [Citation15,Citation17], lamiidoside (2) [Citation15], durantoside III (3) [Citation15,Citation17], durantoside II (4) [Citation15,Citation17], deacetylasperulosidic acid methyl ester (5) [Citation21] and 6′-O-sinapoylgeniposide (6) [Citation21] (). Their structures were determined by modern spectroscopic analysis including 1D and 2D NMR techniques.
Table 1. Antioxidative activity of the solvent fractions.
In the current study we have described the antioxidant activities of the iridoid glycosides (1–6) against four different tests. Of these six compounds, 5 and 6 were isolated for the first time from the genus Duranta.
Materials and methods
Evaluation of antioxidative activity
DPPH (1,1-diphenyl-2-picryl hydrazyl) free radical scavenging activity
The reaction mixture containing 5 μL of test sample (1 mM in DMSO) and 95 μL of DPPH (Sigma, 300 μM) in ethanol. The reaction mixture was transferred to a 96-well microtitre plate (Molecular Devices, USA), incubated by ELISA at 37°C for 30 min and the absorbance measured at 515 nm. The percentage radical scavenging activity was determined by comparison with a DMSO containing control (). The IC50 values represent the concentration of compounds to scavenge 50% of the DPPH radicals. The positive control was 3-t-butyl-4-hydroxyanisole (BHA). All the chemicals used were of analytical grade (Sigma, USA) [Citation22, Citation23].
Table 2. Antioxidative activity of compounds 1–6.
Measurement of the inhibition of total reactive oxygen species (ROS) generation
Rat kidney homogenates prepared from the kidneys of freshly killed maleWistar rats, weighing 130–180 g, were mixed with or without the extracts or test compounds (dissolved in 10% EtOH to a final concentration of 0.4%). The mixtures were then incubated with 12.5 mM 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA, Molecular Probes), which was dissolved in 100% EtOH (final concentration: 0.2%), at 37°C for 30 min. A 50 mM aliquot of phosphate buffer (Wako Pure Chemical Industries, Osaka, Japan) solution at pH 7.4 was also used. DCHF-DA is a stable compound, which is hydrolysed by the intracellular esterase to yield a reduced, non-fluorescent compound, 2′,7′-dichlorodihydrofluorescein (DCHF). The ROS produced by the homogenates oxidises the DCHF to the highly fluorescent compound, 2′,7′-dichlorofluorescein (DCF). The fluorescence intensity of the oxidised DCF was monitored using a microplate fluorescence spectrophotometer (Bio-Tek Instruments, Winooski, VT) with excitation and emission wavelengths of 460 and 530 nm, respectively [Citation24]. Trolox was used as a positive control as it is an effective standard oxidant.
Measurement of the inhibition of hydroxyl radical generation
The extracts or test compounds were dissolved in 10% EtOH (final concentration: 0.4%) were added to 1 mM H2O2 and 0.2 mM FeSO4 (Fisher Scientific, Fair Lawn, N.J.) and incubated at 37°C for 5 min. The esterase-treated 2 M DCHF-DA (Molecular Probes) in 100% EtOH was then added, and the changes in fluorescence were monitored on a microplate fluorescence spectrophotometer (Bio-Tek Instruments, Winooski, VT), with excitation and emission wavelengths of 460 and 530 nm, respectively, for 30 min [Citation25]. Trolox was again used as a positive control.
Measurement of ONOO− scavenging activity
The ONOO− scavenging activity was measured by monitoring the oxidation of dihydrorhodamine 123 (DHR 123, Molecular Probes) using a slight modification of the method reported by Kooy et al. [Citation26]. The solution of DHR 123 (5 mM) in DMF, which was purged with N2, was stored as a stock solution at 80°C. This solution was placed on ice and kept in the dark prior to the study. The buffer consisted of 90 mM NaCl, 50 mM Na3PO4, 5 mM KCl at pH 7.4, and 100 M diethylenetriaminepentaacetic acid (DTPA), each of which was prepared with high-quality deionised H2O and purged with N2. The final concentration of DHR 123 was 5 M. The background and final fluorescent intensities were measured 5 min after treatment with and without the authentic ONOO−. DHR 123 was oxidised rapidly by the authentic ONOO−, and the final fluorescent intensity of the oxidised DHR 123 was measured using a FL 500 microplate fluorescence reader (Bio-Tek Instruments, Winooski, VT) at excitation and emission wavelengths of 480 and 530 nm, respectively. The results were expressed as the mean ± standard error (n = 3) for the final fluorescence intensity minus the background fluorescence. The effects were expressed as the percentage inhibition of DHR 123 oxidation, and the standard oxidant, DL-penicillamine was used as a positive control. The IC50 was defined as the concentrations of sample showing 50% of scavenging activity and were calculated from experiments performed in triplicate for all the three scavenging tests.
Results and discussion
Free radicals and ROS or RNS (reactive nitrogen species), including H2O2, •O2−, •OH, NO•, and ONOO−, play an important role in the etiology of a variety of human degenerative diseases. These reactive species are formed in the body as a consequence of aerobic metabolism and damage the intracellular components, such as nucleic acids, proteins, and lipids. ROS are also implicated in both aging and various degenerative disorders [Citation27].
We have investigated the general antioxidative effects of the compounds to inhibit the •OH, DPPH radical, total ROS and to scavenge authentic ONOO− for both the crude fractions and the isolated compounds 1–6. The EtOAc-soluble fraction of the plant showed a stronger antioxidative activity than that of the other fractions for all four scavenging tests (). A bioassay-directed isolation from the EtOAc-soluble fraction provided compounds 1–6. The compounds with a phenolic hydroxyl groups in the 1,4-orientation of the structure, showed a strong antioxidative activity for all four tests (). Compound 2, with a 1,4-hydroxyphenyliridoid glycoside moiety, showed strong activity with IC50 values of 0.481 ± 0.06, 4.07 ± 0.03, 43.3 ± 0.05 and 3.39 ± 0.02 for the DPPH radical, •OH, total ROS and ONOO− scavenging, respectively. Out of all the scavenging activity tests, the deacetylasperulosidic acid methyl ester (5), with no 1,4-hydroxyy\methoxyphenyl moieties, showed the lowest activity compared with the other compounds tested (). Duraterectoside A (1), which has a 1,4-methoxyphenyl as well 1,3-hydroxyphenyl moieties, showed weaker activity (IC50 values of 0.595 ± 0.02, 7.23 ± 0.05, 63.72 ± 0.07, 8.9 ± 0.04) than lamiidoside (2). Durantoside III (3), durantoside II (4) with 1,4/1,3-dimethoxyphenyl and 1,4-methoxyphenyl moieties respectively, were observed to have the lowest activity of the tested compounds with IC50 values (0.621 ± 0.07, 10.13 ± 0.06, 47.51 ± 0.07, 14.17 ± 0.05 and 0.662 ± 0.05, 10.67 ± 0.02, 90.21 ± 0.03, 16.91 ± 0.09, respectively) for DPPH, •OH, total ROS and to scavenge ONOO−, respectively, but these were still significant activities for all tests.
The antioxidative activity was also examined in terms of chemical structures including those of the functional radical and its orientation. The hydroxy and methoxy groups in the 1,4- and 1,3-orientations are mainly involved in the scavenging of iridoid glycosides (1–6). In particular, this was observed for lamiidoside (2) which contains the hydroxyl group in the 1,4-orientation. Furthermore, the development of scavenging activity was found to be comparatively low for compounds 3 and 4, in which the methoxy group is in the 1,4-orientation. Changing the hydroxyl group to a methoxy group at the 1,4-orientation also decreases the scavenging activity. Generally the ortho substitution with the electron donor methoxy group in the 1,4-oriented phenyl hydroxyl compounds were found to slightly increase the scavenging activity. Based on these results, a benzene ring where the hydroxyl radical is in the 1,4-orientation allowed the oxygen atom to share a positive charge, thereby causing stabilisation through delocalisation. The electron donating effect of the methoxy group in the 1,3-orientation helps to stabilise the positive charge and this is thought to influence the scavenging ability. The substitution radical of the 1,2- or 1,4-orientation generally donates an electron to the aromatic ring to activate it, either by a resonance effect or an inductive effect. This tendency was also found in all the scavenging tests against the iridoid glycosides (1–6).
Compounds 1–6 could be lead compounds in the treatment of oxidative-stress related human diseases. However, further in vivo study would help to explore the pharmacological properties of these compounds.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 1990;87:1620–1624.
- Bohme GA, Bon C, Lemaire M, Reibaud M, Piot O, Stutzmann JM, Doble A, Blanchard JC. Altered synaptic plasticity and memory formation in nitric oxide synthase inhibitor-treated rats. Proc Natl Acad Sci USA 1993;90:9191–9194.
- Ito N, Hirose M. Antioxidants-carcinogenic and chemopreventive properties. Adv Cancer Res 1989;53:247–302.
- Beckman JS, Ye YZ, Anderson PJ, Chen J, Accavitti MA, Tarpey MM, White CR. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe-Seyler 1994;375:81–88.
- Nasir E, Ali SI. Flora of West Pakistan, Department of Botany, University of Karachi. 1974;77:18–19.
- Sastri BN. The Wealth of India. New Delhi, India: Publication and Information Directorate CSIR, 1952:117–119.
- Perry LM, Metzger J. Medicinal Plants of East and Southeast Asia. Cambridge, England: The MIT press, 1980:432.
- Grainge M, Ahmad S. Handbook of Plants with Pest-control Properties. New York: John Wiley & Sons, 1998:118.
- Iqbal K, Anis I, Muhktar N, Malik A. Phosphodiesterase inhibitory coumarinolignoids from Duranta repens. Heterocycles 2003;60:151–157.
- Kuo YH, Chen ZS, Lin YL. Chemical components of the leaves of Duranta repens Linn. Chem Pharm Bull 1996;44:429–436.
- Anis I, Anis E, Ahmed S, Mustafa G, Malik A, Amtul Z, Rahman A-U-. Thrombin inhibitory constituents from Duranta repens. Helv Chim Acta 2001;84:649–655.
- Anis I, Ahmed S, Malik A, Yasin A, Choudhary MI. Enzyme inhibitory constituents from Duranta repens. Chem Pharm Bull 2002;50:515–518.
- Ahmed S, Nizami TA, Nawaz HR, Malik A. A new steroid from Duranta repens. Fitoterapia 1998; LXIX:448–450.
- Lin YL, Kuo YH. A New Glycoside, Brachynoside, isolated from Clerodendron brachyanthum Schauer. Chem Pharm Bull 1992;40:1928–1929.
- Yoshio T, Youko M, Takashi M, Choei O, Eiji H, Anki T, Hideaki O. Iridoid glucosides from the leaves and stems of Duranta erecta. Phytochemistry 1995;39:829–833.
- Hiradate S, Yada H, Ishii T, Nakajima N, Ohnishi-Kameyama M, Sugie H, Zungsontiporn S, Fuji Y. Three plant growth inhibiting saponins from Duranta repens. Phytochemistry 1999;52:1223–1228.
- Rimpler H, Timm H. Iridoids and ecdysones from verbenaceae. V. Iridoids from Duranta repens L. Z. Naturforsch 1974;29c:111–115.
- Rao CB, Rao TN, Kumar VEK, Vijay EKS. Chemical examination of the fruits of Duranta plumeri Jacq. Indian J Chem 1978;16B:844–845.
- Ahmad I, Chen S, Peng Y, Chen S, Xu L. Lipoxygenase inhibiting and antioxidant Iridoids from Buddleja crispa. J Enz Inhib Med Chem 2008;23:140–143.
- Ahmad I, Ahmad N, Wang F. Antioxidant phenylpropanoid glycosides from Buddleja davidii. J Enz Inhib Med Chem 2009;24:993–997.
- Fu X, Chou G, Wang Z. Iridoid glycosides from Gardenia jasminoides ELLIS. Helv Chim Acta 2008;91:646–653.
- Gulcin I. Antioxidant activity of L-adrenaline: A structure-activity insight. Chem Biol Interact 2009;179:71–80.
- Ak T, Gulcin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact 2008;174:27–37.
- Label CP, Bondy SC. Sensitive and rapid quantitation of oxygen reactive species formation in rat synaptosomes. Neurochem Int 1990;17:435–440.
- Nagao A, Seki M, Kobayashi H. Inhibition of xanthine oxidase by flavonoids. Biosci Biotechnol Biochem 1999;63:1787–1790.
- Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radical Biol Med 1994;16:149–156.
- Sgar S, Kallo IJ, Kaul N, Ganguly NK, Sharma BK. Oxygen free radicals in essential hypertension. Mol Cell Biochem 1992;111:103–108.