Abstract
Tetrahydrobiopterin (BH4), methyl-tetrahydropterin (MBH4) and dimethyl-tetrahydropterin (DMBH4) are oxidized by tyrosinase in a process during which the suicide inactivation of tyrosinase may occur. From the kinetic study of this process, ![]() (apparent maximum constant for the suicide inactivation),
(apparent maximum constant for the suicide inactivation), ![]() (Michaelis constant for the substrate) and r (number of turnovers that the enzyme makes before the inactivation) can be obtained. From the results obtained, it can be deduced that the velocity of the inactivation governed by (
(Michaelis constant for the substrate) and r (number of turnovers that the enzyme makes before the inactivation) can be obtained. From the results obtained, it can be deduced that the velocity of the inactivation governed by (![]() ) and the potency of the same (
) and the potency of the same (![]() ) follow the order: BH4 > MBH4 > DMBH4.
) follow the order: BH4 > MBH4 > DMBH4.
Introduction
Tyrosinase or polyphenol oxidase (TYR; EC 1.14.18.1) is a widely distributed enzyme through the animal, vegetal, bacterial and fungal kingdomsCitation1.
In a recent workCitation2, we studied the effect of tetrahydropterines at micromolar concentrations on the monophenolase and diphenolase activities of tyrosinase and demonstrated that they are oxidized by o-dopaquinone and that the systems reach the steady state when all the tetrahydropterines have been exhaustedCitation2. Subsequently, we demonstrated that the effect of tetrahydropterines on the catalytic activities of tyrosinase can occur at three levels: (i) through non-enzymatic inhibition, the tetrahydropterines acting as reductants of o-dopaquinone, (ii) by acting as competitive substrates of TYR and (iii) by acting as irreversible inhibitors of the enzymatic forms deoxy- and met-tyrosinaseCitation3.
Tyrosinase undergoes an inactivation process when it reacts with its phenolic substrates, a phenomenon that has long been knownCitation4. The study of enzymatic inactivation by suicide substrates or mechanism-based inhibitors is of growing importance because of possible pharmacological applications, for studying enzymatic mechanisms and for designing new drugsCitation4,Citation5,Citation6. As regards the possible applications of the suicide substrates of tyrosinase, some of the substrates, such as 7,8,4′-trihydroxyisoflavone and 8-hydroxydaidzein, have been studied for their depigmenting activity, the latter in both mouse melanoma cells and in human volunteersCitation7.
Several mechanisms have been proposed to explain the suicide inactivation of tyrosinase Citation4,Citation8,Citation9.
The objective of this work was the kinetic characterisation of the suicide inactivation of tyrosinase as it acts on tetrahydrobiopterin (BH4), methyl-tetrahydropterin (MBH4) and dimethyl-tetrahydropterin (DMBH4), and we propose a structural mechanism to explain the results.
Material and methods
Reagents
L-tyrosine, 4-tert-butylcatechol (TBC), (6-R)-L-erythro-5,6,7,8-tetrahydro-biopterin dihydrochloride (BH4), 6-(R,S)-methyl 5,6,7,8-tetrahydropterin monohydrochloride (MBH4) and 6,7-(R,S)-dimethyl 5,6,7,8-tetrahydropterin monohydrochloride (DMBH4) were purchased from Sigma (Madrid, Spain; ). Stock solutions of the phenolic substrate were prepared in 0.15 mM phosphoric acid to prevent autooxidation. 6BH4, MBH4 and DMBH4 were prepared in 0.15 mM phosphoric acid, eliminating the oxygen by passing a current of nitrogen.
Enzyme source
Mushroom tyrosinase (TYR, 3300 U/mg) was purchased from Sigma (Madrid, Spain) and purified according to ref. (10). The enzyme concentration was calculated taking the value of Mr as 120,000. Protein content was determined by Bradford’s methodCitation11 using bovine serum albumin as standard. Superoxide dismutase (SOD, 4140 U/mg) was purchased from Sigma (Madrid, Spain).
Monophenolase activity of TYR
The monophenolase activity of TYR was followed spectrophotometrically, measuring the accumulation of dopachrome at a wavelength of 475 nm (![]() = 3500 M−1 cm−1)Citation2 during the oxidation of L-tyrosine, using a Perkin-Elmer Lambda-35 spectrophotometer connected to a PC (Perkin-Elmer, North Billerica, MA). The conditions of the assay are specified in the corresponding figure legends.
= 3500 M−1 cm−1)Citation2 during the oxidation of L-tyrosine, using a Perkin-Elmer Lambda-35 spectrophotometer connected to a PC (Perkin-Elmer, North Billerica, MA). The conditions of the assay are specified in the corresponding figure legends.
Diphenolase activity of TYR
The diphenolase activity of TYR was followed spectrophotometrically, measuring the accumulation of dopachrome at a wavelength of 475 nm (![]() = 3500 M−1 cm−1)Citation12 during the oxidation of L-dopa using the mentioned apparatus. The conditions of the assay are specified in the corresponding figure legends.
= 3500 M−1 cm−1)Citation12 during the oxidation of L-dopa using the mentioned apparatus. The conditions of the assay are specified in the corresponding figure legends.
Kinetics of the suicide inactivation
The kinetics of the suicide inactivation of TYR in its action on tetrahydropterines, ![]() , (BH4, MBH4 and DMBH4) can be followed by measuring the formation of
, (BH4, MBH4 and DMBH4) can be followed by measuring the formation of ![]() (BH2, MBH2 and DMBH2), in a spectrophotometer. The assays were carried out in triplicate for each concentration of
(BH2, MBH2 and DMBH2), in a spectrophotometer. The assays were carried out in triplicate for each concentration of ![]() and enzyme. These spectrophotometric assays were carried out as described above. The experimental data for the formation of
and enzyme. These spectrophotometric assays were carried out as described above. The experimental data for the formation of ![]() (in the other cases is the same) with time follow the equation,
(in the other cases is the same) with time follow the equation,
where ![]() is the instantaneous concentration of
is the instantaneous concentration of ![]() ,
, ![]() is the
is the ![]() formed at the end of the reaction,
formed at the end of the reaction, ![]() , and
, and ![]() the apparent inactivation constant for
the apparent inactivation constant for ![]() in the suicide inactivation of TYR under aerobic conditions.
in the suicide inactivation of TYR under aerobic conditions.
Results and discussion
Under aerobic conditions, tetrahydropterines act as alternative substrates to the physiological substrates of the enzyme (L-tyrosine and L-dopa)Citation3. Under such conditions, the tetrahydropterines may act as inactivators of TYR as a result of the enzyme’s suicide inactivation as it acts on the substratesCitation4–8. This suicide inactivation process is studied below during the action of TYR on BH4, MBH4 and DMBH4.
Tyrosinase inactivation in its action on tetrahydropterines under aerobic conditions: suicide inactivation
The kinetic mechanism proposed to explain the suicide inactivation of tyrosinase acting on tetrahydropterines is described in :
Scheme 2. Kinetic mechanism to explain the pterin oxidase pathway and suicide inactivation pathway of tyrosinase in its action on tetrahydropterines.
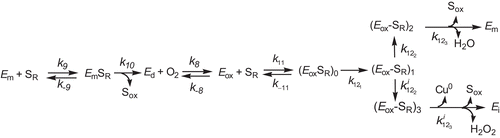
When, >> ![]() and <<
and << ![]() ,
, ![]() , derivation of the analytical expression establishes the accumulation of the product (
, derivation of the analytical expression establishes the accumulation of the product (![]() ) with time, as is detailed in ref. (4).
) with time, as is detailed in ref. (4).
The variation of ![]() with time is given by Eq. (2)Citation4.
with time is given by Eq. (2)Citation4.
When ![]() ,
, ![]() = and according to (4),
= and according to (4),
The velocity of the suicide inactivation is regulated by the apparent inactivation constant, ![]() , whose expression is given by Eq. (4). Taking into account the saturation of tyrosinase by oxygen (
, whose expression is given by Eq. (4). Taking into account the saturation of tyrosinase by oxygen (![]() ), thus according to (4),
), thus according to (4),
The partition ratio, ![]() , between the catalytic and suicide inactivation pathways is,
, between the catalytic and suicide inactivation pathways is,
where ![]() is the substrate binding constant to the copper atom through the C-3 hydroxyl (axial) and
is the substrate binding constant to the copper atom through the C-3 hydroxyl (axial) and ![]() is the rate constant of transfer of a
is the rate constant of transfer of a ![]() to the protonated peroxide (see Scheme 2 and supplementary Scheme 1SM).
to the protonated peroxide (see Scheme 2 and supplementary Scheme 1SM).
Therefore, from Eq. (1–5), we can obtain the kinetic constants that characterise the kinetic behaviour of a suicide substrate: ![]() ,
, ![]() ,
, ![]() and
and ![]() .
.
Experimental study of the suicide inactivation
The study of the suicide inactivation has been developed in three steps using BH4, MBH4 and DMBH4 as substrates of TYR.
Step 1. The suicide inactivation kinetics is corrected when fitting to Eq. (1) so that the non-enzymatic oxidation of the substrates, BH4, MBH4 and DMBH4 is eliminated (results not shown). These corrected recordings were analysed as described in the Kinetic Analysis section. By means of these preliminary studies, the concentration of enzyme was optimised so that ![]() <<
<< ![]() .
.
Step 2. Variation in enzyme concentration. The ![]() -value is varied while the substrate concentration is kept constant. The results obtained are shown in . The concentration of the substrate does not change significantly in these experiments so that
-value is varied while the substrate concentration is kept constant. The results obtained are shown in . The concentration of the substrate does not change significantly in these experiments so that ![]() < 10%
< 10% ![]() . However, the concentration of
. However, the concentration of ![]() does change. In the case of tyrosinase,
does change. In the case of tyrosinase, ![]() is very small (), which permits an oxygen consumption of more than 10%. Note how the values of
is very small (), which permits an oxygen consumption of more than 10%. Note how the values of ![]() are directly proportional to
are directly proportional to ![]() , while
, while ![]() does not vary with
does not vary with ![]() ( Inset A). Inset B shows the stability of BH2 with time, which demonstrates that the product of the reaction of TYR with BH4 during the time that the suicide inactivation kinetics is being measured is stable.
( Inset A). Inset B shows the stability of BH2 with time, which demonstrates that the product of the reaction of TYR with BH4 during the time that the suicide inactivation kinetics is being measured is stable.
Figure 1. Corrected recordings of the appearance of MBH 2 in the suicide inactivation of tyrosinase (TYR) by MBH4 for different enzyme concentrations. Conditions were 30 mM sodium phosphate buffer (pH 7.0), ![]()
![Figure 1. Corrected recordings of the appearance of MBH 2 in the suicide inactivation of tyrosinase (TYR) by MBH4 for different enzyme concentrations. Conditions were 30 mM sodium phosphate buffer (pH 7.0), Display full size = 340 nm, 0.26 mM O2, 0.5 mM [MBH4]0, 414 UI/m [SOD]0, and [E]0 (µM): (a) 0.3, (b) 0.45, (c) 0.5, (d) 0.6, (e) 0.7, and (f) 0.8. Inset A. Representation of the values of [MBH2]∞ (•) and Display full size(O) vs. enzyme concentration. Inset B. Representation of absorbance at λ = 340 nm with time in the reaction of TYR on BH2. The experimental conditions were 30 mM sodium phosphate buffer (pH = 7.0), 25°C, [BH2]0 = 0.2 mM and [E]0 = 0.1 µM.](/cms/asset/d9a6dbef-565e-4f29-b492-a811988bd9eb/ienz_a_548811_f0003_b.gif)
Table 1. Kinetic constants which characterize the suicide inactivation of tyrosinase by tetrahydropterines.
shows the dependence of the values obtained for the product at the end of the reaction, ![]() vs.
vs. ![]() , from whose slopes, and according to Eq. (5),
, from whose slopes, and according to Eq. (5), ![]() can be determined for BH4, MBH4 and DMBH4 (). Note that the value of
can be determined for BH4, MBH4 and DMBH4 (). Note that the value of ![]() is greater in the case of DMBH4 than in that of MBH4 or BH4, indicating that the enzyme needs to undergo a greater number of turnovers before inactivation when it acts on DMBH4 than it does in the case of MBH4 or BH4.
is greater in the case of DMBH4 than in that of MBH4 or BH4, indicating that the enzyme needs to undergo a greater number of turnovers before inactivation when it acts on DMBH4 than it does in the case of MBH4 or BH4.
![Figure 2. Representations of the values of Display full sizevs. enzyme concentration for the different substrates studied (BH4 •, MBH4 O and DMBH4 ▴). Experimental conditions were 30 mM sodium phosphate buffer (pH 7.0), 25°C and 414 UI/ml [SOD]0. The substrates studied were recorded from the appearance of product. The initial concentration for every substrate was 0.8 mM. In every substrate, (—) linear regression fitting of the experimental data points.](/cms/asset/b3d8bd06-f47d-4498-8afd-2895bfb50337/ienz_a_548811_f0004_b.gif)
Step 3. Variation in substrate concentration. The experimental recordings for the appearance of MBH2 during the action of tyrosinase on MBH4 obtained by varying the concentration of substrate are shown in . Fitting by non-linear regression to Eq. (1) gives the apparent inactivation constant ![]() . The dependence of
. The dependence of ![]() vs. [SR]0 is shown in Inset. Due to the high degree of autooxidation of the tetrahydropterines and the long measuring times involved, it is not convenient to increase the substrate concentration by much, so that we shall work in the linear dependence zone, Inset. Fitting these data by linear regression according to Eq. (4) gives the relation
vs. [SR]0 is shown in Inset. Due to the high degree of autooxidation of the tetrahydropterines and the long measuring times involved, it is not convenient to increase the substrate concentration by much, so that we shall work in the linear dependence zone, Inset. Fitting these data by linear regression according to Eq. (4) gives the relation ![]() for each of the isomers ( and ). Bearing in mind the values of
for each of the isomers ( and ). Bearing in mind the values of ![]() calculated from the initial velocity measurements obtained at short timesCitation3, we can obtain the values of
calculated from the initial velocity measurements obtained at short timesCitation3, we can obtain the values of ![]() (). represents the values of
(). represents the values of ![]() vs.
vs. ![]() for the substrates BH4, MBH4 and DMBH4. Note how TYR is not saturated during its action on any tetrahydropterine (). The value of the ratio
for the substrates BH4, MBH4 and DMBH4. Note how TYR is not saturated during its action on any tetrahydropterine (). The value of the ratio ![]() is BH4 > MBH4 > DMBH4.
is BH4 > MBH4 > DMBH4.
Figure 3. Corrected recordings of the appearance of MBH 2 in the suicide inactivation of TYR by different MBH4 concentrations. Conditions were 30 mM sodium phosphate buffer (pH 7.0), 0.26 mM O2, λ = 340 nm, 0.6 µM [TYR]0, and 414 UI/ml [SOD]0. The substrate concentrations were (mM): (a) 0.2, (b) 0.3, (c) 0.4, (d) 0.5, (e) 0.6, (f) 0.7, and (g) 0.8. Inset. Values of ![]()
![Figure 3. Corrected recordings of the appearance of MBH 2 in the suicide inactivation of TYR by different MBH4 concentrations. Conditions were 30 mM sodium phosphate buffer (pH 7.0), 0.26 mM O2, λ = 340 nm, 0.6 µM [TYR]0, and 414 UI/ml [SOD]0. The substrate concentrations were (mM): (a) 0.2, (b) 0.3, (c) 0.4, (d) 0.5, (e) 0.6, (f) 0.7, and (g) 0.8. Inset. Values of Display full size vs. [MBH2]0.](/cms/asset/6ee3d455-110d-4c30-80ea-b90fcf1689d6/ienz_a_548811_f0005_b.gif)
Figure 4. Representation of the values of the inactivation constant ![]()
![Figure 4. Representation of the values of the inactivation constant Display full size vs. [SR]0 for the different substrates studied. Conditions were 30 mM sodium phosphate buffer (pH 7.0), 25°C. Every substrates were recorded from the appearance of product (BH2, MBH2, and DMBH2 for BH4 •, MBH4 O and DMBH4 ▴, respectively). (—), Non-linear regression fitting to Eq. (4) of the data analysis. Enzyme concentration was, in each case, 0.6 µM. The initial concentration of superoxide dismutase (SOD) was 414 UI/ml.](/cms/asset/61334fa1-b762-464c-bc41-947c077ce538/ienz_a_548811_f0006_b.gif)
In confirmation of the inactivation of tyrosinase as it acts on tetrahydropterines, shows the experimental values obtained for the residual diphenolase activity on TBC of the enzyme during the suicide inactivation of TYR with BH4. This figure shows similar results obtained for monophenolase activity in its action on L-tyrosine. Note that the same value for the apparent inactivation constant, ![]() , was obtained in both cases and the same as that in obtained with the same concentration of BH4.
, was obtained in both cases and the same as that in obtained with the same concentration of BH4.
Figure 5. •Suicide inactivation kinetics of TYR in its action on DMBH4. The process was followed by measuring the residual diphenolase activity of the enzyme with time. The experimental conditions were 30 mM sodium phosphate buffer (pH 7.0), [O2]0 = 0.26 mM, [TYR]0 = 0.61 µM, [SOD]0 = 414 UI/ml, and [DMBH4] = 0.8 mM. Aliquots were taken at various times to measure the residual activity with 2.5 mM L-dopa (wavelength 475 nm). ▴Suicide inactivation kinetics of TYR in its action on DMBH2. The process was followed by measurement of the residual monophenolase activity of the enzyme with time. The experimental conditions were 30 mM phosphate buffer (pH 7.0), [O2]0 = 0.26 mM, [TYR]0 = 0.61 µM, [SOD]0 = 414 UI/ml, and [DMBH4] = 0.8 mM. Aliquots were taken at various times to measure the residual activity with 1 mM L-tyrosine and 46 µM L-DOPA (wavelength 475 nm). L-DOPA was added to eliminate the lag phase of the monophenolase activity of TYR.
![Figure 5. •Suicide inactivation kinetics of TYR in its action on DMBH4. The process was followed by measuring the residual diphenolase activity of the enzyme with time. The experimental conditions were 30 mM sodium phosphate buffer (pH 7.0), [O2]0 = 0.26 mM, [TYR]0 = 0.61 µM, [SOD]0 = 414 UI/ml, and [DMBH4] = 0.8 mM. Aliquots were taken at various times to measure the residual activity with 2.5 mM L-dopa (wavelength 475 nm). ▴Suicide inactivation kinetics of TYR in its action on DMBH2. The process was followed by measurement of the residual monophenolase activity of the enzyme with time. The experimental conditions were 30 mM phosphate buffer (pH 7.0), [O2]0 = 0.26 mM, [TYR]0 = 0.61 µM, [SOD]0 = 414 UI/ml, and [DMBH4] = 0.8 mM. Aliquots were taken at various times to measure the residual activity with 1 mM L-tyrosine and 46 µM L-DOPA (wavelength 475 nm). L-DOPA was added to eliminate the lag phase of the monophenolase activity of TYR.](/cms/asset/a423f744-52e2-48de-854e-d596e51cc36d/ienz_a_548811_f0007_b.gif)
The inactivation mechanism, Scheme 2 and supplementary Scheme 1SM, is consistent with the experimental observation that 50% of the copper is lost from the active site during catechol inactivationCitation13, in the form of copper (0), and also with experiments carried outCitation8, concerning the impossibility of reactivating the inactivated enzyme by adding ![]() , which, in turn, suggests the need for a ‘caddie’ proteinCitation14. BH4, MBH4 and DMBH4, therefore, are capable of irreversibly inactivating tyrosinase and may be useful as depigmenting agents or, at least, for designing the same.
, which, in turn, suggests the need for a ‘caddie’ proteinCitation14. BH4, MBH4 and DMBH4, therefore, are capable of irreversibly inactivating tyrosinase and may be useful as depigmenting agents or, at least, for designing the same.
Conclusions
In this article, the suicide inactivation process of tyrosinase acting on tetrahydropterines has been kinetically characterized. From the kinetic constants obtained, it can be deduced that the velocity of the inactivation governed by (![]() ) and its potency (
) and its potency (![]() ) follow the order: BH4 > MBH4 > DMBH4.
) follow the order: BH4 > MBH4 > DMBH4.
Supplementary Material
Download PDF (177.7 KB)Acknowledgements
This article was partially supported by grants from the Ministerio de Educación y Ciencia (Madrid, Spain) Project BIO2009–12956, from the Fundación Séneca (CARM, Murcia, Spain) Projects 08856/PI/08 and 08595/PI/08 and from the Consejería de Educación (CARM, Murcia, Spain) BIO-BMC 06/01-0004. F.G.M. and J.L.M.M. have fellowships from Fundación Caja Murcia (Murcia, Spain).
References
- Solomon EI, Sundaram UM, Machonkin TE. Multicopper Oxidases and Oxygenases. Chem Rev 1996;96:2563–2606.
- García-Molina F, Muñoz JL, Varón R, Rodríguez-López JN, García-Cánovas F, Tudela J. Effect of tetrahydropteridines on the monophenolase and diphenolase activities of tyrosinase. J Enzyme Inhib Med Chem 2007;22:383–394.
- García-Molina F, Munoz-Munoz JL, Acosta JR, García-Ruiz PA, Tudela J, García-Cánovas F et al. Melanogenesis inhibition by tetrahydropterines. Biochim Biophys Acta 2009;1794:1766–1774.
- Muñoz-Muñoz JL, García-Molina F, García-Ruiz PA, Molina-Alarcón M, Tudela J, García-Cánovas F et al. Phenolic substrates and suicide inactivation of tyrosinase: kinetics and mechanism. Biochem J 2008;416:431–440.
- Chang TS. Two potent suicide substrates of mushroom tyrosinase: 7,8,4′-trihydroxyisoflavone and 5,7,8,4′-tetrahydroxyisoflavone. J Agric Food Chem 2007;55:2010–2015.
- Chang TS, Lin MY, Lin HJ. Identifying 8-hydroxynaringenin as a suicide substrate of mushroom tyrosinase. J Cosmet Sci 2010;61:205–210.
- Tai SS, Lin CG, Wu MH, Chang TS. Evaluation of depigmenting activity by 8-hydroxydaidzein in mouse B16 melanoma cells and human volunteers. Int J Mol Sci 2009;10:4257–4266.
- Land EJ, Ramsden CA, Riley PA. The mechanism of suicide-inactivation of tyrosinase: a substrate structure investigation. Tohoku J Exp Med 2007;212:341–348.
- Muñoz-Muñoz JL, Garcia-Molina F, Varon R, Garcia-Ruíz PA, Tudela J, Garcia-Cánovas F et al. Suicide inactivation of the diphenolase and monophenolase activities of tyrosinase. IUBMB Life 2010;62:539–547.
- Rodríguez-López JN, Fenoll LG, García-Ruiz PA, Varón R, Tudela J, Thorneley RN et al. Stopped-flow and steady-state study of the diphenolase activity of mushroom tyrosinase. Biochemistry 2000;39:10497–10506.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- García-Molina F, Muñoz JL, Varón R, Rodríguez-López JN, García-Cánovas F, Tudela J. A review on spectrophotometric methods for measuring the monophenolase and diphenolase activities of tyrosinase. J Agric Food Chem 2007;55:9739–9749.
- Dietler C, Lerch, K. Oxidases and Related Redox Systems. In King TE, Mason HS, Morrison, M, ed. New York, Pergamon Press, 1982:305–317.
- Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H, Sugiyama M. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J Biol Chem 2006;281:8981–8990.