Abstract
The aim of this study was to determine the capacity of some progesterone derivatives, to inhibit the conversion of labeled androstenedione ([3H] 4-dione) to [3H]dihydrotestosterone ([3H]DHT) in prostate nuclear membrane fractions, where the 5α-reductase activity is present. The enzyme 5α-reductase catalyzes the 5α-reduction of 4-dione whereas the 17β-hydroxysteroid dehydrogenase catalyzes the transformation of 4-dione to testosterone or 5α-dione to dihydrotestosterone (DHT). Moreover, we also investigated the role of unlabeled 5α-dione in these pathways. In order to determine the inhibitory effect of different concentrations of the progesterone derivatives in the conversion of [3H] 4-dione to [3H]DHT, homogenates of human prostate were incubated with [3H] 4-dione, NADPH and increasing concentrations of non-labeled 5α-dione. The incubating mixture was extracted and purified using thin layer chromatography. The fraction of the chromatogram corresponding to the standard of DHT was separated and the radioactivity determined. The results showed that the presence of [3H] 4-dione plus unlabelled 5α-dione produced similar levels of DHT as compared to [3H] 4-dione. On the other hand, the results indicated that 17α-hydroxypregn-4-ene-3,20-dione 5 and 4-bromo-17α-hydroxypregn-4-ene-3,20-dione 7b, were the most potent steroids to inhibit the conversion of [3H] 4-dione to [3H]DHT, showing IC50 values of 2 and 1.6 nM, respectively.
Introduction
Testosterone (T) plays an important role in the testes and muscle; on the other hand dihydrotestosterone (DHT) is crucial for the development, function and pathology of the prostate. This tissue produces DHT from the reduction of the circulating T and such conversion is carried out by the 5α-reductase enzyme present in the prostate. T or DHT activate the androgen receptor (AR) which interacts with androgen response elements in DNA to regulate gene transcriptionCitation1.
In addition to T, the adrenal androgen androstenedione (4-dione) is converted to 5α-androstanedione (5α-dione) by the steroid 5α-reductase present in the prostateCitation2. After castration the steroid 4-dione has been implicated as a source of DHT in prostate tissueCitation3. The presence of other steroidogenic enzymes in the prostateCitation4, as well as the availability of various steroid precursors such as 4-dione suggest the existence of additional pathways involved in the biosynthesis and metabolism of DHT ().
Figure 1. Metabolic pathways for the biosynthesis of DHT in the prostate. The steps that do not require the presence of T are indicated in bold lines.
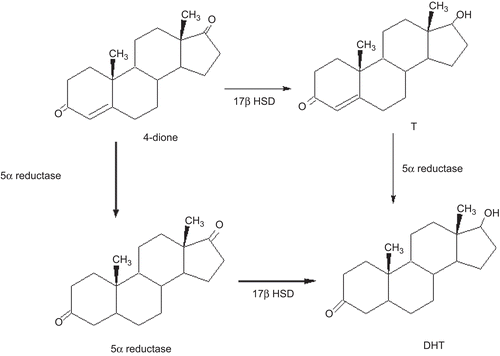
The activity of 5α-reductase enzyme (EC 1.3.99.5) in androgen dependent tissues has long been known. Two isoforms of 5α-reductase had been identified: named 1 and 2, each encoded by different genes, which have been characterized in several speciesCitation5,Citation6. 5α-Reductase type 2 isozyme plays a major role in prostate cancer and benign prostatic hyperplasia as it is predominantly expressed in this tissue. It is well known that type 1 is expressed in prostate epithelial cells while the type 2 is mainly located in the stromal compartmentCitation7,Citation8. 5α-Reductase type 1 is also present in the liver, skin and acts in a neutral or basic medium, whereas type 2 is active in acidic pHCitation5. Recently, a type 3 5α-reductase had been described in prostate cancer cellsCitation9 and in a human sebaceous gland cell lineCitation10.
In this study, we determined the biosynthesis of [3H]DHT from [3H] T or [3H] 4-dione in the presence or absence of non-labeled DHT or 5α-dione in the incubation medium. These studies were carried out using human 5α-reductase from nuclear membrane fractions of prostate. The enzyme 5α-reductase catalyzes the 5α-reduction of 4-dione whereas the 17β-hydroxysteroid dehydrogenase catalyzes the transformation of 4-dione to T or 5α-dione into DHT. Moreover, we assessed the effect of different inhibitors of T 5α-reductase as finasteride and the progesterone derivatives: 3, 20-dioxopregn-4-ene-17-yl acetate 4, 17α-hydroxypregn-4-ene-3,20-dione 5, 17α-hydroxy-4,5-epoxypregnane-3,20-dione 6, 4-chloro-17α-hydroxypregn-4-ene-3,20-dione 7a, 4-bromo-17α-hydroxypregn-4-ene-3,20-dione 7b, 4-chloro-3,20-dioxopregn-4-ene-17-yl-4-ethylbenzoate 8a and 4-bromo-3,20-dioxopregn-4-ene-17-yl-4-ethylbenzoate 8b as 4-dione 5α-reductase inhibitors. These compounds were synthesized and evaluated in our laboratory as inhibitors of both isozymes of T 5α-reductaseCitation11.
Materials and methods
Materials
(1, 2, 6, 7-3H) Testosterone [3H] T specific activity: 95 Ci/ mmol and androstenedione (androst-4-ene-3, 17-dione [1,2,3,7-3H(N)] [3H] 4-dione () specific activity 90 Ci/mmol were provided by Perkin Elmer Life and Analytical Sciences. (Boston, MA). Radioinert T, 5α-DHT, androstenedione and androstanedione were supplied by Steraloids (Wilton, NH). Sigma Chemical Co. (ST. Louis, Mo) provided NADPH. Finasteride was obtained by extraction from Proscar (Merck, Sharp and Dohme). The tablets were crushed, extracted with chloroform and the solvent was eliminated in vacuum; the crude product was purified by silica gel column chromatography. The melting point of the isolated finasteride (252–254°C) was identical to that reported in the literature.
Steroids 4–6, 7a, 7b, 8a and 8b () were synthesized according to the method of Bratoeff et al.Citation11
Method
The prostate of a man 37-years old, who died from gastrinoma was extirpated and the tissue was immediately chilled in ice-cold 150 mM NaCl and stored at −20°C. Frozen human prostate was thawed on ice and minced with a tissue mill IKA A11 basic. Unless specified, the following procedures were carried out at 4°C.
Human prostate tissue was homogenized in 2 volumes of buffer A (20 mM sodium phosphate, pH 6.5 containing 0.32 M sucrose, 0.1 mM dithiothreitol Sigma-Aldrich Inc.) with a tissue homogenizer Ultra-Turrax IKA, T18 basic. (Wilmington, NC). Homogenates were centrifuged at 1500g for 60 min at 0°CCitation12,Citation13 in a SW 60 Ti rotor (Beckman instruments, Palo Alto, CA). The pellets were separated, suspended in medium A and kept at −70°C. The suspension, 5 mg of protein/mL for human prostates, determined by the Bradford methodCitation14 was used as source of 5α-reductase.
Determination of 5α-reductase activity
The activity of the T 5α-reductase or 4-dione 5α-reductase was assayed as previously describedCitation12,Citation13. The reaction mixtures contained a final volume of 1 mL, 1 mM DTT, sodium phosphate buffer 40 mM, at pH 6.5, 2 mM, NADPH, 2 nM [1,2,6,7-3H]T or [1,2,3,7-3H(N)] 4-dione and the prostatic enzyme fractions. The amount of prostatic enzyme fractions was determined to adjust the rate of conversion of T to DHT or 4-dione to 5α-dione to around 28%. T, DHT, unlabeled 4-dione or 5α-dione concentrations were adjusted to 25–250 nM in the incubating medium, by adding one of the following reagents cold T, DHT, unlabeled 4-dione or 5α-dione. The reactions in duplicate were started when added to the enzymatic fraction (400 µg protein in a volume of 80 µL) incubated at 37°C for 60 minCitation13 and stopped by mixing with 1 mL of dichloromethane. Incubation without tissue was used as a control. All reactions were carried out in two different times by duplicate.
Extraction and purification of steroids formed from T and 4-dione
The mixtures (incubation medium/dichloromethane), were agitated on a vortex for 1 min and the dichloromethane phase was separated and placed in another tube. This procedure was repeated four more times. The dichloromethane extract was evaporated to dryness under a nitrogen stream and suspended in 50 µL of methanol that was spotted on HPTLC Keiselgel 60 F254 plates. T, DHT, 4-dione and 5α-dione were used as standard carriers and were applied in different lanes on both lateral sides of the plates (T, T+DHT, DHT and 4-dione, 4-dione+5α-dione, 5α-dione). The plates were developed in chloroform-acetone 9:1 and were air-dried; this procedure was repeated two more times. The steroidal standard DHT carrier was detected using phosphomolibdic acid reagent DHT; T and 4-dione with an UV lamp (254 nm). The 5α-dione standard was detected by the Zimmermann reaction; the lines of the plate corresponding to 5α-dione were sprayed with a freshly prepared mixture of equal volume of a 2% solution of m-dinitrobenzene in absolute ethanol and a 2.5 N solution of potassium hydroxide. After the plates were segmented in areas of one cm each, they were cut off and the strips soaked in 5 mL of Ultima Gold (Packard). The radioactivity was determined in a scintillation counter (Packard tri-carb 2100 TR). in the segment corresponding to T, DHT, 4-dione and 5α-dione carriers. The radioactivity that had identical chromatographic behavior as the T, DHT and 5α-dione standards was considered as the T, DHT or 5α-dione transformation. Control incubations, chromatography separations and identifications, were carried out in the same manner as described above except that the tubes did not contain tissue. The T, DHT or 5α-dione transformation yields were calculated from the strips, taken into account the entire radioactivity on the plate.
The T 5α-reductase or 4-dione 5α-reductase activity was calculated from the percentage of labeled T, DHT, or 5α-dione formed, taking into consideration recovery, blank values, the specific activity of [3H] T or [3H] 4-dione and the radio of added [3H] T or [3H] 4-dione to unlabeled T, 4-dione, DHT or 5α-dione. Km and Vmax values were derived from Linewear–Burk plots and determined also by the Michaelis–Menten expressionCitation15,Citation16.
The efficiency of T 5α-reductase and 4-dione 5α-reductase were estimated according to Weisser and Krieg reportCitation2.
Determination of 50% of the inhibitory concentration of finasteride and steroids 4, 5, 6, 7a, 7b, 8a and 8b in 5α-reductase activities
In order to calculate the IC50 values (the concentration of finasteride and steroids 4, 5, 6, 7a, 7b, 8a and 8b, ) required to inhibit 50% of the activity 5α-reductase, six series of tubes containing increasing concentrations of these steroids (10−11 – 10−3 M) were incubated in duplicate, in the presence of: 1 mM of dithiothreitol, 40 mM sodium phosphate buffer pH of 6.5; 2 mM NADPH, 2 nM [3H] 4-dione plus 125 nM of 5α-dione and 400 μg of protein from the enzymatic fraction obtained from human prostate as described above.
The reactions were carried out in duplicate at 37°C for 60 min and 1 mL of dichloromethane was added to stop the reaction. The extraction and the chromatographic procedures were carried out as described above. The radioactivity contained in the fraction corresponding to DHT carrier was determined using the above procedure.
Results
In vitro experiments
5α-reductase activity
Human prostatic 5α-reductase from nuclear membrane fractions of prostate affects the conversion of T to DHT in the presence of NADPH (). Furthermore, labeled T in the presence of increasing concentrations of unlabeled DHT showed higher conversion to labeled DHT (higher 5α-reductase activity) Vmax =103.9 pmol/mg of protein/h as compared to the experiment with increasing concentrations of T (Vmax = 59.6 pmol/mg of protein/h). On the other hand, the 5α-reductase enzyme showed a higher affinity (minor Km value, ) when DHT was present in the incubating medium as compared to the presence of T (major Km value, ).
Table 1. DHT and 5α-dione production from different concentrations of labeled T and 4-dione by human prostate homogenates. Effect of increasing concentrations of unlabeled DHT and 5α-dione on the prostatic 5α-reductase activity.
It was observed that after the incubation with labeled 4-dione, a radioactive zone that had identical chromatographic behavior as the standard of 5α-dione (Rf value of 0.87), was found, thus demonstrating the activity of 5α-reductase (). When increasing concentrations of unlabeled 5α-dione were added to the incubation medium, a higher production of labeled 5α-dione (67.5 pmol/mg of protein/h) was observed (). However, when increasing concentrations of labeled and unlabeled 4-dione were incubated, a lower rate of 5α-dione formation was observed (26.10 pmol/mg of protein/h), . In addition, the Km values for 5α-reductase in the presence of unlabeled 5α-dione were lower as compared to the Km values in the absence of this steroid (). On the other hand, 4-dione possesses a lower affinity than T for 5α-reductase as shown by their Km values of 176 nM for 4-dione and 96.29 nM for T ().
When prostatic nuclear membrane fraction was incubated in the presence of labeled 4-dione plus increasing concentrations of unlabeled 5α-dione, a slightly higher conversion to labeled DHT was found. The Vmax of conversion to DHT was in the range of 19.2 pmol/mg of protein/h, when increasing concentrations of 4-dione was incubated alone a similar conversion to DHT was found; the Vmax determined for DHT was of 18 pmol/ mg of protein/h. (). Therefore it appears that the 5α-dione is converted to DHT by a catalytic reaction mediated by 17β-HSDCitation4.
Table 2. T and DHT production from different concentrations of labeled 4-dione by human prostate homogenates. Effect of increasing concentrations of unlabeled 5α-dione.
In addition to the labeled DHT formed from labeled 4-dione, we also found a radioactive zone that had identical chromatographic behavior as the carrier T (Rf value of 0.59); these results suggest that 17β-HSD () is present in the prostate tissue as had been previously reportedCitation17. However the Vmax value (9.7 pmol/mg of protein/h) for T produced from labeled 4-dione was higher than the Vmax value (1.9 pmol/mg of protein/h) for T obtained, when unlabeled 5α-dione was present in the incubated medium (). These results imply that 5α-dione could have inhibited the 17β-HSD activity. However the presence of unlabeled 5α-dione increased the affinity of 17β-HSD for its substrate (minor Km value) as compared to the Km value obtained when T was produced from 4-dione alone (see ). It is remarkable to indicate that 17β-HSD is a labile enzyme, whose activity can easily be lost in the homogenizing processCitation18; this fact could explain the low rate of conversion of 4-dione to T.
The 5α-reductase’s enzymatic efficiency (Vmax/Km)Citation16,Citation19 obtained from the rate between maximal velocities (Vmax) of production of DHT or 5α-dione, using different substrates and the Michaelis constants (Km) are shown in . Vmax/Km had up to 1.9 fold higher efficiency when [3H]T plus DHT were used as substrate as compared to T alone. Furthermore Vmax/Km had up to approximately 9 fold higher efficiency when [3H]4-dione plus 5α-dione were used as substrate as compared to 4-dione. The experimental results in this study indicated that human prostate 5α-reductase converts in a similar manner 4-dione to 5α-dione as compared with T to DHT under these experimental conditions ().
Figure 2. Enzymatic efficiency (Vmax/Km) was obtained from the rate between maximal velocities (Vmax) of DHT or 5α-dione produced, using different substrates and the Michaelis constants (Km). Vmax/Km was higher when [3H]T+DHT were used as substrate than when T was used as a substrate. Furthermore, Vmax/Km was higher also when [3H]4-dione+5α-dione were used as substrate than when 4-dione was used. However, human prostate 5α-reductase enzyme converts 4-dione to 5α-dione in a similar manner as compared to T to DHT.
![Figure 2. Enzymatic efficiency (Vmax/Km) was obtained from the rate between maximal velocities (Vmax) of DHT or 5α-dione produced, using different substrates and the Michaelis constants (Km). Vmax/Km was higher when [3H]T+DHT were used as substrate than when T was used as a substrate. Furthermore, Vmax/Km was higher also when [3H]4-dione+5α-dione were used as substrate than when 4-dione was used. However, human prostate 5α-reductase enzyme converts 4-dione to 5α-dione in a similar manner as compared to T to DHT.](/cms/asset/1601eac0-733e-427e-8263-aa8fd0cf0eb3/ienz_a_548330_f0002_b.gif)
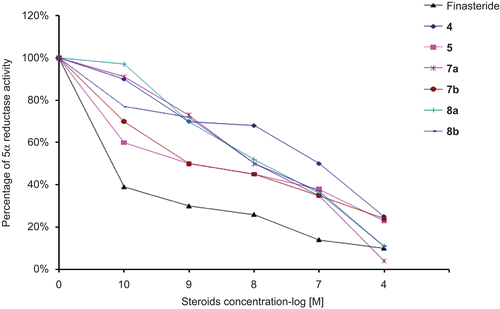
IC50 values of steroidal compounds in human prostate
The concentrations of finasteride and the progesterone derivatives 4, 5, 6, 7a, 7b, 8a and 8b required for inhibiting 4 dione 5α-reductase activity by 50% (IC50) were determined from the inhibition plots using different concentrations of the tested steroids (); these results are shown in . The data in this table show very clearly the inhibitory effect of finasteride as well as steroids 4, 5, 7a, 7b, 8a and 8b on human prostate 5α-reductase when labeled 4-dione plus 125 nM of unlabeled 5α-dione were used as substrate. However compound 6 did not showed any inhibition of the enzyme activity.
Table 3. Effect of different compounds as inhibitors of the activity of 5α-reductase.
Figure 3. Inhibition plots using different concentrations of the tested steroids against the percentage of activity of 4-dione 5α-reductase. These plots were used for the determination of the concentrations of finasteride as well as the progesterone derivatives 4, 5, 6, 7a, 7b, 8a and 8b required for inhibiting 4-dione 5α-reductase activity by 50%.
Discussion
In this article, we demonstrated that T and 4-dione 5α-reductase activity, obtained from nuclear membrane fractions of prostate was increased in the presence of its own products of reaction, such as DHT or 5α-dione. Furthermore, the efficiency of these reactions was higher in the presence of their products; this evidence indicates that 5α-reductase is an allosteric enzymeCitation16 activated by its own products of reaction. This fact could have important implications since overabundance of DHT has been implicated in the pathogenesis of benign prostatic hyperplasia and prostate cancerCitation20. 5α-Reductase has a biologically important role since it is responsible for concentrate intraprostatic DHT when/if serum T levels are physiologically lowCitation21. Experimental studies had suggested that T is more potent than DHT in stimulating the expression of many androgen-response genes in regressed prostateCitation22. The expression of these androgen-response genes is likely to be associated with androgen-dependent prostate preservation, since these genes preserve the capacity of the prostatic cells to undergo apoptosis and are less associated with a stimulating proliferation during prostate growth. On the other hand, high levels of intracellular DHT result in cellular proliferation and delay in cellular differentiationCitation22.
Some evidences indicated that 5α-reductase prefers 4-dione as a substrate than TCitation23,Citation24. This is evidenced by the low free levels of T in the circulation compared to 4-dione and also by higher affinity of T to AR than for the 5α-reductases. These data suggest that the 5α-reductase activity step precedes the 17β-HSD activityCitation4,Citation18 according to the pathway 4-dione →5α-dione →DHT. On the basis of this phenomenon, it is important to consider this source of DHT in benign prostatic hyperplasia and prostate cancer. In this sense the results presented in this article indicated that human prostate 5α-reductase converts T to DHT more efficiently than 4-dione to 5α-dione; these data are in agreement with those previously reported by Weisser and KriegCitation2; however in contrast to our data, the studies of Andersson and RussellCitation22 using cloned and expressed human steroid 5α-reductases indicated that human 5α-reductase catalyzes more efficiently the conversion of 4-dione than that for T. These differences in the results could be explained by considering the differences in the experimental protocols, such as: pH, the use of the nuclear membrane fractions from human prostate instead off transfected cells. However, the results obtained in this study showed that the 4-dione possesses a lower affinity than T for 5α-reductase as indicated by their Km values.
It is important to observe that in this experiment the Vmax of 4-dione conversion to T was low (9.7 pmol/mgof protein/h), probably due to the labile nature of 17βHSDCitation18. Furthermore, Vmax was still lower when 5α-dione was present in the incubating medium (1.9 pmol/mg of protein/h); this result can be explained on the grounds that 5α-dione could inhibit the activity of the 17βHSD enzyme. However, in this experiment, the amount of T obtained from 4-dione was so low under our experimental conditions that it contributed very little to the pool of DHT quantified in this experiment.
Previously, it had been reported that in benign prostatic hyperplasia’s stroma cells produce more 5α-reduced metabolites (DHT and 5α-dione) than normal prostatic’s stroma cellsCitation2. On this basis and taking into account our results, it could be assumed that a positive feedback of 5α-reductase activity could be occurring in this pathology, since a higher amount of reduced metabolites produced enhanced 5α-reductase activity. As a result of this, increased concentrations of DHT could produce a cellular proliferation and delay in cellular differentiationCitation22.
The results obtained in these experiments showed also, that 4-dione 5α-reductase from normal prostate was inhibited by finasteride and steroids 4, 5, 7a, 7b, 8a and 8b, which have demonstrated its activity as inhibitors of T 5α-reductase types 1 and 2Citation11. On the other hand the inhibitory effect of finasteride on 5α-reduction of T and 4-dione had previously been reported in benign prostatic hyperplasia, BPHCitation25–27. Finasteride is a better inhibitor of T 5α-reductase type 2 than type 1Citation5; in this experiment we found a lower IC50 value for finasteride as compared to 4-dione when it was used as substrate. When T was used as substrate, under the same experimental conditions (type 2 enzyme) a higher IC50 value was obtainedCitation11.
In the experimental conditions steroids, 17α-hydroxypregn-4-ene-3,20-dione 5 and the 4-bromo-17α-hydroxypregn-4-ene-3,20-dione 7b were the most potent steroids for the inhibition of the transformation of [3H] 4-dione to [3H]DHT, showing IC50 values of 2 and 1.6 nM, respectively.
Acknowledgements
We would like to thank CONACYT for its support for the project No 54853.
Declaration of interest
We report in this manuscript that we don’t have any conflict of interest with this research work.
References
- Lee HJ, Chang C. Recent advances in androgen receptor action. Cell Mol Life Sci 2003;60:1613–1622.
- Weisser H, Krieg M. Kinetic analysis of androstenedione 5α-reductase in epithelium and stroma of human prostate. Steroids 1997;62:589–594.
- Bélanger B, Bélanger A, Labrie F, Dupont A, Cusan L, Monfette G. Comparison of residual C-19 steroids in plasma and prostatic tissue of human, rat and guinea pig after castration: unique importance of extratesticular androgens in men. J Steroid Biochem 1989;32:695–698.
- Luu-The V, Bélanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab 2008;22:207–221.
- Russell DW, Wilson JD. Steroid 5α-reductase: two genes/two enzymes. Annu Rev Biochem 1994;63:25–61.
- Liang T, Cascieri MA, Cheung AH, Reynolds GF, Rasmusson GH. Species differences in prostatic steroid 5α-reductases of rat, dog, and human. Endocrinology 1985;117:571–579.
- Thomas LN, Lazier CB, Gupta R, Norman RW, Troyer DA, O’Brien SP et al. Differential alterations in 5α-reductase type 1 and type 2 levels during development and progression of prostate cancer. Prostate 2005;63:231–239.
- Bonkhoff H, Stein U, Aumüller G, Remberger K. Differential expression of 5α-reductase isoenzymes in the human prostate and prostatic carcinomas. Prostate 1996;29:261–267.
- Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y et al. Novel 5α-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer. Cancer Sci 2008;99:81–86.
- Samson M, Labrie F, Zouboulis CC, Luu-The V. Biosynthesis of dihydrotestosterone by a pathway that does not require testosterone as an intermediate in the SZ95 sebaceous gland cell line. J Invest Dermatol 2010;130:602–604.
- Bratoeff E, García P, Heuze Y, Soriano J, Mejía A, Labastida AM et al. Molecular interactions of progesterone derivatives with 5α-reductase types 1 and 2 and androgen receptors. Steroids 2010;75:499–505.
- Hirosumi J, Nakayama O, Fagan T, Sawada K, Chida N, Inami M et al. FK143, a novel nonsteroidal inhibitor of steroid 5α-reductase: (1) In vitro effects on human and animal prostatic enzymes. J Steroid Biochem Mol Biol 1995;52:357–363.
- Bratoeff E, Sainz T, Cabeza M, Heuze I, Recillas S, Pérez V et al. Steroids with a carbamate function at C-17, a novel class of inhibitors for human and hamster steroid 5α-reductase. J Steroid Biochem Mol Biol 2007;107:48–56.
- Bradford MM. A rapid and sensitive method for the quantization of micrograms quantities of protein utilizing the principle of protein dye binding. Anal Biochem 1986;72:248–254.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem 1934;54:658–666.
- Segel IH. Enzyme kinetics. Behavior and analysis of rapid equilibrium and steady state enzyme systems. New York: John Wiley & Sons, INC. New York, 1993:37–51.
- Pelletier G. Expression of steroidogenic enzymes and sex-steroid receptors in human prostate. Best Pract Res Clin Endocrinol Metab 2008;22:223–228.
- Dufort I, Rheault P, Huang XF, Soucy P, Luu-The V. Characteristics of a highly labile human type 5 17β-hydroxysteroid dehydrogenase. Endocrinology 1999;140:568–574.
- Krieg M, Weisser H, Tunn S. Potential activities of androgen metabolizing enzymes in human prostate. J Steroid Biochem Mol Biol 1995;53:395–400.
- Raynaud JP. Prostate cancer risk in testosterone-treated men. J Steroid Biochem Mol Biol 2006;102:261–266.
- Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS. Androgen-induced regrowth in the castrated rat ventral prostate: role of 5α-reductase. Endocrinology 1999;140:4509–4515.
- Dadras SS, Cai X, Abasolo I, Wang Z. Inhibition of 5α-reductase in rat prostate reveals differential regulation of androgen-response gene expression by testosterone and dihydrotestosterone. Gene Expr 2001;9:183–194.
- Andersson S, Russell DW. Structural and biochemical properties of cloned and expressed human and rat steroid 5α-reductases. Proc Natl Acad Sci USA 1990;87:3640–3644.
- Sugimoto Y, López-Solache I, Labrie F, Luu-The V. Cations inhibit specifically type I 5α-reductase found in human skin. J Invest Dermatol 1995;104:775–778.
- Hudson RW, Wherrett D. Comparison of the nuclear 5α-reduction of testosterone and androstenedione in human prostatic carcinoma and benign prostatic hyperplasia. J Steroid Biochem 1990;35:231–236.
- Weisser H, Tunn S, Debus M, Krieg M. 5α-reductase inhibition by finasteride (Proscar) in epithelium and stroma of human benign prostatic hyperplasia. Steroids 1994;59:616–620.
- Weisser H, Krieg M. In vitro inhibition of androstenedione 5α-reduction by finasteride in epithelium and stroma of human benign prostatic hyperplasia. J Steroid Biochem Mol Biol 1998;67:49–55.
