Abstract
In the present work, a new bis heterocyclic compound comprising both the piperidone and thiohydantoin nuclei namely 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one was synthesised and characterised with the help of mp, elemental analysis, FT-IR, MS and one-dimensional NMR (1H and 13C) spectra. The inhibitory effect of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one on 7,12-dimethylbenz[a]anthracene (DMBA) induced buccal pouch carcinogenesis was investigated in Syrian male hamsters. All the hamsters that were painted with DMBA on their buccal pouches for 14 weeks developed squamous cell carcinoma. Administration of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one effectively suppressed the oral carcinogenesis initiated with the DMBA as revealed by a reduced incidence of neoplasms. Lipid peroxidation, glutathione (GSH) content and the activities of glutathione peroxidase (GPx), glutathione S-transferase (GST) were used to biomonitor the chemopreventive potential of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one. Lipid peroxidation was found to be significantly decreased, whereas GSH, GPx, GST and GGT were elevated in the oral mucosa of tumour bearing animals. Our data suggest that 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one may exert its chemopreventive effects in the oral mucosa by modulation of lipid peroxidation, antioxidants and detoxification systems.
Introduction
The sulphur analogues of hydantoins with one or both carbonyl groups replaced by thiocarbonyl groups are known as thiohydantoins. There has been much interest in the synthesis and properties of 2-thiohydantoin derivatives as useful synthetic intermediates for a wide range of applications [Citation1,Citation2] as hypolipidaemic, anticarcinogenic, antimutagenic, antithyroidal, antiviral (e.g. against herpes simplex virus, HSV, human immunodeficiency virus (HIV), tuberculosis, antimicrobial (antifungal and antibacterial), anti-ulcer and anti-inflammatory agents, as well as pesticides.
Among the nitrogen containing heterocyclic compounds, piperidones have gained considerable importance owing to their varied biological properties and therapeutic importance. Baliah et al. reviewed the importance of piperidin-4-ones as intermediates in the synthesis of several physiologically active compounds [Citation3,Citation4]. In corollary of their interesting biological and pharmaceutical properties and synthetic utility, substantial interest has been demonstrated towards piperidones; this substructure is widely present in numerous alkaloids and synthetically derived molecules of biological importance [Citation5].
Squamous cell carcinoma of the oral cavity is the sixth most common malignant neoplasm worldwide and constitutes 47% of all cancers in the Indian subcontinent [Citation6]. Despite advances in cancer detection and therapy, the mortality rate of oral cancer remains high and the 5 year survival rate is among the lowest of the major cancers [Citation7]. Patients with oral squamous cell carcinoma are susceptible to multiple primary and secondary tumours due to the phenomenon of ‘field cancerisation’ [Citation8,Citation9]. Furthermore, treatment of these tumours often results in severe disfigurement and functional compromise.
Chemoprevention offers a novel approach to control the incidence of oral cancer. The buccal mucosa of the Syrian hamster is an excellent model for the investigation of oral cancer development and intervention by chemopreventive agents. Squamous cell carcinomas induced by the application of 7,12-dimethylbenz[a]anthracene (DMBA) to the cheek pouch of the Syrian hamsters are morphologically and histologically similar to human tumours [Citation10,Citation11]. In addition, hamster tumours express many metabolic and molecular markers that are expressed in human oral cancer [Citation12–14].
Several markers have been developed to biomonitor chemoprevention. These are based on the fact that chemopreventive agents can exert their anticarcinogenic effects by one or a combination of the following mechanisms: inhibiting formation of reactive carcinogenic metabolites, induction of enzymes that detoxify carcinogens, scavenging reactive oxygen species, influencing apoptosis and inhibiting cell proliferation [Citation15,Citation16]. The tripeptide glutathione, a physiologically important nucleophile and the enzymes glutathione peroxidase (GPx), glutathione S-transferase (GST) and gamma-glutamyltranspeptidase (GGT), which utilise reduced glutathione (GSH) as their substrate are significant as biomarkers of chemoprevention owing to their antioxidant and detoxification properties [Citation17–19].
A large number of chemopreventive agents have been identified in epidemiological and experimental studies, preclinical systems and clinical observations [Citation20,Citation21]. However, the toxic side effects produced by some of these agents have limited their extensive use [Citation22]. There is therefore a need to identify synthetic compounds that have significant chemopreventive potential. In view of our continued interest in the development of simpler and more convenient synthetic routes for achieving the biologically challenging hybrid heterocyclic systems [Citation23–31] and as part of our ongoing research programme, we have designed a system, which combines both bioactive thiohydantoin and piperidone nuclei together to give a compact structure like the title compound, 3-(2,6-bis(4-fluorophenyl)-3-methylpiperin-4-ylidene)-2-thioxoimidazolidin-4-one and we investigated the effects of 3-(2,6-bis(4-fluorophenyl)-3-methylpiperin-4-ylidene)-2-thioxoimidazolidin-4-one in a hamster buccal pouch carcinogenesis model using lipid peroxidation, GSH, GPx, GST and GGT as biochemical end points of chemoprevention.
Materials and methods
Chemicals
General remarks
We used thin layer chromatograhy (TLC) to assess both the reactions and the purity of the products. The reported melting point was taken in open capillaries and was uncorrected. IR spectra were recorded in KBr (pellet forms) on a Thermo Nicolet-Avatar–330 FT-IR spectrophotometer (Thermo Fisher Scientific Inc, Waltham, USA) and only note worthy absorption values (cm−1) were listed. One dimensional 1H and 13C NMR spectra were recorded at 400 MHz and 100 MHz respectively on a Bruker AMX 400 NMR spectrometer (Bruker Biospin International, Ag, Aegeristrasse, Switzerland) using DMSO-d as solvent. The electron spray impact (ESI) positive (+ve) mass (MS) spectrum was recorded on a Bruker Daltonics LC-MS spectrometer. Satisfactory microanalysis was obtained on a Carlo Erba 1106 CHN analyser (Fisher Scientific Inc, Waltham, USA). All the chemicals were purchased from Fluka, Sigma-Aldrich chemicals (St. Louis, USA) and Spectrochem Pvt. Ltd., (Mumbai, India).
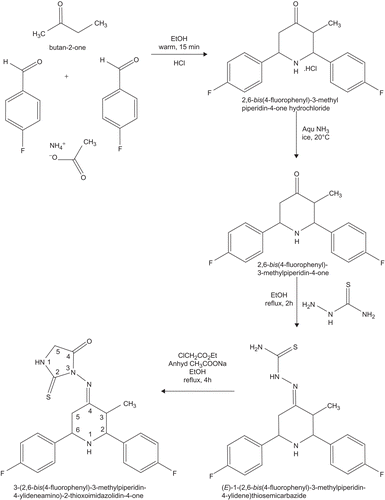
The parent compounds 2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-one and 2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-one thiosemicarbazone were prepared according to the literature [Citation3,Citation4].
Typical procedure for the synthesis of 2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-one
A mixture of ammonium acetate (0.1mol), 4-fluorobenzaldehyde (0.2mol) and butan-2-one (0.1mol) were dissolved in 95% alcohol (80 mL) and the solution was heated on a hot plate with gentle swirling until the colour of the mixture changed to orange. The mixture was cooled and poured into diethyl ether (100 mL) and concentrated hydrochloric acid (14 mL) was added. The precipitated hydrochloride was collected by filtration and recrystallised from the ethanol-ether mixture. The hydrochloride was dispersed in acetone and concentrated aqueous ammonia was added dropwise until a clear solution was obtained. The clear solution was poured into cold water and the solid precipitation was collected and recrystallised from ethanol.
Typical procedure for the synthesis of 2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-one thiosemicarbazone
A mixture of 2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-one (0.01mol) and thiosemicarbazide (0.01 mol) in ethanol (60 mL) was refluxed for two hours on a steam bath, cooled, then the separated solid was filtered and washed with water. The solid was subjected to column chromatography using benzene:chloroform (1:1) as eluent to afford the 2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-one thiosemicarbazone.
Typical procedure for the synthesis of novel new 3-(2,6-bis(4-fluorophenyl)-3-methylpiperin-4-ylidene)-2-thioxoimidazolidin-4-one
To a well stirred solution of 2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-one thiosemicarbazone (0.01mmol) and anhydrous sodium acetate (0.01 mol) in 30 mL of ethanol, was added chloroethyl acetate (0.01 mmol in 15 mL of ethanol) drop wise through the addition funnel for about 10 min. Then the reaction mixture was refluxed further for 4 h. After completion of the reaction, the reaction mixture was poured into ice cold water and the solid mass was collected and recrystallised twice from the ethanol to give the title compound with a yield of 78% at a mp of 182°C.
Spectral data of 3-(2,6-bis(4-fluorophenyl)-3-methylpiperin-4-ylidene)-2-thioxo imidazolidin-4-one
IR (KBr) (cm−1): 3412, 3304, 3071, 3030, 2985, 2931, 2869, 1717, 1635, 1597, 1221, 1030, 535, 836, 766; MS: m/z = 415, [M+1]+. Molecular formula C21H20F2N4OS; Elemental analysis: Carbon 60.85cal. (60.77found), Hydrogen 4.86cal. (4.75found), Nitrogen 13.52cal. (13.44found); 1H NMR (δ ppm): 0.78–0.8 (d, 3H, CH3 at C-3, JCH3,3a=6.58Hz), 2.07–2.09 (m, 1H, H5a), 2.54–2.59 (m, 1H, H3a), the signal for 1H, NH of piperidine merged with water peak, 2.67–2.79 (m, 1H, H5e), 3.46–3.48 (d, 1H, H2a, J2a,3a=10.61 Hz), 3.54–3.58 (dd, 1H, H6a, J6a,5e=2.46 Hz, J6a,5a=12.46 Hz), 3.78 (s, 2H, CH2 of imidazolidine), 7.12–7.51 (m, 8H, Ar-H’s), 11.72 (s, NH of imidazolidine); 13C NMR (δ ppm): 11.8 -CH3 at C-3, 32.5 C-5, 37.2 C-3, 44.5 CH2 of imidazolidine, 61 C-6, 67.7 C-2, 114.6–160.7 Ar-C’s, 162.4, 162.5, ipso-C, 163.4 C=N, 169.2 C=O, 173.9 C=S.
Animals
Male Syrian hamsters aged 6–10 weeks and weighing between 90–110 g were obtained from the Central Animal House, Annamalai University, India, for use in this study. The animals were housed six to a polypropylene cage and provided with food and water ad libitum. The animals were maintained in a controlled environment under standard conditions of temperature and humidity with an alternating 12 h light:dark cycle. All animals were fed with a standard pellet diet (Mysore Snack Feed Ltd., Mysore, India).
Treatment Schedule
The animals were randomised into experimental and control groups and divided into four groups of six animals each. Animals in group 1 were painted with a 0.5% solution of DMBA in liquid paraffin on their right buccal pouches using a number 4 brush, three times per week for 14 weeks, with each application leaving approximately 0.4 mg DMBA [Citation32]. Group 2 animals were painted with DMBA as in group 1 but with the addition of the oral administration of the title compound, 3-(2,6-bis(4-fluorophenyl)-3-methylpiperin-4-ylidene)-2-thioxoimidazolidin-4-one at a concentration of 1 mg/kg body weight, three times per week and on alternate days to the DMBA application. Animals in group 3 received only 3-(2,6-bis(4-fluorophenyl)-3-methylpiperin-4-ylidene)-2-thioxoimidazolidin-4-one as in group 2. Group 4 were the untreated control animals received neither DMBA nor 3-(2,6-bis(4-fluorophenyl)-3-methylpiperin-4-ylidene)-2-thioxoimidazolidin-4-one. The experiment was terminated at 14 weeks and all animals were sacrificed by cervical dislocation after an overnight fast. Fresh tissue was used for estimations.
Estimations
The thiobarbituric acid reactive substances (TBARS) released from the endogenous lipid peroxides reflecting the lipid peroxidation process were assayed in tissues as described by Ohkawa et al. [Citation33]. Superoxide dismutase activity was assayed by the method of Oberley et al. [Citation34], based on the inhibition of the formation of NADPH phenazine methosulphate and nitroblue tetrazolium. Catalase was assayed colorimetrically by the method of Sinha. [Citation35]. Reduced GSH was determined using the method of Beutler and Kelley [Citation36]. GPx activity was assayed by following the utilisation of hydrogen peroxide according to the method of Rotruck et al. [Citation37]. The activity of GST was assayed using the method of Habig et al. [Citation38] using 1-chloro, 2,4-dinitrobenzene (CDNB) as the substrate. Tissue protein was estimated by the method of Lowry et al. [Citation39] using bovine serum albumin as the standard.
Statistical analysis
The data have been expressed as mean ± standard deviation (SD). Statistical analysis on the data for body weights and tumour burden was carried out using Student’s t test. Tumour incidence was statistically compared using χ2-test. Statistical analysis on the data for biochemical assays was analysed using analysis of variance (ANOVA) and the group means were compared by the least significant difference test (LSD). The results were considered statistically significant if p < 0.05.
Results and discussion
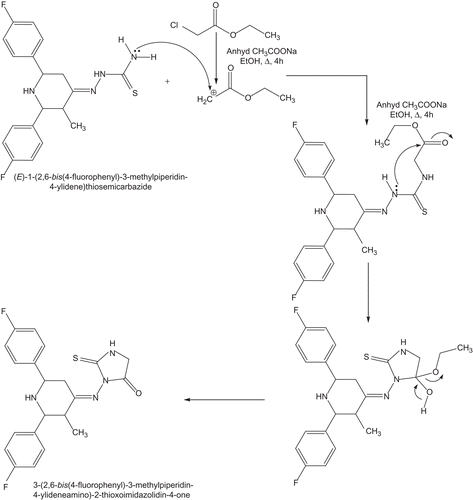
In the present work, a new series of bis heterocycles comprising both piperidine and thiohydantoin nuclei namely 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one were synthesised by the treatment of the respective thiosemicarbazones with chloroethyl acetate and anhydrous sodium acetate in refluxing ethanol for 4 h. The synthetic route for the formation of title compound is given in and a reaction mechanism is shown in . The structure of the synthesised compound is characterised with the help of mp, elemental analysis, FT-IR, MS, one-dimensional NMR (1H, 13C) spectra.
Scheme 1. Synthetic rote for the formation of 3-(2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino)-2-thioxoimidazolidin-4-one
The body weights, tumour incidence, tumour multiplicity, tumour burden. incidence of preneoplastic and neoplastic lesions in the control and experimental animals are shown in . The mean final body weights were significantly decreased in group 1 compared to the controls (group 4). No significant differences in body weight were observed in groups 2 and 3. In the DMBA painted animals (group 1), the incidence of squamous cell carcinoma (SCC) was 100% with a tumour multiplicity of 1.17 per hamster. These tumours were large and exophytic with a mean tumour burden of 208 mm3. In group 2, only one animal developed SCC, while the others exhibited moderate to severe dysplasia without infiltration. Although no tumours were observed in group 3, histopathological examination of the pouches revealed varying degrees of hyperplasia, hyperkeratosis and dysplasia. While administration of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one (1 and 10 mg/kg body weight (BW) decreased the tumour incidence as well as any preneoplastic lesions, the inhibitory effect was more pronounced at 10 mg/kg BW of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one. In group 4, the epithelium was found to be normal, intact and continuous.
Table 1. Body weight, tumour incidence, tumour multiplicity, tumour burden and incidence of preneoplastic and neoplastic lesions in control and experimental animals (mean ± SD; n = 6).
The levels of TBARS and the activities of SOD, catalase, GPx and GST on the buccal pouches of the control and experimental animals are shown in . The changes in the levels of TBARS, GSH and the activities of GPx and GST were significantly increased with a decrease in SOD and catalase activities in the DMBA painted animals (group 1) compared to the control (group 4). The administration of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one significantly enhanced the lipid peroxidation and the antioxidant activity in the buccal pouches of the group 2 and 3 animals compared to group 1. However, the antioxidant enhancing effects were more significant in group 3 (10 mg/kg BW) compared to the other groups. DMBA, an aryl hydrocarbon, is a potent carcinogen that induces significant histological and biochemical changes during HBP carcinogenesis. In the present study, topical application of DMBA to the cheek pouch for 14 weeks resulted in well-differentiated SCCs with a very high tumour burden.
Table 2. The cellular redox status in the buccal pouches of experimental and control animals (mean ± SD; n = 10).
In the DMBA-induced HBP tumours, a low lipid level was associated with enhanced activities of the GSH-redox cycle antioxidants. Lipid peroxidation, which prolongs the G1 phase of the cell cycle, has been suggested to control cell division. Das have demonstrated that tumour cells are more resistant to lipid peroxidation than normal cells [Citation40]. Several studies have documented decreased lipid peroxidation in rapidly proliferating tumours compared with their normal counterparts [Citation40,Citation41]. The thiol antioxidant GSH, which provides cellular protection against ROS in conjunction with GPx, γ-glutamyl tranferase (GGT) and glutathione reductase (GR) is recognised to play a key role in regulating cell proliferation [Citation42]. The overexpression of the GSH- and GSH-dependent enzymes has been reported in a wide range of tumours including oral squamous cell carcinoma (OSCC) [Citation43]. The elevated GSH redox cycle of antioxidants may serve to maintain a reduced environment providing a selective growth advantage for the hamster buccal pouch (HBP) tumours. Our results are in agreement with the hypothesis of Slater et al. [Citation44] that an increase in antioxidant capacity is associated with a decrease in lipid peroxidation. In contrast to GSH-dependent antioxidants, the activities of the antioxidant enzymes SOD and CAT were decreased in the HBP tumours. An increase in the activities of GPx with decreased SOD and CAT has been reported in various tumours, including the hamster cheek pouch carcinoma cell line HCPC-1 [Citation45–47]. It has been reported that reduced activities of SOD and CAT in fast growing tumor tissues can cause accumulation of the superoxide anion (02•-) and H2O2 with deleterious consequences including oxidation of critical sulphydryl groups and conformational changes in functional proteins as well as DNA strand breaks leading to oxidative stress. Oxidative stress has been implicated in cancer progression [Citation48].
Administration of 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one at two different doses (1 and 10 mg/kg BW) significantly suppressed tumour incidence, multiplicity, and tumour burden in the HBP by modulating the oxidant-antioxidant status. The results of the present study substantiate the anticarcinogenic effects of bioactive piperidones and imidazole derivatives as reported in the literature [Citation5,Citation49]. It was found that the administration of synthetic compounds could reverse the susceptibility to lipid peroxidation while simultaneously increasing the antioxidant status in the buccal pouch. These findings support reports by us and other workers that chemopreventive agents exert an “electrophilic counterattack response” characterised by the elevation of antioxidant enzymes [Citation50]. Out of the two doses used in the present study, the maximum dose of 10 mg/kg BW was more effective in chemoprevention. We have demonstrated that 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one suppresses DMBA-induced oral neoplasms as revealed by the reduced incidence of carcinomas. The present preliminary study suggest that 3-[2,6-bis(4-fluorophenyl)-3-methylpiperidin-4-ylideneamino]-2-thioxoimidazolidin-4-one has potential anticarcinogenic properties in experimental animals and could be a promising candidate for chemoprevention.
Acknowledgements
Authors are thankful to NMR Research Centre, Indian Institute of Science, Bangalore for recording spectra. V. Kanagarajan is grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, Republic of India for providing financial support in the form of CSIR-Senior Research Fellowship (SRF) in Organic Chemistry. J. Thanusu is thankful to the Annamalai University authorities for providing University Studentship.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Cherouvrier JR, Carreaux F, Bazureau JP. Reactivity of 2-thiohydantoins towards various electrophilic reagents: Applications to the synthesis of new 2-ylidene-3,5-dihydro-4H-imidazol-4-ones. Molecules 2004;9:867–875.
- Wang ZD, Sheikh SO, Zhang Y. A simple synthesis of 2-thiohydantoins. Molecules 2006;11:739–750.
- Baliah V, Jeyaraman, R, Chandrasekaran L. Synthesis of 2,6-disubstituted piperidones, oxanes and thianes. Chem Rev 1983;83:379–423.
- Noller CR, Baliah V. The preparation of some piperidine derivatives by the Mannich reaction. J Am Chem Soc 1948;70:3853–3854.
- Angle SR, Breitenbucher JG. In: Atta-ur-Rahman, (ed.) Studies in Natural Products Chemistry, Stereoselective Synthesis Vol 16, New York: Elsevier, 1995:453.
- Johnson NW. Epidemiology of Oral Cancer in Risk Markers of Oral Disease. In: Johnson NW, (ed.) Oral Cancer Vol 2. Cambridge: Cambridge University Press, 1991:3–26.
- Boring CC, Squires TS, Tong T. Cancer statistics. CA Cancer J Clin 1992;42:19–38.
- Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium: clinical implications of multicentric origin. Cancer 1953;6:963–968.
- Licciardello JTW, Spitz MR, Hong WK. Multiple primary cancer of the head and neck, esophagus and lung. International J Rad Oncol Biol Physics 1989;17:467–476.
- Gimenez-Conti IB, Slaga TJ. The hamster cheek pouch model of carcinogenesis and chemoprevention. Adv Expt Med Biol 1992:320:63–67.
- Gimenez-Conti IB, Slaga TJ. The hamster cheek pouch carcinogenesis model. J Cell Biochem Suppl 1993;17F:83–90.
- Solt DB, Shklar G. Rapid induction of gamma glutamyl transpeptidase rich intraepithelial clones in 7,12-dimethylbenz[a]anthracene treated hamster buccal pouch. Cancer Res 1982;45:285–291.
- Kwong YY, Husain Z, Biswas DK. C-Ha-ras gene mutation and activation precede pathological changes in DMBA-induced in vivo carcinogenesis. Oncogene 1992;7:1481–1489.
- Chang KW, Lin SC, Koos S, Pather K, Solt D. P53 and Ha-ras mutations in chemically induced hamster buccal pouch carcinomas. Carcinogenesis 1996;17:595–600.
- Wattenberg LW. Chemoprevention of cancer. Cancer Res 1985;45:1–8.
- Sharma S, Stutzman JD, Kelloff GJ, Steele VE. Screening of potential chemopreventive agents using bio chemical markers of carcinogenesis. Cancer Res 1994;54:5848–5855.
- Anderson ME, Meister A. Transport and direct utilization of gamma-glutamyl cyst(e)ine for glutathione synthesis. Proc Natl Acad Sci USA 1983;80:707–711.
- Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis. Mutation Res 1988;202:343–361.
- Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprevention and drug resistance. Critical Rev Biochem Mol Biol 1995;30:445–600.
- Boone CW, Kelloff GJ, Malone WE. Identification of candidate cancer chemopreventive agents and their evaluation in animal models and human clinical trials: A review. Cancer Res 1990;50:2–9.
- Lippman SM, Benner SE, Hong WK. Cancer chemoprevention. J Clin Oncol 1994;12:851–873.
- Ito N, Fukushima S, Tsuda H. Carcinogenicity and modification of the carcinogenic response by BHA, BHT and other antioxidants. CRC Critical Rev Toxicol 1985;15:109–150.
- Gopalakrishnan M, Kanagarajan V, Thanusu J. Novel one-pot synthesis of 1,2,4-triazolidin-3-thiones comprising piperidine moiety. Green Chem Lett Rev 2008;1:241–246.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J, Govindaraju R.A simplified green chemistry approaches to organic synthesis in solid media. activated fly ash, an industrial waste (pollutant) as an efficient and novel catalyst for some selected organic reactions in solvent-free conditions under microwave irradiation. Arkivoc 2006;13:130–141.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J, Govindaraju R, Ezhilarasi MR. Microwave-promoted facile and rapid solvent-free procedure for the efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones and -thiones using ZrO2/SO42- as a reusable heterogeneous catalyst. Lett Org Chem 2006;3:484–488.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Aluminium metal powder (atomized) catalyzed Friedel-Crafts acylation in solvent-free conditions: A facile and rapid synthesis of aryl ketones under microwave irradiation. Catalysis Commun 2005;6:753–756.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J, Govindaraju R. Silica gel supported sodium hydrogen sulfate as an efficient and reusable heterogeneous catalyst for the synthesis of imines in solvent-free conditions under microwave irradiation. J Chem Res 2005;5:299–303.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Organic synthesis in solid media. Alumina supported sodium hydrogen sulfate as an effective and reusable catalyst for ‘one-pot’ synthesis of amides from ketones in dry media under microwave irradiation. Lett Org Chem 2005;2:444–446.
- Gopalakrishnan M, Sureshkumar P, Kanagarajan V, Thanusu J. Design, ‘one-pot’ synthesis, characterization, antibacterial and antifungal activities of novel 6-aryl-1,2,4,5-tetrazinan-3-thiones in dry media. J Sulfur Chem. 2007;8:383–392.
- Gopalakrishnan M, Sureshkumar P, Thanusu J, Kanagarajan V, Govindaraju R, Jayasri G. A convenient ‘one-pot’ synthesis and in vitro microbiological evaluation of novel 2,7-diaryl-[1,4]-diazepan-5-ones. J Enz Inhib Med Chem 2007;22:709–715.
- Gopalakrishnan M, Thanusu J, Kanagarajan V. Heterogeneous NaHSO4.SiO2 catalyzed ‘one-pot’ synthesis and in vitro antibacterial and antifungal activities of pyridino-1,2,3-thiadiazoles. J Sulfur Chem 2008;29:179–185.
- Shklar G. Experimental oral pathology in the Syrian hamster. Progress in Expt Tumour Res 1972;16:518–538.
- Ohkawa H, Ohisi N, Yagi K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt Biochem 1979;95:351–358.
- Oberley LW, Spitz DR. In: ed. Methods in Enzymology. New York: Academic Press, 1984:457.
- Sinha AK. Colorimetric assay of Catalase. Anal Biochem 1972;47:389.
- Beutler E, Kelley BM. The effect of sodium nitrate on RBC glutathione. Experimentia 1963;29:96–97.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: Biochemical roles as a component of glutathione peroxidase. Science 1973;179:588–590.
- Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases, the first enzymatic step in mercapturic acid formation. J Biol Chem 1974;249:7130–7139.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folins phenol reagent. J Biol Chem 1951;193:265–275.
- Das UN. A radical approach to cancer. Med Sci Monit 2002;8:79.
- Subapriya R, Bhuvaneswari V, Ramesh V, Nagini S. Ethanolic Neem (Azadirachta indica) leaf extract induces apoptosis in the hamster buccal pouch carcinogenesis model by modulation of Bcl-2, Bim, caspase 8 and caspase 3. Cell Biochem Func 2005;23:229–234.
- Kohno Y, Patel V, Kim Y, Tsuji T, Chin BR, Sun M, Bruce Donoff R, Kent R, Wong D, Todd R. Oncogene - P12CDK2-AP1 mediates DNA damage responses induced by DMBA. Oral Oncol 2002;38:274–280.
- Ghalia AHB, Fouad IM. Glutathione and its metabolizing enzymes in patients with different benign and malignant diseases. Clin Biochem 2000;33:657.
- Slater TF, Benedetto C, Burton GW, Cheeseman KH, Ingold KU, Nodes JT. Lipid peroxidation in animal tumours: A disturbance in the control of cell division. In: Thaler-Dao H, Paoletti R, Crastes de Paulet A, eds. Icosanoids and Cancer. New York: Raven Press, 1984:21–29.
- Chandra Mohan KVP, Nagini S. Combination chemoprevention by tomato and garlic in the hamster buccal pouch carcinogenesis model. Nutr Res 2003;23:1403–1406.
- Moghadasian MH, Freeman HJ, Godin DV. Endogenous antioxidant status in neoplastic and adjacent tissues in 1,2-dimethylhydrazine-induced colon cancer in rats: Effects of olsalazine. Carcinogenesis 1996;17:983–987.
- Lam EWN, Zwacka R, Seftor EA, Nieva DRC, Davidson BL, Engelhardt JF, Hendrix MJC, Oberley LW. Effects of antioxidant enzyme overexpression on the invasive phenotype of hamster cheek pouch carcinoma cells. Free Radic Biol 1999;27:572–579.
- Cheng SC, Prakash AS, Pigott MA, Hilton BD, Lee H, Harvey RG, Dipple A. A metabolite of the carcinogen 7,12-dimethylbenz[a]anthracene that inititate carcinogenesis by causing mutation. Carcinogenesis 1988;9:1721–1723.
- Sinhababu AK, Thakker DR, Prodrugs of anticancer agents. Adv Drug Delivery Rev 1996;19:241–273.
- Prestera T, Zhang Y, Spencer SR, Wilezak C, Talalay P. The electrophile counterattack response: protection against neoplasia and toxicity. Adv Enzyme Regul 1993;33:281–296.