Abstract
A series of novel 2-substituted-thio-1,3,4-thiadiazoles bearing a 5-nitroaryl moiety including nitrofuran, nitrothiophene or nitroimidazole at the 5-position and a bulky residue attached to the 2-position of the thiadiazole ring were synthesised as potential antileishmanial agents. The target compounds were evaluated against the promastigote form of Leishmania major using the tetrazolium bromide salt (MTT) colorimetric assay. All test compounds exhibited high activity against L. major promastigotes with 50% inhibitory concentrations (IC50) ranging from 1.11 to 3.16 μM. The structure-activity relationship study indicated that the S-pendant group attached to the 2-position of the thiadiazole ring has a high flexibility for structural alteration therefore retaining good antileishmanial activity.
Introduction
Leishmaniasis is caused by the Leishmania parasite which is transmitted to humans by sandflies [Citation1]. The term leishmaniasis comprises three different clinical manifestations: generalised visceral (kala-azar), cutaneous, and mucocutaneous leishmaniasis [Citation2]. While cutaneous leishmaniasis poses essentially cosmetic problems, mucocutaneous leishmaniasis leads to painful disfiguration, social stigmatisation and often severe secondary infections, visceral leishmaniasis is generally lethal if left untreated [Citation3]. It is estimated that over 350 million people live at risk of infection [Citation4]. In spite of the socioeconomic importance of this tropical infection, effort directed toward the discovery of new drugs and/or a vaccine against it, are underdeveloped [Citation5,Citation6].
The recommended drugs for treatment of leishmaniasis are the pentavalent antimonials, including sodium stibogluconate (Pentostam®) and meglumine antimoniate (Glucantime®) [Citation3]. These drugs have been used clinically for more than 50 years and are still the first choice drug, for the treatment of leishmania [Citation7]. The development of resistance against the antimonial compounds is of great concern, and poses a major impediment in the successful therapy of the disease [Citation8]. In unresponsive cases, there are some alternative drugs to antimonial compounds such as amphotericine B, pentamidine [Citation9] and in the case of visceral leishmaniasis the only orally adminstered drug is miltefosine [Citation10]. However all these drugs are expensive, potentially toxic and require long term treatment. In addition, the development of drug resistance by the pathogens especially in an HIV-leishmania co-infection has aggravated the public health risk [Citation11].
Thus the lack of an alternative chemotherapeutic approach to the treatment of leishmania requires urgent attention and a great number of synthetic compounds have been evaluated in recent years in antileishmanial assays [Citation12–14].
In this regard, the use of nitroheterocycle scaffolds such as 5-nitrofurans, 5-nitrothiophenes and 5-nitroimidazoles in the development of anti-parasitic agents has been well established [Citation15,Citation16]. On the other hand, the anti-parasitic property of 1,3,4-thiadiazoles has been well documented and their attachment to other heterocycles often alters the bioactivity, depending upon the type of substituent and the position of the attachment [Citation17,Citation18]. Accordingly, in continuation of our previous papers [Citation10,Citation19] which were mostly devoted to the synthesis of diverse heterocycles with the emphasis on the role of 2,5-disubstituted-1,3,4-thiadiazole derivatives as anti-parasitic drugs, we decided to focus our attention toward the synthesis of new structures of 5-(5-nitroaryl)-2-substitutedthio-1,3,4-thiadiazoles to evaluate their antileishmanial activity against the promastigote form of Leishmania major.
Experimental
Chemistry
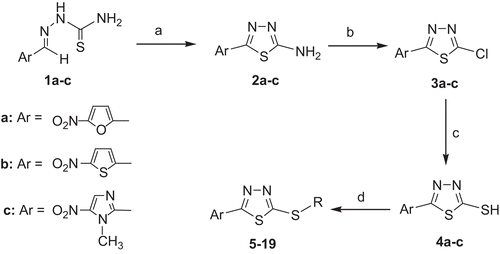
All the starting materials, reagents and solvents were purchased from Merck (Darmstadt, Germany). As illustrated in , the key intermediate compounds, 4a–c were synthesised according to the general methods previously described by us [Citation20]. The purity of the synthesised compounds was confirmed by thin layer chromatography (TLC) using various solvents of different polarities. Merck silica gel 60 F254 plates were used for analytical TLC. The melting points were determined on a Kofler hot stage apparatus (C. Reichert, Vienna, Austria) and were uncorrected. The 1H-NMR spectra were recorded using a Bruker 400 spectrometer (Rheinstatten, Germany), and chemical shifts expressed as δ (ppm) with tetramethylsilane (TMS) as the internal standard. The IR spectra were obtained on a Shimadzu 470 (Shimadzu, Tokyo, Japan) spectrophotometer (potassium bromide disks). The mass spectra were run on a Finigan TSQ-70 spectrometer (Finigan, San Jose, CA, USA) at 70 eV. Elemental analyses were carried out on a CHN-O-rapid elemental analyser (Heraeus, Hanau, Germany) for C, H and N, and the results are within ± 0.4% of the theoretical values.
General procedure for preparation of compounds 5–13
To a mixture of compounds 4a–c (1 mmol) and KOH (1 mmol) in EtOH, the appropriate 2-bromo-1-(chlorophenyl) ethanone (1 mmol) were added. Then, the reaction mixture was allowed to stir overnight. The reaction was followed by TLC, which was accompanied by a colour change from red to yellow. The solvents were removed under reduced pressure, the residue was washed with water and crystallised from ethanol to give compounds 5–13.
1-(2-Chlorophenyl)-2-(5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (5)
Yield 79%; mp 188–189°C; 1H NMR (400 MHz, CDCl3) δ: 8.02 (m, 1H, H6 phenyl), 7.96–7.92 (m, 1H, H3 phenyl), 7.9 (d, 1H, J = 4.4 Hz, H4 thiophene), 7.64–7.6 (m, 1H, H4 phenyl), 7.51–7.48 (m, 1H, H5 phenyl), 7.36 (d, 1H, J = 4.4 Hz, H3 thiophene), 5 (s, 2H, S-CH2-CO). IR (KBr, cm−1) νmax: 1699 (C=O), 1517 and 1339 (NO2). Anal. Calcd for C14H8ClN3O3S3: C, 42.26; H, 2.03; N, 10.56. Found: C, 42.55; H, 2.16; N, 10.23.
1-(2-Chlorophenyl)-2-(5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (6)
Yield 54%; mp 178–179°C; 1H NMR (400 MHz, CDCl3) δ: 8.03–7.92 (m, 2H, H4 and H6 phenyl), 7.64 (d, 1H, J = 7.2 Hz, H3 phenyl), 7.56–7.48 (m, 2H, H5 phenyl and H4 furan), 7.34 (d, 1H, J = 3.6 Hz, H3 furan), 5.04 (s, 2H, S-CH2-CO). IR (KBr, cm−1) νmax: 1705 (C=O), 1506 and 1347 (NO2). MS (m/z, %): 381 (M+, 81), 331 (10), 138 (100), 111 (80). Anal. Calcd for C14H8ClN3O4S2: C, 44.04; H, 2.11; N, 11.01. Found: C, 44.22; H, 2.32; N, 10.85.
1-(2-Chlorophenyl)-2-(5-(1-methyl-5-nitro-1H-imidazol-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (7)
Yield 93%; mp 178–180°C; 1H NMR (400 MHz, CDCl3) δ: 8.1 (s, 1H, H imidazole), 8.03 (m, 1H, H6 phenyl), 7.98–7.92 (m, 1H, H3 phenyl), 7.66–7.6 (m, 1H, H5 phenyl), 7.53–7.46 (m, 1H, H4 phenyl), 5.03 (s, 2H, S-CH2-CO), 4.53 (s, 3H, N-CH3). IR (KBr, cm−1) νmax: 1684 (C=O), 1523 and 1338 (NO2). Anal. Calcd for C14H10ClN5O3S2: C, 42.48; H, 2.55; N, 17.69. Found: C, 42.37; H, 2.62; N, 17.72.
1-(3-Chlorophenyl)-2-(5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (8)
Yield 43%; mp 185–187°C; 1H NMR (400 MHz, CDCl3) δ: 8.02 (s, 1H, H2 phenyl), 7.96–7.91 (m, 1H, H6 phenyl), 7.9 (d, 1H, J = 4.4 Hz, H4 thiophene), 7.64–7.59 (m, 1H, H4 phenyl), 7.52–7.46 (m, 1H, H5 phenyl), 7.35 (d, 1H, J = 4.4 Hz, H3 thiophene), 5 (s, 2H, S-CH2-CO). IR (KBr, cm−1) νmax: 1693 (C=O), 1518 and 1344 (NO2). Anal. Calcd for C14H8ClN3O3S3: C, 42.26; H, 2.03; N, 10.56. Found: C, 42.37; H, 1.94; N, 10.63.
1-(3-Chlorophenyl)-2-(5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (9)
Yield 84%; mp 177–179°C; 1H NMR (400 MHz, CDCl3) δ: 8.12–8.08 (m, 1H, H2 phenyl), 8.04–8 (m, 1H, H6 phenyl), 7.89 (d, 1H, J = 4 Hz, H4 furan), 7.8–7.74 (m, 1H, H5 phenyl), 7.63 (d, 1H, J = 8 Hz, H4 phenyl), 7.6 (d, 1H, J = 4 Hz, H3 furan), 5.22 (s, 2H, S-CH2-CO). IR (KBr, cm−1) νmax: 1698 (C=O), 1503 and 1347 (NO2). MS (m/z, %): 381 (M+, 8), 331 (23), 139 (100). Anal. Calcd for C14H8ClN3O4S2: C, 44.04; H, 2.11; N, 11.01. Found: C, 44.15; H, 2.06; N, 10.94.
1-(3-Chlorophenyl)-2-(5-(1-methyl-5-nitro-1H-imidazol-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (10)
Yield 52%; mp 189–191°C; 1H NMR (400 MHz, CDCl3) δ: 8.1 (s, 1H, H imidazole), 8.04 (s, 1H, H2 phenyl), 7.98–7.92 (m, 1H, H6 phenyl), 7.66–7.6 (m, 1H, H4 phenyl), 7.52–7.46 (m, 1H, H5 phenyl), 5.03 (s, 2H, S-CH2-CO), 4.53 (s, 3H, N-CH3). IR (KBr, cm−1) νmax: 1685 (C=O), 1522 and 1344 (NO2). Anal. Calcd for C14H10ClN5O3S2: C, 42.48; H, 2.55; N, 17.69. Found: C, 42.37; H, 2.34; N, 17.82.
1-(4-Chlorophenyl)-2-(5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (11)
Yield 62%; mp 182–184°C; 1H NMR (400 MHz, CDCl3) δ: 8 (d, 2H, J = 8.8 Hz, H2 and H6 phenyl), 7.9 (d, 1H, J = 4.4 Hz, H4 thiophene), 7.51 (d, 2H, J = 8.8 Hz, H3 and H5 phenyl), 7.35 (d, 1H, J = 4.4 Hz, H3 thiophene), 5 (s, 2H, S-CH2-CO). IR (KBr, cm−1) νmax: 1747 (C=O), 1522 and 1379 (NO2). Anal. Calcd for C14H8ClN3O3S3: C, 42.26; H, 2.03; N, 10.56. Found: C, 42.13; H, 2.27; N, 10.37.
1-(4-Chlorophenyl)-2-(5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (12)
Yield 90%; mp 187–189°C; 1H NMR (400 MHz, CDCl3) δ: 8.03 (d, 2H, J = 8.4 Hz, H2 and H6 phenyl), 7.58–7.5 (m, 3H, H4 furan and H3 and H5 phenyl), 7.34 (d, 1H, J = 3.6 Hz, H3 furan), 5.04 (s, 2H, S-CH2-CO). IR (KBr, cm−1) νmax: 1672 (C=O), 1527 and 1347 (NO2). MS (m/z, %): 381 (M+, 3), 138 (100), 110 (27). Anal. Calcd for C14H8ClN3O4S2: C, 44.04; H, 2.11; N, 11.01. Found: C, 43.84; H, 2.33; N, 11.23.
1-(4-Chlorophenyl)-2-(5-(1-methyl-5-nitro-1H-imidazol-2-yl)-1,3,4-thiadiazol-2-ylthio)ethanone (13)
Yield 91%; mp 202–205°C; 1H NMR (400 MHz, CDCl3) δ: 8.28 (s, 1H, H imidazole), 8.1 (d, 2H, J = 8.8 Hz, H2 and H6 phenyl), 7.67 (d, 2H, J = 8.8 Hz, H3 and H5 phenyl), 5.23 (s, 2H, S-CH2-CO), 4.33 (s, 3H, N-CH3). IR (KBr, cm−1) νmax: 1674 (C=O), 1588 and 1359 (NO2). Anal. Calcd for C14H10ClN5O3S2: C, 42.48; H, 2.55; N, 17.69. Found: C, 42.16; H, 2.74; N, 17.51.
General procedure for the preparation of compounds 14–19
To a mixture of compounds 4a,b (1 mmol) and KOH (1 mmol) in EtOH, 3- chloro-1-phenylpropan-1-one or 2-chloro-1-phenylpropan-1-one or (1-chloroethyl) benzene (1 mmol) were added and the reaction mixture was allowed to stir overnight. The progress of the reaction was followed by TLC, which was accompanied by a colour change from red to yellow. The solvents were removed under reduced pressure and the residue was washed with water and crystallised from ethanol to give compounds 14–19.
2-(5-(5-Nitrothiophen-2-yl)-1,3,4-thiadiazol-2-ylthio)-1-phenylpropan-1-one (14)
Yield 68%; mp 158°C; 1H NMR (400 MHz, CDCl3) δ: 8.06 (d, 2H, J = 7.6 Hz, H2 and H6 phenyl), 7.9 (d, 1H, J = 4.4 Hz, H4 thiophene), 7.64 (t, 1H, J = 7.6 Hz, H4 phenyl), 7.52 (t, 2H, J = 7.6 Hz, H3 and H5 phenyl), 7.35 (d, 1H, J = 4.4 Hz, H3 thiophene), 5.9 (q, 1H, J = 7.2 Hz, CHMe), 1.8 (d, 3H, J = 7.2 Hz, CH3). IR (KBr, cm−1) νmax: 1671 (C=O), 1510 and 1352 (NO2). Anal. Calcd for C15H11N3O3S3: C, 47.73; H, 2.94; N, 11.13. Found: C, 47.55; H, 3.13; N, 10.98.
2-(5-(5-Nitrofuran-2-yl)-1,3,4-thiadiazol-2-ylthio)-1-phenylpropan-1-one (15)
Yield 45%; mp 182–183°C; 1H NMR (400 MHz, CDCl3) δ: 7.47 (d, 2H, J = 7.4 Hz, H2 and H6 phenyl), 7.45 (d, 1H, J = 3.8 Hz, H4 furan), 7.39–7.34 (m, 2H, H3 and H5 phenyl), 7.33–7.28 (m, 2H, H4 phenyl and H3 furan), 5.13 (q, 1H, J = 7 Hz, CHMe), 1.87 (d, 3H, J = 7 Hz, CH3). IR (KBr, cm−1) νmax: 1685 (C=O), 1530 and 1347 (NO2). Anal. Calcd for C15H11N3O4S2: C, 49.85; H, 3.07; N, 11.63. Found: C, 50.03; H, 3.19; N, 11.87.
3-(5-(5-Nitrothiophen-2-yl)-1,3,4-thiadiazol-2-ylthio)-1-phenylpropan-1-one (16)
Yield 64%; mp 187°C; 1H NMR (400 MHz, CDCl3) δ: 8 (d, 2H, J = 7.6 Hz, H2 and H6 phenyl), 7.85 (d, 1H, J = 4.4 Hz, H4 thiophene), 7.61 (t, 1H, J = 7.6 Hz, H4 phenyl), 7.5 (t, 2H, J = 7.6 Hz, H3 and H5 phenyl), 7.18 (d, 1H, J = 4.4 Hz, H3 thiophene), 4.8 (t, 2H, J = 7.2 Hz, S-CH2), 3.6 (t, 2H, J = 7.2 Hz, CH2-CO). IR (KBr, cm−1) νmax: 1685 (C=O), 1514 and 1351 (NO2). Anal. Calcd for C15H11N3O3S3: C, 47.73; H, 2.94; N, 11.13. Found: C, 47.51; H, 2.87; N, 11.47.
3-(5-(5-Nitrofuran-2-yl)-1,3,4-thiadiazol-2-ylthio)-1-phenylpropan-1-one (17)
Yield 59%; mp 177°C; 1H NMR (400 MHz, CDCl3) δ: 8 (d, 2H, J = 7.6 Hz, H2 and H6 phenyl), 7.61 (t, 1H, J = 7.6 Hz, H4 phenyl), 7.5 (t, 2H, J = 7.6 Hz, H3 and H5 phenyl), 7.41 (d, 1H, J = 4 Hz, H4 furan), 7 (d, 1H, J = 4 Hz, H3 furan), 4.82 (t, 2H, J = 7.2 Hz, S-CH2), 3.65 (t, 2H, J = 7.2 Hz, CH2-CO). IR (KBr, cm−1) νmax: 1685 (C=O), 1526 and 1324 (NO2). Anal. Calcd for C15H11N3O4S2: C, 49.85; H, 3.07; N, 11.63. Found: C, 49.74; H, 3.15; N, 11.39.
2-(5-Nitrothiophen-2-yl)-5-(1-phenylethylthio)-1,3,4-thiadiazole (18)
Yield 41%; mp 96–97°C; 1H NMR (400 MHz, CDCl3) δ: 7.88 (d, 1H, J = 4 Hz, H4 thiophene), 7.46 (d, 2H, J = 7.2 Hz, H2 and H6 phenyl), 7.4–7.32 (m, 3H, H3, H4 and H5 phenyl), 7.31 (d, 1H, J = 4 Hz, H3 thiophene), 5.16 (q, 1H, J = 7.2 Hz, SCH), 1.87 (d, 3H, J = 7.2 Hz, CH3). IR (KBr, cm−1) νmax: 1507 and 1347 (NO2). Anal. Calcd for C14H11N3O2S3: C, 48.12; H, 3.17; N, 12.02. Found: C, 47.82; H, 3.06; N, 12.17.
2-(5-Nitrofuran-2-yl)-5-(1-phenylethylthio)-1,3,4-thiadiazole (19)
Yield 54%; mp 125–126°C; 1H NMR (400 MHz, CDCl3) δ: 7.46 (d, 2H, J = 7.8 Hz, H2 and H6 phenyl), 7.43 (d, 1H, J = 3.2 Hz, H4 furan), 7.38–7.33 (m, 2H, H3 and H5 phenyl), 7.32–7.28 (m, 2H, H4 phenyl and H3 furan), 5.12 (q, 1H, J = 6.8 Hz, SCH), 1.86 (d, 3H, J = 6.8 Hz, CH3). IR (KBr, cm−1) νmax: 1536 and 1355 (NO2). Anal. Calcd for C14H11N3O3S2: C, 50.44; H, 3.33; N, 12.6. Found: C, 50.71; H, 3.28; N, 12.45.
In vitro antileishmanial activity
The vaccine strain of L. major (MRHO/IR/75/ER) was obtained from the Pasteur Institute (Tehran, Iran). The promastigote form of the parasite was grown in blood agar cultures at 25°C. For the experiments described here, the stationary phase of the promastigotes were washed with phosphate buffered saline and then re-cultured in RPMI 1640 medium (Sigma, St Louis, MO, USA) at a density of 2 × 106 cells/mL, supplemented with 10% of heat-inactivated fetal bovine serum, 2 mM glutamine (Sigma), pH ∼7.2, 100 U/mL penicillin (Sigma) and 100 µg/mL streptomycin (Sigma). The growth curve of the L. major strain was determined daily using a light microscope and counting in a Neubauer’s chamber. To determining the 50% inhibitory concentrations (IC50), the tetrazolium bromide salt (MTT) assay was used. Briefly, promastigotes (2 × 106 /mL) from the early log phase of growth were seeded in 96-well plastic cell culture trays, containing serial dilutions of the compounds and phenol red free RPMI 1640 medium, supplemented with 10% of FCS, 2 mM glutamine, pH ∼7.2 and antibiotics, in a volume of 200 µL. After 24 h of incubation at 25°C, the media was renewed with 100 µg /well of MTT (0.5 mg/mL) and the plates were further incubated for 4 h at 37°C. The plates were centrifuged (2000 rpm × 5 min) and the pellets were dissolved in 200 µL of DMSO. The samples were read using an ELISA plate reader (Bio-Rad Laboratories, Hercules, CA, USA) at a wavelength of 492 nm. Two or more independent experiments in triplicate were performed to determine the sensitivity to each drug, the IC50 were calculated by linear regression analysis, expressed as the mean. Control cells were incubated with culture medium supplemented with DMSO [Citation21].
Results and discussion
Chemistry
The synthesis of the target compounds 5–19 was accomplished through an efficient process as outlined in . The starting compounds 1a and 1b were obtained from the corresponding 5-nitro-2-arylidene diacetate; whereas the starting compound 1c was prepared from 1-methyl-5-nitroimidazole-5-carboxaldehyde according to the previously described procedure [Citation22,Citation23]. The oxidative cyclisation of 1a–c in the presence of NH4Fe(SO4)2 caused the formation of 2-amino-1,3,4-thiadiazoles 2a–c. The diazotation and the subsequent chlorination of 2a–c in hydrochloric acid in the presence of NaNO2 and copper powder afforded 3a–c, which then reacted with an equivalent amount of thiourea in refluxing EtOH to produce 4a–c [Citation20]. The reaction of compound 4a–c with appropriate phenacyl bromide in stirring KOH/EtOH gave the target compounds 5–13; while the treatment of 4a,b with 3-chloro-1-phenylpropan-1-one, 2-chloro-1-phenylpropan-1-one or (1-chloroethyl)benzene in stirring KOH/EtOH afforded target compounds 14–19.
Antileishmanial activity
The antileishmanial activity of all the target compounds 5–19 against Leishmania major is shown in . In addition, the activity of the clinically used drug Glucantime® is also shown as a standard drug. The inhibitory concentrations for 50% of inhibition (IC50) of parasitic growth, at the third day of incubation, were calculated based on a linear regression and reported as a mean.
Table 1. Structures and in vitro activities of compounds 5–19 against the promastigote form of L. major.
These data indicated that all the compounds exhibited a high activity against L. major promastigotes with IC50 values ranging from 1.11 to 3.16 μM. For the structure-activity relationship study, the type of nitroheterocyle and pendant bulky group attached to the 1,3,4-thiadiazole ring was varied. The comparison of IC50 values for the different nitroaryl derivatives including furan, thiophene, and N-methylimidazole revealed that these compounds are close in activity and the differences observed were not very significant. Regio-isomeric chlorine substitution and α-methyl branching of the phenacylthio-pendant group did not improve activity at concentrations less than 1.11 μM. The results for the two α-methyl benzyl derivatives (compounds 18 and 19) and the α-methylphenacyl analogs (compounds 14 and 15) suggested that the carbonyl group may not be essential for optimum activity. Replacement of the phenacyl group with a propiophenone homologue retained the activity for compounds 16 and 17.
In conclusion, we have identified a series of 2,5-disubstituted-1,3,4-thiadiazoles bearing a 5-nitroaryl moiety including nitrofuran, nitrothiophene or nitroimidazole at the 5-position and a bulky residue attached to the 2-position of thiadiazole ring as promising antileishmanial agents. The structure-activity relationships for this series indicated that in all type of 5-(5-nitroaryl)-2-thio-1,3,4-thiadiazoles, the S-pendant group have a high flexibility with the structural alteration retaining a good antileishmanial activity.
Declaration of interest
The authors have declared no conflict of interest.
References
- Navarro M, Cisneros Fajardo EJ, Fernandez Mestre M, Arrieche D, Marchan E. Synthesis, characterization, DNA binding study and biological activity against Leishmania mexicana of [Cu(dppz)2]BF4. J Inorg Biochem 2003;97:364–369.
- Rocha LG, Almeida JRGS, Macêdo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine 2005;12:514–535.
- Kolodziej H, Kiderlen AF. Antileishmanial activity and immune modulatory effects of tannins and related compounds on Leishmania parasitised RAW 264.7 cells. Phytochemistry 2005;66:2056–2071.
- WHO. http://www.who.int/leishmaniasis/burden/en.
- Croft SL. The current status of antiparasite chemotherapy. Parasitology 1997;114:S3–S15.
- Engers HD, Bergquist R, Moddaber F. Progress on vaccines against parasites. Dev Biol Stand 1996;87:73–84.
- Brenzan MA, Nakamura CV, Dias Filho BP, Ueda-Nakamura T, Young MCM, Côrrea AG, Júnior JA, dos Santos AO, Cortez DAG. Structure-activity relationship of (-) mammea A/BB derivatives against Leishmania amazonensis. Biomed Pharmacother 2008;62:651–658.
- Mishra Bhuwan B, Kale Raju R, Singh Rakesh K, Tiwari Vinod K. Alkaloids: Future prospective to combat leishmaniasis. Fitoterapia 2009;2:81–90.
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Diseases 2004;27:305–318.
- Poorrajab F, Kabudanian Ardestani S, Emami S, Behrouzi Fardmoghadam M, Shafiee A, Foroumadi A. Nitroimidazolyl-1,3,4- thiadiazole-based anti-leishmanial agents: Synthesis and in vitro biological evaluation. Eur J Med Chem 2009;44:1758–1762.
- Mayence A, Vanden Eynde JJ, LeCour Jr L, Walker LA, Tekwani BL, Huang TL. Piperazine-linked bisbenzamidines: a novel class of antileishmanial agents. Eur J Med Chem 2004;39:547–553.
- Khan F, Choudhary M, Rasheed M, Zia Ullah S, Hayat S, Kaukab F, Choudhary MI. Attaur Rahman M, Perveen S. Synthesis and in vitro leishmanicidal activity of some hydrazides and their analogues. Bioorg Med Chem 2003;11:1381–1387.
- Khan KM, Mughal UR, Ambreen N, Samreen Perveen, S, Choudhary MI. Synthesis and leishmanicidal activity of 2,3,4-substituted-5-imidazolones. J Enzyme Inhib Med Chem 2010;25:29–37.
- Pires JR, Saito C, Gomes SL, Giesbrecht AM, Amaral A. Investigation of 5-nitrofuran derivatives: synthesis, antibacterial activity, and quantitative structure-activity relationships. J Med Chem 2001;22:3673–3681.
- Muelas-Serrano S, Pérez-Serrano J, Gómez-Barroi A, Arán VJ, Rodríguez-Caabeiro F. Ultrastructural alterations induced by nifurtimox and another nitro derivative on epimastigotes of Trypanosoma cruzi. Parasitol Res 2002;2:97–101.
- Rodrigues RF, da Silva EF, Echevarria A, Fajardo Bonin R, Amaral VF, Leon LL, Canto Cavalheiro MM. A comparative study of mesoionic compounds in Leishmania sp. and toxicity evaluation. Eur J Med Chem 2007;42:1039–1043.
- da Silva EF, Canto Cavalheiro MM, Braz VR, Cysne Finkelstein L, Leon LL, Echevarria A. Synthesis, and biological evaluation of new 1,3,4-thiadiazolium-2-phenylamine derivatives against Leishmania amazonensis promastigotes and amastigotes. Eur J Med Chem 2002;37:979–984.
- Foroumadi A, Mansouri S, Kiani Z, Rahmani A. Synthesis and in vitro antibacterial evaluation of N-[5-(5-nitro-2-thienyl)-1,3,4-thiadiazole-2-yl] piperazinyl quinolones. Eur J Med Chem 2003;38:851–854.
- Behrouzi-Fardmoghadam M, Poorrajab F, Ardestani SK, Emami S, Shafiee A, Foroumadi A. Synthesis and in vitro anti-leishmanial activity of 1-[5-(5-nitrofuran-2-yl)-1,3,4-thiadiazol-2-yl]- and 1-[5-(5-nitrothiophen-2-yl)-1,3,4-thiadiazol-2-yl]-4-aroylpiperazines. Bioorg Med Chem 2008;16:4509–4515.
- Foroumadi A, Rineh A, Emami S, Siavoshi F, Massarrat S, Safari F, Rajabalian S, Falahati M, Lotfali E, Shafiee A. Synthesis and anti-Helicobacter pylori activity of 5-(nitroaryl)-1,3,4-thiadiazoles with certain sulfur containing alkyl side chain. Bioorg Med Chem Lett 2008;18:3315–3320.
- Poorrajab F, Ardestani SK, Foroumadi A, Emami S, Kariminia A, Behrouzi-Fardmoghadam M, Shafiee A. Selective leishmanicidal effect of 1,3,4-thiadiazole derivatives and possible mechanism of action against Leishmania species. Exp Parasitol 2009;121:323–330.
- Foroumadi A, Mirzaei M, Shafiee A. Antituberculosis agents II. Evaluation of in vitro antituberculosis activity and cytotoxicity of some 2-(1-methyl-5-nitro-2-imidazolyl)-1,3,4-thiadiazole derivatives. Il Farmaco 2001;56:621–623.
- Foroumadi A, Daneshtalab M, Shafiee A. Synthesis and in vitro antifungal activity of 2-aryl-5-phenylsulfonyl-1,3,4-thiadiazole derivatives. Arzneim-Forsch Drug Res 1999;49:1035–1038.