Abstract
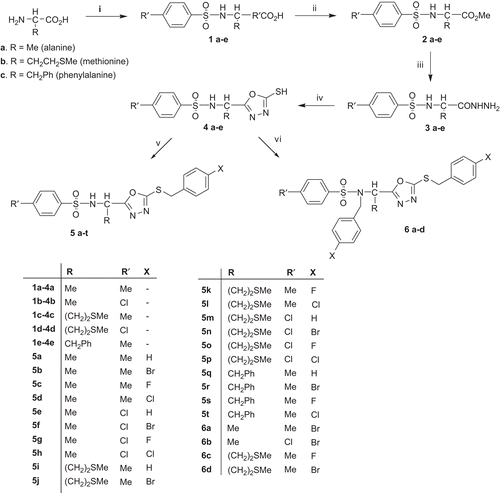
2-(1-[(4-Chloro/methylphenylsulfonylamino)alkyl]-5-thioxo-4,5-dihydro-1,3,4-oxadiazoles (4a–e) were synthesized, in four steps, via the sulfonyl derivatives of l-amino acids (l-alanine, l-methionine and l-phenylalanine) 1a–e, the esters 2a–e, the hydrazides 3a–e and finally the cyclization to 4a–e. Alkylation of 4a–e with 1.0 mole eq. of substituted benzyl halides furnished S-benzyl derivatives 5a–t, while 1.1 mole eq. yielded major 5a–t and minor amount of 6a–d. Alternatively, treatment of 4a–e with 2.0 mole eq. of substituted benzyl halides furnished 6a–d only. The structures of 5b and 5l were further confirmed by single crystal X-ray analysis. Compounds 5a–t and 6a–d showed no selective inhibition against HIV-1 and HIV-2 replication in MT-4 cells. However, 5f and 5j–5q exhibited some inhibitory activity against both types with EC50 values (>11.50 − >13.00 µg/mL). These results suggest that the structural modifications of these compounds might lead to the development of new antiviral agents. The quantum structure-activity relationship of these novel structural congeners is discussed.
Introduction
Among the various viral human ailments, acquired immunodeficiency syndrome is perhaps the most complicated disease, and as yet no effective drugs or methods of control are available owing to the mutational changes in HIV virusCitation1. In spite of the beneficial effects of the drugs in use, the side effects are intensified with the combination therapyCitation2. Therefore, synthesis or design of novel potent, selective, and less toxic drugs remains one of the most challenging tasks that chemists are facing. 1,3,4-oxadiazole is a versatile moleculeCitation3–6 for designing potential antiviral agents. The safety and efficacy of RaltegravirCitation7, a new anti-HIV drug containing the 1,3,4-oxadiazole moiety, has recently been described. On the other hand, sulphonamides attract significant attention because of their chemotherapeutic importanceCitation8–11. Cyclotriazadisulphonamide compounds are new effective HIV entry inhibitorsCitation12. We selected in the present work, two backbones: 1,3,4-oxadiazole and a sulphonamide since both having potential anti-HIV activity, which might lead to a remarkable potent anti-HIV agent with high therapeutic index. In continuation of our interest in the synthesis of biologically active azolesCitation13–17, we report here the synthesis of chiral sulphonamides bearing 1,3,4-oxadiazole derivatives and evaluation of their anti-HIV activity.
Experimental section
General
Melting points were measured on a Gallenkamp melting point apparatus (MP-D) and are uncorrected. The Rf values were determined using pre-coated silica gel aluminium packed plates, Kieselgel 60 HF254 from Merck (Germany). Infrared (IR) spectra were recorded on a FTS 3000 MX, Bio-RAD Merlin spectrophotometer (Excalibur Model, USA). Nuclear magnetic resonance (NMR) spectra were recorded on a 300 (1H) and 75 MHz (13C) NMR spectrometer (Bruker Avance, Switzerland) with tetramethylsilane as internal standard on a δ scale in ppm, multiplicities are abbreviated as s = singlet, d = doublet, t = triplet, q = quartet, ad = apparent doublet, aq = apparent quartet, qn = quintet and m = multiplet. Electron impact (EI) mass spectra were recorded on a Agilent technologies 6890N (GC) mass spectrometer and an inert selective detector 5973 (Agilent Technologies, USA). Elemental analyses were recorded on CHNS-932 Leco (Leco Corporation, USA).
General procedure for synthesis of the hydrazides (3a–e)
The hydrazides 3a–e were synthesized from the appropriate amino acids via three steps of sulfonylation furnishing 1a–e, followed by esterfication with acidic MeOH to give 2a–e and finally treatment with the hydrazine hydrate. The hydrazides were characterized by comparison of their physical data with the literature valuesCitation3,Citation17.
General procedure for the synthesis of N-[1-(5-mercapto-1,3,4-oxadiazol-2-yl)alkyl]-4-chloro/methylbenzenesulphonamides (4a-e)
A mixture of 2-(4-chloro/methylphenylsulfonylamino)alkane hydrazide (5.40 mmol), CS2 (10.80 mmol) and KOH (10.80 mmol) in MeOH (25 mL) was heated under reflux for 18–20 h. The solvent was evaporated to 5 mL, poured into ice-cooled water, and acidified with HOAc to pH 5. The resulting precipitate was collected, dried and recrystallized from aq. EtOH, except 4d, which was purified by column chromatography using n-hexane and ethyl acetate (8:2) as an eluent.
N-[1-(5-Mercapto-1,3,4-oxadiazol-2-yl)ethyl]-4-methylbenzenesulphonamide (4a)
Yield: 0.93 g (58%); m.p. 190–192°C; Rf: 0.39 (n-hexane: ethyl acetate 3:2); IR (υmax, cm−1): 3278, 2925, 2936, 1474, 1327, 1261, 1160; 1H-NMR (300 MHz, acetone-d6): δ 12.86 (1H, s, N-H), 7.73 (2H, d, J = 8.4 Hz, Ar-H), 7.37 (2H, d, J = 7.8 Hz, Ar-H), 7.31 (1H, d, J = 8.4 Hz, N-H), 4.65 (1H, q, J = 7.2 Hz, CH), 2.41 (3H, s, CH3), 1.49 (3H, d, J = 7.2Hz, CH3). 13C-NMR (75 MHz, acetone-d6): δ 178.6, 162.8, 143.5, 137.8, 129.6, 126.8, 45.5, 20.6, 18.2. Anal. calcd. for C11H13N3O3S2 (299.37): C, 44.13; H, 4.38; N, 14.04%. Found: C, 44.24; H, 4.33; N, 13.57%. EI-MS [m/z (%)]: 299 [M+].
4-Chloro-N-[1-(5-mercapto-1,3,4-oxadiazol-2-yl)ethyl]benzenesulphonamide (4b)
Yield: 0.94 g (52%); m.p. 191–193°C; Rf:0.39 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3297, 2939, 1496, 1333, 1261, 1168. 1H-NMR (300 MHz, acetone-d6): δ 9.30 (1H, s, N-H), 7.85 (2H, d, J = 8.7 Hz, Ar-H), 7.61 (2H, d, J = 8.7 Hz, Ar-H), 7.54 (1H, d, J = 8.1 Hz, N-H), 4.71 (1H, aq, J = 7.2 Hz, CH), 1.52 (3H, d, J = 6.9 Hz, CH3). 13C-NMR (75 MHz, acetone-d6): δ 178.6, 162.6, 139.7, 138.5, 129.3, 128.6, 45.5, 18.2. EI-MS [m/z (%)] 286 (55), 218 (100), 175 (75), 111 (70), 75 (20). Anal. calcd. for C10H10N3O4S2Cl (335.79): C, 37.56; H, 3.15; N, 13.1%4. Found: C, 38.06; H, 3.37; N, 12.60%.
N-[1-(5-Mercapto-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-methylbenzenesulphonamide (4c)
Yield: 1.24 g (64%); m.p. 144–146°C; Rf: 0.41 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3261, 2910, 1472, 1331, 1291, 1160, 1086. 1H-NMR (300 MHz, acetone-d6): δ 14.35 (1H, s, N-H), 8.65 (1H, d, J = 8.4 Hz, N-H), 7.60 (2H, d, J = 8.4 Hz, Ar-H), 7.33 (2H, d, J = 8.1 Hz, Ar-H), 4.50 (1H, aq, J = 7.8 Hz, CH), 2.50 (2H, m, -CH2), 2.36 (3H, s, CH3), 2.03–1.93 (2H, m, CH2), 1.93 (3H, s, CH3). 13C-NMR (75 MHz, acetone-d6): δ 178.1, 170.5, 143.6, 137.8, 130.0, 126.9, 48.3, 31.6, 29.2, 21.5, 14.7. Anal. calcd. for C13H17N3O3S3 (359.49): C, 45.52; H, 3.80; N, 8.33%. Found: C, 46.02; H, 3.87; N, 8.35%. EI-MS [m/z (%)]: 258 (52), 171 (5), 155 (32), 91 (100), 73 (86), 61 (83).
4-Chloro-N-[1-(5-mercapto-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]benzenesulphonamide (4d)
Yield: 1.37 g (67%); m.p. 148–150°C; Rf: 0.40 (n-hexane: ethyl acetate 3:2); IR (υmax, cm−1): 3256, 1467, 1336, 1278, 1161, 1090. 1H-NMR (300 MHz, DMSO-d6): δ 14.38 (1H, s, N-H) 8.67 (1H, d, J = 8.4 Hz, N-H), 7.72 (2H, d, J = 8.7 Hz, Ar-H), 7.61 (2H, d, J = 8.7 Hz, Ar-H), 4.55 (1H, q, J = 8.1 Hz, CH), 2.50–2.35 (2H, m, -CH2), 2.01–1.91 (2H, m, CH2), 1.95 (3H, s, CH3). 13C-NMR (75 MHz, DMSO-d6): δ 178.0, 161.9, 139.5, 138.2, 129.7, 128.7, 48.3, 31.5, 29.2, 14.8. EI-MS [m/z (%)]: 191 (33), 175 (30), 144 (8), 128 (28), 111 (100), 75 (82), 61 (5). Anal. calcd. for C12H14N3O3S3Cl (379.91): C, 37.94; H, 3.71; N, 11.06%. Found: C, 37.55; H, 3.61; N, 11.03%.
N-[1-(5-Mercapto-1,3,4-oxadiazol-2-yl)-2-phenylethyl]-4-methylbenzenesulphonamide (4e)
Yield: 1.45 g (72%); m.p. 150–152°C; Rf: 0.42 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3279, 2950, 1462, 1334, 1270, 1156, 1085. 1H-NMR (300 MHz, DMSO-d6): δ 13.80 (1H, s, N-H), 8.67 (1H, d, J = 8.4 Hz, N-H), 7.72 (2H, d, J = 8.7 Hz, Ar-H), 7.61 (2H, d, J = 8.7 Hz, Ar-H), 7.21–7.11 (5H, m, Ar-H), 4.55 (1H, aq, J = 8.1 Hz, CH), 2.50–2.35 (2H, m, CH2), 2.01–1.91 (2H, m, CH2), 1.95 (3H, s, CH3). 13C-NMR (75 MHz, DMSO-d6): δ 178.0, 161.6, 143.4, 137.6, 136.0, 129.8, 129.6, 128.8, 128.4, 127.4, 126.6, 51.1, 38.2, 21.5. EI-MS [m/z (%)]: 274 (82), 155 (72), 91 (100), 77 (5), 65 (25). Anal. calcd. for C17H17N3O3S2 (375.47): C, 54.38; H, 4.56; N, 11.19%. Found: C, 54.56; H, 4.61; N, 11.03%.
N-[1-(5-benzylthio/4-halobenzylthio)-1,3,4-oxadiazol-2-yl)alkyl]-4-methyl/4-halobenzenesulphonamides (5a-t)
A mixture of 4a–e (0.92 mmol), 4-halobenzyl halide (0.92 mmol) and K2CO3 (2.76 mmol) was stirred in acetone (30 mL) at room temperature for 3–4 h. The reaction mixture was filtered, the filtrate concentrated and poured into ice-cold water. The resulting solid was filtered and recrystallized from acetone - water or purified by column chromatography using n-hexane and ethyl acetate (4: 1) as eluent.
N-[1-(5-Benzylthio-1,3,4-oxadiazol-2-yl)ethyl]-4-methylbenzenesulphonamide (5a)
Yield: 0.308 g (86%); m.p. 98–100°C; Rf: 0.57 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3275, 1596, 1570, 1328, 1153, 1087. 1H-NMR (300 MHz, CDCl3): δ 7.70 (2H, d, J = 8.4 Hz, Ar-H), 7.41–7.31 (5H, m, Ar-H), 7.24 (2H, d, J = 7.8 Hz, Ar-H), 4.75 (1H, q, J = 7.2 Hz, CH), 4.36 (3H, s, NH, CH2), 2.39 (3H, s, CH3), 1.54 (3H, d, J = 7.2 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.8, 164.6, 143.9, 136.6, 135.6, 129.7, 129.1, 128.8, 128.2, 127.1, 45.5, 36.7, 21.6, 20.4. EI-MS [m/z (%)]: 389 [M+], 234 (2), 198 (10), 155 (18), 91 (100), 77 (4), 65 (20). Anal. calcd. for C18H19N3O3S2 (389.49): C, 55.51; H, 4.92; N, 10.79%. Found: C, 55.70; H, 4.99; N, 10.52%.
N-[1-(5-(4-Bromobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-methylbenzenesulphonamide (5b)
Yield: 0.335 g (80%); m.p. 135–137°C; Rf: 0.56 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3142, 1593, 1578, 1334, 1167, 1093, 1032. 1H-NMR (300 MHz, CDCl3): δ 7.70 (2H, d, J = 8.1 Hz, Ar-H), 7.46 (2H, d, J = 8.4 Hz, Ar-H), 7.24–7.30 (4H, m, Ar-H), 4.74 (1H, q, J = 6.9 Hz, CH), 4.50 (1H, s, NH), 4.31 (2H, s, CH2), 2.39 (3H, s, CH3), 1.53 (3H, d, J = 6.9 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 167.0, 164.3, 143.9, 136.6, 134.5, 131.9, 130.8, 129.7, 127.1, 122.2, 45.5, 35.9, 21.6, 20.3; EI-MS [m/z (%)]: 467 /469 [M+], 314/312 (3), 298 (1), 198 (41), 171 /169 (90), 155 (53), 91 (100), 65 (27). Anal. calcd. for C18H18BrN3O3S2 (456.38): C, 46.16; H, 3.87; N, 8.97%. Found: C, 45.47; H, 3.96; N, 8.76%.
N-[1-(5-(4-Fluorobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-methylbenzenesulphonamide (5c)
Yield: 0.36 g (96%); m.p. 106–108°C; Rf: 0.57 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3283, 1597, 1575, 1336, 1223, 1150, 1086. 1H-NMR (300 MHz, CDCl3): δ 7.71 (2H, d, J = 8.4 Hz, Ar-H), 7.36–7.40 (2H, m, Ar-H), 7.25 (2H, d, J = 8. Hz, Ar-H), 7.05 (2H, at, J = 8.4 Hz, Ar-H), 4.74 (1H, q, J = 6. Hz, CH), 4.41 (2H, s, CH2), 4.35 (1H, s, NH), 2.39 (3H, s, CH3), 1.54 (3H, d, J = 6.9 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 167.0, 164.5, 162.0 (J = 246.0 Hz), 144.0, 136.6, 131.2 (J = 3.7 Hz), 130.9 (J = 8.2 Hz), 129.0, 127.0, 115.7 (2J = 21.7 Hz), 45.5, 35.8, 21.6, 20.4. EI-MS [m/z (%)]: 407 (1), 392 (1), 252 (2), 198 (20), 155 (25), 109 (100), 91 (32), 65 (15). Anal. calcd. for C18H19FN3O3S2 (408.49): C, 53.06; H, 4.45; N, 10.31%. Found: C, 52.48; H, 4.53; N, 10.09%.
N-[1-(5-(4-Chlorobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-methylbenzenesulphonamide (5d)
Yield: 0.319 g (82%); m.p. 103–105°C; Rf: 0.57 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3134, 1597, 1578, 1334, 1167, 1129, 1092, 1032. 1H-NMR (300 MHz, CDCl3): δ 7.71 (2H, d, J = 8.4 Hz, Ar-H), 7.35 (2H, d, J = 8.7 Hz Ar-H), 7.30 (2H, d, J = 8.7 Hz, Ar-H), 7.25 (2H, d, J = 8.1 Hz, Ar-H), 4.75 (1H, q, J = 6.9 Hz, CH), 4.61 (1H, s, NH), 4.33 (2H, s, CH2), 2.39 (3H, s, CH3), 1.53 (3H, d, J = 7.2 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.9, 164.4, 143.9, 136.6, 134.1, 134.0, 130.5, 129.7, 128.9, 127.1, 45.5, 35.8, 21.6, 20.3. EI-MS (m/z %) 423/425 [M+], 268 (1), 198 (19), 155 (32), 125/127 (100), 113/111 (2), 91 (60), 65 (17). Anal. calcd. for C18H18ClN3O3S2 (423.94): C, 51.00; H, 4.28; N, 9.91%. Found: C, 51.00; H, 4.31; N, 9.83%.
N-[1-(5-Benzylthio-1,3,4-oxadiazol-2-yl)ethyl]-4-chlorobenzenesulphonamide (5e)
Yield: 0.233 g (62%); m.p. 98–100°C; Rf: 0.49 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3243, 1580, 1568, 1330, 1165, 1083. 1H-NMR (300 MHz, CDCl3): δ 7.75 (2H, d, J = 8.7 Hz, Ar-H), 7.44 (2H, d, J = 8.7 Hz, Ar-H), 7.31–7.40 (5H, m, Ar-H), 4.81 (1H, s, NH), 4.75 (1H, q, J = 7.2 Hz, CH), 4.39 (2H, s, CH2), 1.58 (3H, d, J = 7.2 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.5, 164.9, 139.5, 138.2, 135.1, 129.4, 129.1, 128.8, 128.6, 128.2, 45.6, 36.7, 20.3. EI-MS [m/z (%)]: 409 [M+], 332 (1), 218 (10), 177/175 (16), 113/111 (25), 91 (100), 77 (6), 65 (16). Anal. calcd. for C17H16ClN3O3S2 (409.91): C, 49.81; H, 3.93; N, 10.25%. Found: C, 49.67; H, 4.00; N, 10.00%.
N-[1-(5-(4-Bromobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-chlorobenzenesulphonamide (5f)
Yield: 0.292 g (65%); m.p. 114–116°C; Rf: 0.50 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3225, 1585, 1510, 1342, 1171, 1083, 1032. 1H-NMR (300 MHz, CDCl3): δ 7.75 (2H, d, J = 8.4 Hz, Ar-H), 7.44 (4H, d, J = 9.0 Hz, Ar-H), 7.29 (2H, d, J = 8.1 Hz, Ar-H), 5.83 (1H, d, J = 8.7 Hz, NH), 4.78 (1H, aq, J = 6.9 Hz, CH), 4.33 (2H, s, CH2), 1.57 (3H, d, J = 7.2 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.6, 164.6, 139.5, 138.1, 134.3, 131.9, 130.8, 129.4, 128.6, 122.3, 45.6, 35.9, 20.2. EI-MS [m/z (%)]: 489 /487 [M+], 288 /286 (2), 218 (13), 175 (22), 171 /169 (100), 111 (48), 75 (20), 28 (17). Anal. calcd. for C17H15BrClN3O3S2 (488.81): C, 41.77; H, 3.09; N, 8.60%. Found: C, 42.01; H, 3.15; N, 8.80%.
N-[1-(5-(4-Fluorobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-chlorobenzenesulphonamide (5g)
Yield: 0.232 g (59%); m.p. 92–94°C; Rf: 0.48 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3274, 1599, 1569, 1331, 1158, 1229, 1089. 1H-NMR (300 MHz, CDCl3): δ 7.75 (2H, d, J = 8. Hz, Ar-H), 7.43 (2H, d, J = 8.7 Hz, Ar-H), 7.36–7.39 (2H, m, Ar-H), 7.03 (2H, at, J = 8.4 Hz, Ar-H), 6.05 (1H, d, J = 8.1 Hz, NH), 4.79 (1H, at, J = 7.2 Hz, CH), 4.35 (2H, s, CH2), 1.57 (3H, d, J = 6.9 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.6, 164.7, 162 (1J = 246 Hz), 139.5, 138.2, 131.0 (overlapped), 130.9 (3J = 8.2 Hz), 129.4, 128.6, 115.8 (2J = 21.7 Hz), 45.5, 35.9, 20.1. EI-MS [m/z (%)]: 427 [M+], 332 (1), 252 (2), 218 (8), 175 (12), 109 (100); Anal. calcd. for C17H15ClFN3O3S2 (427.9): C, 47.72; H, 3.53; N, 9.82%. Found: C, 48.08; H, 3.79; N, 9.65%.
N-[1-(5-(4-Chlorobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-chlorobenzenesulphonamide (5h)
Yield: 0.236 g (63%); m.p. 87–89°C; Rf: 0.47 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): δ3129, 1577, 1332, 1170, 1083, 1032. 1H-NMR (300 MHz, CDCl3): δ 7.74 (2H, d, J = 8.7 Hz, Ar-H), 7.44 (2H, d J = 8.7 Hz Ar-H), 7.31 (2H, d, J = 8.7 Hz, Ar-H);7.36 (2H, d, J = 8.7 Hz, Ar-H), 5.58 (1H, s, NH), 4.75 (1H, q, J = 6.9 Hz, CH); 4.35 (2H, s, CH2), 1.58 (3H, d, J = 7.2 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.5, 164.6, 139.6, 138.1, 134.2, 133.7, 130.6, 129.4, 129.0, 128.6, 45.5, 35.9, 20.3. EI-MS [m/z (%)]: 218 (12), 175 (24), 159/157 (4), 127/125 (100), 113/111 (39). Anal. calcd. for C17H15ClN3O3S2 (408.9): C, 45.95; H, 3.40; N, 9.46%. Found: C, 46.29; H, 3.65; N, 8.94%.
N-[1-(5-Benzylthio-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-methylbenzenesulphonamide (5i)
Yield: 0.256 g (62%); m.p. 120–122°C; Rf: 0.52 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3280, 1596, 1327, 1153, 1083. 1H-NMR (300 MHz, CDCl3): δ 7.68 (2H, d, J = 8.4 Hz, Ar-H), 7.31–7.41 (5H, m, Ar-H), 7.23 (2H, d, J = 8.1 Hz, Ar-H), 5.70 (1H, d, J = 9.3 Hz, NH), 4.83 (1H, m, CH), 4.36 (2H, s, CH2), 2.55 (2H, m, CH2), 2.38 (3H, s, CH3), 2.16–2.08 (2H, m, CH2), 2.05 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.0, 164.6, 143.9, 136.5, 135.0, 129.7, 129.1, 128.8, 128.2, 127.1, 48.6, 36.7, 33.2, 29.6, 21.6, 15.3. EI-MS [m/z (%)]: 358 (4), 326 (3), 312 (25), 171 (2), 155 (11), 109 (100), 91 (40), 75 (10), 61 (38). Anal. calcd. for C20H23N3O3S3 (449.61): C, 53.43; H, 5.16; N, 9.35%. Found: C, 53.45; H, 5.15; N, 9.10%.
N-[1-(5-(4-Bromobenzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-methylbenzenesulphonamide (5j)
Yield: 0.33 g (68%); m.p. 119–121°C; Rf: 0.55 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3264, 1597, 1327, 1153, 1080, 1069. 1H-NMR (300 MHz, CDCl3): δ 7.70 (2H, d, J = 8.4 Hz, Ar-H), 7.47 (2H, d, J = 8.4 Hz, Ar-H), 7.22–7.29 (4H, m, Ar-H), 5.70 (1H, d, J = 9.3 Hz, NH), 4.83 (1H, m, CH), 4.31 (2H, s, CH2), 2.53 (2H, t, J = 6.9 Hz, CH2), 2.38 (3H, s, CH3), 2.16–2.08 (2H, m, CH2), 2.04 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.3, 164.3, 143.9, 136.5, 134.5, 131.9, 130.8, 129.7, 127.1, 122.2, 48.5, 35.9, 33.1, 29.6, 21.6, 15.3. EI-MS [m/z (%)]: 372 /370 (27), 300 /298 (36), 258 (5), 169 /171 (85), 91 (100), 75 (18), 65 (21), 61 (82), 28 (95). Anal. calcd. for C20H22BrN3O3S3 (528.51): C, 45.45; H, 4.20; N, 7.95%. Found: C, 45.32; H, 4.24; N, 7.60%.
N-[1-(5-(4-Fluorobenzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-methylbenzenesulphonamide (5k)
Yield: 0.342 g (78%); m.p. 108–110°C; Rf: 0.45 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3262, 1597, 1569, 1326, 1154, 1142, 1089. 1H-NMR (300 MHz, CDCl3): δ 7.70 (2H, d, J = 8.4 Hz, Ar-H), 7.35–7.40 (2H, m, Ar-H), 7.24 (2H, d, J = 8.1 Hz, Ar-H), 7.03 (2H, at, J = 8.7 Hz, Ar-H), 5.42 (1H, s, NH), 4.83 (1H, at, J = 7.2 Hz, CH), 4.34 (2H, s, CH2), 2.54 (2H, t, J = 6.7 Hz, CH2), 2.39 (3H, s, CH3), 2.16–2.06 (2H, m, CH2), 2.04 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.2, 164.5, 162.0 (J = 246 Hz), 149.3, 136.5, 131.1 (J = 3.0 Hz), 130.9 (J = 8.2 Hz), 129.7, 127.1, 115.7 (J = 21.7 Hz), 48.6, 35.9, 33.1, 29.6, 21.6, 15.3. EI-MS [m/z (%)]: 358 (4), 326 (3), 312 (25), 171 (2), 155 (11), 109 (100), 91 (40), 75 (10), 61 (38). Anal. calcd. for C20H22FN3O3S3 (467.6): C, 51.37; H, 4.74; N, 8.99%. Found: C, 51.39; H, 4.91; N, 8.77%.
N-[1-(5-(4-Chlorobenzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-methylbenzenesulphonamide (5l)
Yield: 0.338 g (76%); m.p. 114–116°C; Rf: 0.45 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3261, 1596, 1567, 1325, 1153, 1089, 1039. 1H-NMR (300 MHz, CDCl3): δ 7.71 (2H, d, J = 8.4Hz, Ar-H), 7.35 (2H, d, J = 8.1 Hz, Ar-H), 7.31 (2H, d, J = 9.0 Hz, Ar-H), 7.24 (2H, d, J = 8.1 Hz, Ar-H), 4.83 (1H, at, J = 6.9 Hz, CH), 4.81 (1H, s, NH), 4.33 (2H, s, CH2), 2.53 (2H, t, J = 6.9 Hz, CH2), 2.39 (3H, s, CH3), 2.16–2.06 (2H, m, CH2), 2.04 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.2, 164.4, 143.9, 136.5, 134.1, 133.9, 130.5, 129.7, 129.1, 127.1, 48.6, 35.9, 33.1, 29.6, 21.6, 15.3; EI-MS [m/z (%)]: 358 (2), 328 (28), 313 (2), 155 (15), 125 (100), 91 (72), 65 (12), 61 (65). Anal. calcd. for C20H22ClN3O3S3 (484.05): C, 49.63; H, 4.58; N, 8.68%. Found: C, 50.00; H, 4.73; N, 8.73%.
N-[1-(5-Benzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-chlorobenzenesulphonamide (5m)
Yield: 0.25 g (58%); m.p. 102–104°C; Rf: 0.48 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3132, 1579, 1338, 1167, 1083. 1H-NMR (300 MHz, CDCl3): δ 7.74 (2H, d, J = 8.7 Hz, Ar-H), 7.44 (2H, d, J = 8.7Hz, Ar-H), 7.33–7.38 (5H, m, Ar-H), 5.71 (1H, d, J = 9.3 Hz, NH), 4.88 (1H, m, CH), 4.39 (2H, s, CH2), 2.58 (2H, t, J = 7.2 Hz, CH2), 2.10–2.18 (2H, m, CH2), 2.08 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 165.5, 165.0, 139.6, 137.9, 134.9, 129.4, 129.1, 128.8, 128.6, 128.2, 48.6, 36.8, 33.0, 29.6, 15.4. EI-MS [m/z (%)]: 294 (52), 111 (5), 91 (100), 65 (20), 28 (51). Anal. calcd. for C19H20ClN3O3S3 (470.03): C, 48.55; H, 4.29; N, 8.94%. Found: C, 47.98; H, 4.38; N, 8.52%.
N-[1-(5-(4-Bromobenzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-chlorobenzenesulphonamide (5n)
Yield: 0.308 g (61%); m.p. 98–100°C; Rf: 0.48 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3150, 1575, 1330, 1163, 1084, 1070. 1H-NMR (300 MHz, CDCl3): δ 7.74 (2H, d, J = 8.7 Hz, Ar-H), 7.48 (2H, d, J = 8.4 Hz, Ar-H), 7.43 (2H, d, J = 8.4 Hz, Ar-H), 7.29 (2H, d, J = 8.4 Hz, Ar-H), 4.86 (1H, at, J = 6.9 Hz, CH), 4.45 (1H, s, NH), 4.33 (2H, s, CH2), 2.54 (2H, t, J = 6.6 Hz, CH2), 2.09–2.16 (2H, m, CH2), 2.05 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.2, 164.6, 139.5, 138.2, 134.2, 132.0, 129.4, 128.9, 128.6, 122.3, 48.7, 36.0, 33.0, 29.6, 15.3; EI-MS [m/z (%)]: 346 (3), 171/169 (100), 111 (10), 75 (16), 28 (62). Anal. calcd. for C19H19BrClN3O3S3 (548.92): C, 41.57; H, 3.49; N, 7.65%. Found: C, 41.60; H, 3.52; N, 7.55%.
N-[1-(5-(4-Fluorobenzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-chlorobenzenesulphonamide (5o)
Yield: 0.246 g (55%); m.p. 94–96°C; Rf: 0.48 (n-hexane: ethyl acetate 3: 2); IR (υmax, cm−1): 3226, 1573, 1326, 1227, 1165, 1084. 1H-NMR (300 MHz, CDCl3): δ 7.75 (2H, d, J = 8.7 Hz, Ar-H), 7.43 (2H, d, J = 8.7 Hz, Ar-H), 7.37–7.40 (2H, m, Ar-H), 7.04 (2H, at, J = 8.4 Hz, Ar-H), 4.88 (1H, at, J = 7.2 Hz, CH), 4.37 (3H, s, CH2, NH), 2.57 (2H, t, J = 6.9 Hz, CH2), 2.10–2.18 (2H, m, CH2), 2.07 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 165.8, 164.8, 163.0 (J = 246 Hz), 139.6, 138.0, 130.9 (J = 8.4 Hz), 312 (21), 191 (3), 175 (10), 109 (100), 111 (20), 75 (10), 61 (35). 130.9, 129.4, 128.6, 115.8 (J = 21 Hz), 48.6, 35.9, 32.9, 29.6, 15.4. EI-MS [m/z (%)]: 378 (3), 346 (2). Anal. calcd. for C19H19ClFN3O3S3 (488.02): C, 46.76; H, 3.92; N, 8.61%. Found: C, 46.96; H, 3.90; N, 8.49%.
N-[1-(5-(4-Chlorobenzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-chlorobenzenesulphonamide (5p)
Yield: 0.233 g (54%); m.p. 85–87°C; Rf: 0.48 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3241, 1574, 1326, 1190, 1165, 1093; 1H-NMR (300 MHz, CDCl3): δ 7.75 (2H, d, J = 8.7 Hz, Ar-H), 7.43 (2H, d, J = 8.7 Hz, Ar-H), 7.35 (2H, d, J = 8.7 Hz, Ar-H), 7.31 (2H, d, J = 8.7 Hz, Ar-H), 4.92 (1H, s, NH), 4.88 (1H, at, J = 7.5 Hz, CH), 4.35 (2H, s, CH2), 2.65 (2H, t, J = 6.9 Hz, CH2), 2.10–2.18 (2H, m, CH2), 2.06 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 165.9, 164.7, 139.6, 137.9, 134.2, 133.7, 130.5, 129.4, 129.0, 128.6, 48.5, 35.9, 32.9, 29.6, 15.4. EI-MS [m/z (%)]: 278 (12), 175 (14), 127/125 (10), 113/111 (45), 73 (91), 61 (100), 28 (35). Anal. calcd. for C19H20ClN3O3S3 (470.03): C, 45.52; H, 3.80; N, 8.33%. Found: C, 46.02; H, 3.87; N, 8.35%.
N-[1-(5-Benzylthio)-1,3,4-oxadiazol-2-yl)-2-phenylethyl]-4-methylbenzenesulphonamide (5q)
Yield: 0.325 g (76%); m.p. 155–157°C; Rf: 0.47 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3210, 1570, 1330, 1163, 1088. 1H-NMR (300 MHz, CDCl3): δ 7.56 (2H, d, J = 8.4 Hz, Ar-H), 7.34–7.38 (5H, m, Ar-H), 7.21–7.24 (3H, m, Ar-H), 7.17 (2H, d, J = 8.1 Hz, Ar-H), 6.98–7.01 (2H, m, Ar-H), 5.02 (1H, s, NH), 4.88 (1H, aq, J = 6.9 Hz, CH), 4.33 (2H, s, CH2), 3.16 (2H, m, CH2), 2.37 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 165.8, 164.5, 143.9, 136.1, 135.2, 134.1, 129.7, 129.3, 129.1, 128.9, 128.8, 128.3, 127.6, 127.1, 50.7, 40.4, 36.7, 21.6. EI-MS [m/z (%)]: 374 (20), 310 (13), 274 (3), 155 (24), 91 (100), 77 (3), 65 (14). Anal. calcd. for C24H23N3O3S2 (465.59): C, 61.91; H, 4.98; N, 9.03%. Found: C, 62.46; H, 5.37; N, 9.02%.
N-[1-(5-(4-Bromobenzylthio)-1,3,4-oxadiazol-2-yl)-2-phenylethyl]-4-methylbenzenesulphonamide (5r)
Yield: 0.37 g (74%); m.p. 150–152°C; Rf: 0.47 (n-hexane: ethyl acetate; 3: 2); IR (υmax, cm−1): 3256, 1595, 1567, 1330, 1162, 1091, 1075. 1H-NMR (300 MHz, CDCl3): δ 7.56 (2H, d, J = 8.4 Hz, Ar-H), 7.48 (2H, d, J = 8.4 Hz, Ar-H), 7.22–7.25 (3H, m, Ar-H), 7.18 (2H, d, J = 8.1 Hz, Ar-H), 7.05 (2H, d, J = 8.7 Hz, Ar-H), 6.97–7.01 (2H, m, Ar-H), 5.18 (1H, d, J = 8.7 Hz, NH), 4.87 (1H, aq, J = 6.9 Hz, CH), 4.29 (2H, s, CH2), 3.09–3.22 (2H, m, CH2), 2.38 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.0, 164.2, 143.9, 136.1, 134.5, 134.1, 132.0, 130.8, 129.7, 129.3, 128.9, 127.6, 127.1, 122.3, 50.7, 40.3, 35.9, 21.6. EI-MS (m/z %) 274 (3), 171/169 (100), 155 (11), 91 (60), 65 (22). Anal. calcd. for C24H22BrN3O3S2 (544.48): C, 52.94; H, 4.07; N, 7.72%. Found: C, 52.94; H, 4.30; N, 7.57%.
N-[1-(5-(4-Fluorobenzylthio)-1,3,4-oxadiazol-2-yl)-2-phenylethyl]-4-methylbenzenesulphonamide (5s)
Yield: 0.355 g (80%); m.p. 140–142°C; Rf: 0.47 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3129, 1599, 1540, 1331, 1226, 1157, 1089. 1H-NMR (300 MHz, CDCl3): δ 7.57 (2H, d, J = 8.4 Hz, Ar-H), 7.36 (2H, m, Ar-H), 7.22–7.24 (3H,m, Ar-H), 7.18 (2H, d, J = 8.1 Hz, Ar-H), 7.03 (2H, at, J = 8.7 Hz, Ar-H), 6.97–6.99 (2H, m, Ar-H), 5.18 (1H, d, J = 8.4 Hz, NH), 4.87 (1H, aq, J = 6.9 Hz, CH), 4.32 (2H, s, CH2), 3.09–3.22 (2H, m, CH2), 2.38 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 165.9, 164.3, 162.5 (J = 243 Hz), 143.9, 136.1, 134.1, 131.2 (J = 3.7 Hz), 130.9 (J = 7.5 Hz), 129.7, 129.3, 128.9, 127.6, 127.1, 50.7, 40.3, 35.9, 21.6. EI-MS [m/z (%)]: 483 [M+], 392 (22), 328 (18), 274 (5), 155 (51), 109 (100), 91 (95), 65 (15). Anal. calcd. for C24H22FN3O3S2 (483.58): C, 59.61; H, 4.59; N, 8.69%. Found: C, 60.03; H, 4.74; N, 8.75%.
N-[1-(5-(4-Chlorobenzylthio)-1,3,4-oxadiazol-2-yl)-2-phenylethyl]-4-methylbenzenesulphonamide (5t)
Yield: 0.345 mg (75%); m.p: 146–148° C; Rf: 0.47 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 3139, 1598, 1568, 1331, 1163, 1090, 1030. 1H-NMR (300 MHz, CDCl3): δ 7.57 (2H, d, J = 8.1Hz, Ar-H), 7.30 (4H, m, Ar-H), 7.21–7.23 (3H, m, Ar-H), 7.18 (2H, d, J = 8.4 Hz, Ar-H), 6.97–6.99 (2H, m, Ar-H), 5.18 (1H, d, J = 8.4 Hz, NH), 4.87 (1H, at, J = 6.9 Hz, CH), 4.31 (2H, s, CH2), 3.08–3.23 (2H, m, CH2), 2.38 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 166.1, 164.2, 143.9, 136.2, 134.2, 134.1, 133.9, 130.5, 129.7, 129.3, 129.0, 128.9, 127.6, 127.1, 50.7, 40.3, 35.9, 21.6.; EI-MS [m/z (%)]: 499 [M+], 410/408 (23), 344 (15), 155 (48), 127/125 (80), 91 (100), 65 (14). Anal. calcd. for C24H22ClN3O3S2 (500.03): C, 57.65; H, 4.43; N, 8.40%. Found: C, 57.03; H, 4.51; N, 8.26%.
Synthesis of 2-{N-[4-halobenzyl]-1-(4-chloro/methyl-phenylsulphonylamino)alkyl}-5-benzylthio-1,3,4-oxadiazoles (6a-d)
Compounds 6a–d were prepared by following the same procedure as for the preparation of 5a–d from treatment of 4a–e with 1.1 mol. eq. of 4-halobenzyl halides to give a mixture of mono- and disubstituted products. Compounds 5 and 6 were separated by SiO2 column chromatography, using n-hexane and ethyl acetate (4:1) as an eluent.
N-(4-Bromobenzyl)-N-[1-(5-(4-bromobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-methylbenzenesulphonamide (6a)
Yield: 0.135 g (23%); m.p. 156–158°C; Rf: 0.64 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 2982, 1593, 1565, 1486, 1469, 1327, 1155, 1070. 1H-NMR (300 MHz, CDCl3): δ 7.71 (2H, d, J = 8.1 Hz, Ar-H), 7.49 (2H, d, J = 8.4 Hz, Ar-H), 7.30–7.36 (6H, m, Ar-H), 7.10 (2H, d, J = 8.1 Hz, Ar-H), 5.36 (1H, q, J = 6.9 Hz, CH), 4.47 (1H, d, J = 15.9 Hz, CH), 4.30 (1H, d, J = 15.2 Hz, CH), 4.26 (2H, s, CH2), 2.45 (3H, s, CH3), 1.46 (3H, d, J = 6.9 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 165.8, 164.7, 144.1, 136.9, 135.3, 134.5, 131.9, 130.9, 129.9, 129.8, 127.2, 122.2, 121.7, 48.5, 47.3, 35.8, 21.6, 15.7. EI-MS [m/z (%)]: 281 (6), 212/210 (11), 198 (25), 171/169 (65), 155 (145), 133 (27), 91 (100), 73 (10), 65 (18), 28 (85). Anal. calcd. for C25H23Br2N3O3S2 (639.42): C, 47.11; H, 3.64; N, 6.59%. Found: C, 45.37; H, 3.45; N, 5.94%.
N-(4-Bromobenzyl)-N-[1-(5-(4-bromobenzylthio)-1,3,4-oxadiazol-2-yl)ethyl]-4-chlorobenzenesulphonamide (6b)
Yield: 0.125 g (19%); m.p. 144–146°C; Rf: 0.65 (n-hexane: ethyl acetate; 3:2); IR (υmax, cm−1): 2990, 1596, 1570, 1480, 1468, 1331, 1156, 1069. 1H-NMR (300 MHz, CDCl3): δ 7.75 (2H, d, J = 8.7 Hz, Ar-H), 7.48 (4H, d, J = 8.7 Hz, Ar-H), 7.37 (2H, d, J = 8.4 Hz, Ar-H), 7.31 (2H, d, J = 8.4 Hz, Ar-H), 7.11 (2H, d, J = 8.1 Hz, Ar-H), 5.35 (1H, q, J = 7.2 Hz, CH), 4.32 (1H, d, J = 15.7 Hz, CH), 4.31 (1H, d, J = 17.1 Hz, CH), 4.30 (2H, s, CH2), 1.49 (3H, d, J = 7.2 Hz, CH3). 13C-NMR (75 MHz, CDCl3): δ 165.5, 164.9, 139.7, 138.3, 134.9, 134.4, 131.9, 131.6, 130.9, 129.8, 129.6, 128.6, 122.2, 121.9, 48.8, 47.6, 35.8, 16.2; EI-MS [m/z (%)]: 300/298 (70), 212/210 (30), 183 (5), 171/169 (100), 143 (12), 89 (31), 75 (15), 63 (20). Anal. calcd. for C24H20Br2ClN3O3S2 (659.84): C, 43.82; H, 3.06; N, 6.39%. Found: C, 44.14; H, 3.47; N, 6.03%.
N-(4-Fluorobenzyl)-N-[1-(5-(4-fluorobenzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl]-4-methylbenzenesulphonamide (6c)
Yield: 0.143 g (27%); brownish oil; Rf: 0.65 (n-hexane: ethyl acetate 3:2); IR (υmax, cm−1): 2950, 1597, 1560, 1486, 1472, 1340, 1158, 1069. 1H-NMR (300 MHz, CDCl3): δ 7.72 (2H, d, J = 8.4 Hz, Ar-H), 7.48 (2H, d, J = 8.7 Hz, Ar-H), 7.37–7.42 (2H, m, Ar-H), 7.30 (2H, d, J = 8.1 Hz, Ar-H), 7.05 (2H, at, J = 8.4 Hz, Ar-H), 6.9 (2H, at, J = 8.7 Hz, Ar-H), 5.39 (1H, t, J = 7.5 Hz, CH), 4.38 (1H, d, J = 15.9 Hz, CH), 4.30 (1H, d, J = 15.2 Hz, CH), 4.30 (2H, s, CH2), 2.30–2.55 (3H, m, CH2, CH), 2.43 (3H, s, CH3), 1.90–1.96 (1H, m, CH), 1.96 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 164.4, 164.0, 162.5 (J = 246 Hz), 162.3 (J = 245 Hz), 144.1, 136.8, 131.8 (J = 3.7 Hz), 131.1 (J = 3.7 Hz), 130.5 (J = 8.2 Hz), 130.4 (J = 8.2 Hz), 129.8, 127.3, 115.8 (J = 21.7 Hz), 115.3 (J = 21.7 Hz), 51.7, 48.4, 35.7, 30.2, 29.7, 21.6, 15.2. EI-MS [m/z (%)]: 500 (3), 420 (11), 297 (2), 155 (3), 109 (100), 91 (8), 65 (5), 61 (10).
N-(4-bromobenzyl)-N-[1-(5-(4-bromobenzylthio)-1,3,4-oxadiazol-2-yl)-3-(methylthio)propyl-4-methylbenzenesulphonamide] (6d)
Yield: 0.109 g (17%); brownish oil; Rf: 0.63 (n-hexane: ethyl acetate 3:2); IR (υmax, cm−1): 2917, 1601, 1562, 1508, 1472, 1340, 1155, 1220. 1H-NMR (300 MHz, CDCl3): δ 7.71 (2H, d, J = 8.4 Hz, Ar-H), 7.48 (2H, d, J = 8.4 Hz, Ar-H), 7.37 (2H, d, J = 8.4 Hz, Ar-H), 7.28–7.32 (4H, m, Ar-H), 7.15 (2H, d, J = 8.4 Hz, Ar-H), 5.39 (1H, at, J = 7.5 Hz, CH), 4.30 (1H, d, J = 13.5 Hz, CH), 4.29 (1H, d, J = 13.5 Hz, CH), 4.27 (2H, s, CH2), 2.52–2.23 (3H, m, CH2, CH), 2.43 (3H, s, CH3), 1.89–1.96 (1H, m, CH), 1.95 (3H, s, CH3). 13C-NMR (75 MHz, CDCl3): δ 164.7, 144.1, 136.7, 135.5, 134.4, 131.9, 131.6, 130.9, 130.1, 129.9, 127.3, 122.3, 121.9, 51.8, 48.4, 35.8, 29.7, 21.6, 15.2. EI-MS [m/z (%)]: 697/695 (2), 276/274 (2), 186/184 (95),157/155 (20), 75 (31), 65 (45), 28 (15).
X-ray structure determinations
Crystal data and refinement details are presented in . Data collection and reduction: Crystals were mounted in inert oil on glass fibres and transferred to the cold gas stream of an Oxford Diffraction diffractometer (5b: Xcalibur S with monochromated Mo-Kα radiation, λ = 0.71073 Å; 5l: Xcalibur Nova E with mirror-focussed Cu-Kα radiation, λ = 1.54184 Å). Absorption corrections were performed on the basis of multi-scans. Structure refinement: The structures were refined anisotropically against F2 (program SHELXL-97Citation19). Hydrogens of NH groups were refined freely; methyl groups were refined as idealized rigid groups allowed to rotate but not tip; other hydrogen atoms were included with a riding model. For 5b, restraints to displacement parameters were employed to improve stability of refinement. For both structures, the absolute configuration was confirmed by the Flack parameter.
Table 1. Crystallographic data for compounds 5b and 5l.
Complete crystallographic data (excluding structure factors) have been deposited at the Cambridge Crystallographic Data Centre under the numbers CCDC-762468 (5b) and 762469 (5l). Copies may be requested free of charge from http://www.ccdc.cam.ac.uk/products/csd/request/.
Results and discussion
Three different amino acids: l-alanine, l-methionine and l-phenylalanine (a–c) were selected in our present work to synthesize the desired chiral compounds. The l-amino acids were converted into the corresponding sulphonamides 1a–e by reaction with 4-chlorobenzenesulfonyl chloride and 4-methylbenzenesulfonyl chloride (Scheme 1). Compounds 1a–e were converted into their respective methyl esters 2a–eCitation20, followed by treatment with the hydrazine hydrate to yield the corresponding acid hydrazides 3a–eCitation21. The hydrazides 3a–e were cyclized to 1,3,4-oxadiazoles 4a–eCitation22, using CS2 in the presence of KOH. The 1,3,4-oxadiazoles 4a–e were further derivatized with 4-substituted benzylhalidesCitation13. Thus, treatment of 4a–e with a slight excess (1.1 equiv.) of 4-substituted benzyl halides resulted in the substitution at S- as well as N-(sulphonamide), giving a mixture of two products: S-substituted and S,N-disubstituted products. However, repetition of the experiment using equimolar ratios of 4-substituted benzylhalide and 1,3,4-oxadiazoles led to the S-substitution only and furnished 5a–t.
The structures of 4a–e were confirmed from the NMR, IR and mass spectra. In the IR spectra, the C=N absorption appeared in the region υmax 1496–1462 cm−1 at the expense of strong carbonyl absorption of the hydrazides 3a–e (υmax 1686–1664 cm−1). The weak absorption for C-S in the range of υmax 1291–1261 cm−1 was a further support for formation of the desired molecules. In the 1H-NMR spectra, the signals at δ 14.38–9.30 ppm were assigned to the N-H proton. The two signals in the 13C-NMR spectra at δ 178.6–178.0 ppm and δ 170.5–161.6 ppm were assigned to the C-2 and C-5 of the oxadiazole ring respectively. The mass spectra demonstrated a common fragment for 4a, 4c and 4e at m/z 155 and for 4b and 4d at m/z 175, resulting by cleavage of the sulphonamide linkage. The base peak observed for compounds 4a–e was attributed to the tropyllium cation at m/z 91 or the chlorotropyllium cation at m/z 127/125. Analogously, the structures of 5a–t were confirmed by the 1H-, 13C-NMR and mass spectra. In the 1H-NMR spectra of 5a–t, two signals for four aromatic protons in the range δ 7.75–7.03 ppm together with two protons singlet (δ 4.88–4.74 ppm) assigned to the benzylic protons were observed. The 13C-NMR spectra showed new signals corresponding to the methylene carbons of the benzyl group resonating in the range δ 48.6–45.5 ppm. In the mass spectra, the most abundant fragments were observed at m/z 91 or 90 + X (X = Cl). The fragments at m/z 155 (R′ = CH3) and m/z 175 (R′ = Cl) were generated due to the cleavage of the sulphonamide moiety.
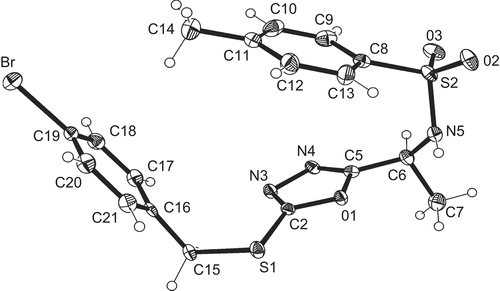
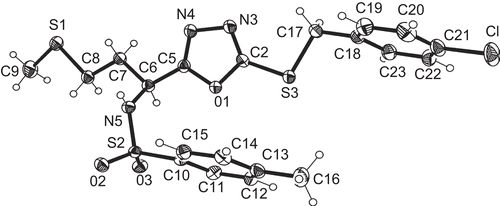
The synthesis of compounds 5a–t was further confirmed by the single crystal X-ray structure analysis of compounds 5b and 5l. Compound 5b () is a disc-shaped molecule in which all three rings lie at the periphery of the disc and are approximately perpendicular to the mean molecular plane (interplanar angles 86° to the five-membered ring, 81° to the ring C8–13, 86° to the ring C16–21). Compound 5l is also disc-shaped, the height of the disc being approximately the breadth of a phenyl ring (); the rings C10–15 and C18–23 subtend angles of 86° and 84°, respectively, to the mean molecular plane, but the angle from the five-membered ring is 27°. A least-squares fit of both molecules in the region C-5,6,7 and N-tosyl gives a root mean squared (RMS) deviation of 0.13 Å. shows clearly that the molecules differ significantly in the torsion angles involving the rotation of the five-membered ring (N5-C6-C5-O1 = -47.5° for 5b and 65.9° for 5l) and about the short C-S chain (C2-S1-C15-C16 -84.7° for 5b, C2-S3-C17-C18 169.8° for 5l). The crystallographic data for 5b and 5l are listed in .
The structures of the disubstituted 1,3,4-oxadiazole derivatives 6a–d were confirmed by the NMR, IR and mass spectra. The IR spectra demonstrated the disappearance of NH stretchings in the range of υmax 3256–3297 cm−1 with the appearance of the strong C–X (X = Cl, Br, F) absorptions in the range υmax 1220–1069 cm−1. The 1H-NMR spectra of 6a–d demonstrated eight additional aromatic protons in the range of δ 7.75–6.90 ppm, the singlets’ oriented in the region δ 4.51–4.19 ppm corresponding to CH2 protons of the benzylthio group, and the two doublets (Citation2J couplings) in the region δ 4.47–4.30 ppm attributed to the N-benzyl group. In the Citation13C-NMR spectra, eight new signals corresponding to aromatic portion of halobenzyl groups were observed. Further, two methylene carbons appeared in the range of δ 51.7–47.3 ppm. In the mass spectra, the most abundant fragments were observed at m/z 91 or 90 + X.
In vitro anti-HIV assay
Compounds 5a–t and 6a–d were tested for their anti-HIV-1 and HIV-2 activity, in vitro, using IIIB and ROD strain in human T-lymphocyte (MT-4) cells, and the results are summarized in , in which the data for Nevirapine (BOE/BIRG587Citation23) and azidothymidine (DDN/AZTCitation24) have been included for comparison purposes. Compound-induced cytotoxicity was also measured in MT-4 cells parallel with the antiviral activity. None of the new 1,3,4-oxadiazole derivatives were found to inhibit HIV-1 or HIV-2 replication, in vitro, at EC50 lower than the CC50 in comparison to the Nevirapine and AZT.
Table 2. In vitro anti-HIV-1a and HIV-2b of some new sulphonamide derivatives.
Theoretical calculations and quantum structure-activity relationship
Semi-empirical self-consistent-field molecular orbital (SCF-MO) method at PM3Citation18 level within restricted Hartree–FockCitation25. Formalism has been considered to optimize fully the geometry of the 5-benzylthio-1,3,4-oxadiazole molecule in its ground state. Geometry optimization was carried out by using a conjugate gradient method (Polak-Ribiere algorithmCitation26). The SCF convergence was set at 0.001 kcal/mol and the RMS gradient was set to 0.001 kcal/(mol) in the calculations.
We performed all the calculations using the HyperChem-7.52 program (Hypercube Inc., USA). In addition, the correlation analysis and the regression analysis for quantum parameters were performed by using Minitab program release 11.11 (Minitab Inc., USA). all calculations were performed on a windows XP workstation in Pentium IV PC.
Acceptability of the regression model was judged by examining the correlation coefficient (r), squared correlation coefficient (R2), Fisher’s value (F) and standard deviation (s). The selected descriptors have obtained and listed in and .
Table 3. Calculated value of descriptors.
Table 4. Mulliken charges of the selected atoms.
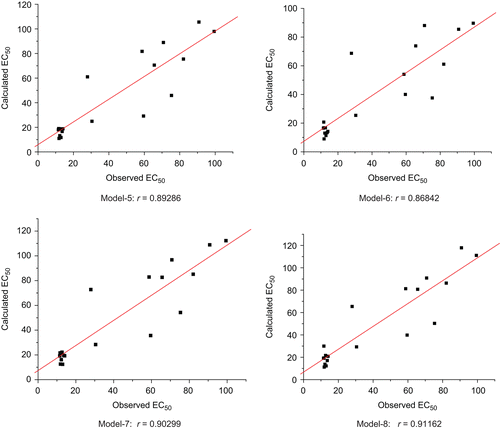
A data set of twenty compounds (5a–5t) concerning their anti-HIV activity was used for the present quantum structure-activity relationship (QSAR) study. QSAR studies of the 5-benzylthio-1,3,4-oxadiazoles series resulted in several QSAR equations. The four best equations are:
In the above equations, n is the number of compounds used to derive the model and q2 is the predictive capability.
All the four models have one outlier’s compounds 6, because their residual values exceeded twice the standard error of estimate. When this outlier has been removed from the data set, four highly significant equations (5, 6, 7 and 8 respectively) have been obtained.
Model–5 shows a good correlation coefficient (r) of 0.913 between descriptors (LogP, EHOMO, μ, and N10) and the anti-HIV activity. Squared correlation coefficient (r2) of 0.834 explains 83.7% variance in biological activity. This model also indicates statistical significance > 99.9% with values F = 17.64. Cross-validated squared correlation coefficient (q2) of this model was 0.787, which shows remarkable internal predication power of this model.
Similarly, model–6 shows a remarkable correlation coefficient (r) of 0.909 between descriptors (ΔE, P, S, and N10) and the anti-HIV activity. Squared correlation coefficient (r2) of 0.826 explains 82.6% variance in biological activity. This model also indicates statistical significance > 99.9% with values F = 16.56. Cross-validated squared correlation coefficient (q2) of this model was 0.776, which shows the good internal predication power of this model.
Further, model–7 demonstrates an interesting correlation coefficient (r) of 0.919 between descriptors (EHOMO, P, S, and N10) and anti-HIV activity. Squared correlation coefficient (r2) of 0.845 explains 84.5% variance in biological activity. This model also indicates statistical significance > 99.9% with values F = 19.09. Cross-validated squared correlation coefficient (q2) of this model was 0.801, which shows the good internal predication power of this model.
Finally, model–8 shows a desirable correlation coefficient (r) of 0.913 between descriptors (EHOMO, μ, S and N10) and the anti-HIV activity, where squared correlation coefficient (r2) of 0.834 explains 83.4% variance in biological activity. This model also indicates statistical significance > 99.9% with values F = 17.64. Cross-validated squared correlation coefficient (q2) of this model was 0.787, which shows reasonable internal predication power of this model.
According to 5, 6, 7, and 8 models, the calculated and experimental activities (EC50) of the title compounds were obtained and listed in . These models showed good correlation between the experimental and calculated EC50 (r = 0.893, 0.868, 0.903, and 0.912 for 5, 6, 7 and 8 models, respectively). Models 7 and 8 can be considered as most suitable models for predicting the anti-HIV activity with both statistical significant and excellent predictive ability ().
Table 5. Observed and calculated anti-HIV activity (EC50) of given series of compounds.
Conclusion
In conclusion, the above data showed no selective anti-HIV activity. However, compounds 5f, j–5q did show some inhibitory activity against both HIV-1 and HIV-2 with EC50 value ranging from >11.50 to >14.08 µg/mL, but with Si <1.
Acknowledgements
The authors thank Professor C. Pannecouque of Rega institute for medical research, Katholieke Universiteit Leuven, Belgium for the anti-HIV screening.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Hijikata M, Ohta Y, Baba K. Iwata K, Matsumoto M, Mishiro S, Kanai K. Instability of the NS5A ISDR of hepatitis C virus during natural course: take-over of wild type by mutant type or vice-versa driven by immune pressure. Hepatology Res 1998;11:19–25.
- Bartenschlager R, Lohmann V. Novel cell culture systems for the hepatitis C virus. Antiviral Res 2001;52:1–17.
- Zareef M, Iqbal R, Al-Masoudi NA, Zaidi JH, Arfan M, Shahzad SA. Synthesis, anti-HIV and antifungal activity of new benzensulphonamides bearing 2,5-disubstituted 1,3,4-oxadiazole moiety. Phosphorus Sulfur Silicon Relat Elem 2007;182:281–298.
- Iqbal R, Zareef M, Ahmed S, Zaidi JH, Arfan M, Shafique M, Al-Masoudi NA. Synthesis, antimicrobial and anti-HIV activity of some novel benzenesulfonamides bearing 2,5-disubstituted-1,3,4-oxadiazole moiety. J Chin Chem Soc 2006;53:689–696.
- El-Emam AA, Al-Deeb OA, Al-Omar M, Lehmann J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg Med Chem 2004;12:5107–5113.
- Johns BA, Weatherhead JG, H. Allen, SH, Thompson JB, Garvey EP, Foster SA, Jeffrey JL, Miller WH. The use of oxadiazole and triazole substituted naphthyridines as HIV-1 integrase inhibitors. Part 1: Establishing the pharmacophore. Bioorg Med Chem Lett 2009;19:1802–1806.
- Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS et al.; STARTMRK investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet 2009;374:796–806.
- Yan S, Appleby T, Larson G, Wu JZ, Hamatake RK, Hong Z et al. Thiazolone-acylsulfonamides as novel HCV NS5B polymerase allosteric inhibitors: convergence of structure-based drug design and X-ray crystallographic study. Bioorg Med Chem Lett 2007;17:1991–1995.
- Yannopoulos CG, Xu P, Ni F, Chan L, Pereira OZ, Reddy TJ et al. HCV NS5B polymerase-bound conformation of a soluble sulfonamide inhibitor by 2D transferred NOESY. Bioorg Med Chem Lett 2004;14:5333–5337.
- Selvam P, Murugesh N, Chandramohan M, Sidwell RW, Wandersee MK, Smee DF. Anti-influenza virus activities of 4-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)amino]-N-(4,6-dimethyl-2-pyrimidin-2-yl)benzenesulphonamide and its derivatives. Antiviral Chem Chemother 2006;17:269–274.
- Li X, Yang X, Liang X, Kai Z, Yuan H, Yuan D, Zhang J, Wang R, Ran F, Qi S, Ling Y, Chen F, Wang D. Synthesis and biological activities of 2-oxocycloalkylsulfonamides Bioorg Med Chem 2008;16:4538–4544.
- Vermeire K, Schols D. Cyclotriazadisulfonamides: promising new CD4-targeted anti-HIV drugs. J Antimicrob Chemother 2005;56:270–272.
- Akhtar T, Hameed S, Al-Masoudi NA, Loddo R, La Colla P. In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione. Acta Pharm 2008;58:135–149.
- Akhtar T, Hameed S, Al-Masoudi NA, Khan KM. Synthesis, and anti-HIV activity of new chiral 1,2,4-triazoles and 1,3,4-thiadiazoles. Heteroatom Chem 2007;18:316–322.
- Zahid M, Yasin KA, Akhtar T, Rama NH, Hameed S, Al-Masoudi NA., Loddo R, La Colla P. New 2-(4-aryl)-5-(2-adamantylthiazol-4-yl)-1,3,4-oxadiazoles as potential antiproliferative and antiviral agents, ARKIVOC 2009;xi: 85–93.
- Serwar M, Akhtar T, Hameed S, Khan KM. Synthesis, urease inhibition and antimicrobial activities of some chiral 5-aryl-4-(1-phenylpropyl)-2H-1,2,4-triazole-3-(4H)-thione. ARKIVOC 2009;vii:210–221.
- Akhtar T, Hameed S, Khan KM, Choudhary MI. Syntheses, urease inhibition, and antimicrobial studies of some chiral 3-substituted-4-amino-5-thioxo-1H,4H-1,2,4-triazoles. Med Chem 2008;4:539–543.
- Stewart JJP. Optimization of parameters for semi-empirical methods. I Method J Comput Chem 1989;10:209–220.
- Sheldrick GMA. Short history of SHELX. Acta Cryst 2008;A64:112–122.
- Zareef M, Iqbal R, De Dominguez NG, Rodrigues J, Zaidi JH, Arfan M, Supuran CT. Synthesis andantimalarial activity of novel chiral and achiral benzenesulfonamides bearing 1, 3, 4-oxadiazole moieties. J Enz Inhib Med Chem 2007;22:301–308.
- Zarghi A, Faizi M, Shafaghi B, Ahadian A, Khojastehpoor HR, Zanganeh V et al. Design and synthesis of new 2-substituted-5-(2-benzylthiophenyl)-1,3,4-oxadiazoles as benzodiazepine receptor agonists. Bioorg Med Chem Lett 2005;15:3126–3129.
- Koparir M, Cetin A, Cansiz A. 5-Furan-2-yl[1,3,4]oxadiazole-2-thiol, 5-furan-2yl-4H [1,2,4] triazole-3-thiol and their thiol-thione tautomerism. Molecules 2005;10:475–480.
- Hargrave KD, Proudfoot JR, Grozinger KG, Cullen E, Kapadia SR, Patel UR et al. Novel non-nucleoside inhibitors of HIV-1 reverse transcriptase. 1. Tricyclic pyridobenzo- and dipyridodiazepinones. J Med Chem 1991;34:2231–2241.
- Mitsuya H, Weinhold KJ, Furman PA, St Clair MH, Lehrman SN, Gallo RC et al. 3′-Azido-3′-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci USA 1985;82:7096–7100.
- Fletcher P. Practical Methods of Optimization. New York: Wiley, 1990.
- Rui OM, Qian L. I. 2D-QSAR studies on phenoxybenzoic acid derivatives: A novel class of 5α -reductase inhibitors. Chinese J Struct Chem 2008; 27: 105–111.