Abstract
Estrogen receptor beta (ERβ) selective ligands have attracted much attention recently in the design of anti-cancer drugs that are devoid of the common side effects of estrogen. Structural studies of estrogen receptor alpha (ERα) and β revealed that there were considerable differences in their ligand-binding cavity and in their volume. Hence, the present study has hypothesized that size and shape descriptors can influence the affinity/selectivity of the ligands towards ERβ. To prove the same, quantitative structure-activity relationship (QSAR) analyses were carried out using multiple regression analysis on 2-phenylquinoline, tetrahydrofluorenone and 3-hydroxy-6H-benzo[c]chromen-6-one series. Results indicate that increased lipophilicity, decrease in ellipsoidal volume and width of substituents, presence of halogen atoms was essential for the ligands to have high affinity/selectivity towards ERβ. QSAR models obtained were both internally and externally validated. The study delineates that the size and shape descriptors are best modulators of ERβ affinity/selectivity. Docking studies were performed to support our QSAR results.
Introduction
The biological effects of estrogens are mediated through its interaction with estrogen receptors (ERs) two forms of which are known as estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ)Citation1. ERβ, as a molecularly distinct receptor, was identified and cloned from rat prostate and ovarian cellsCitation2, closely followed by cloning of the human and mouse homologuesCitation3–5. ERα has 595 amino acids, whereas ERβ is 485 amino acids long. The ER isoforms share 95% and 58% amino acid sequence identity in their DNA binding and ligand-binding domains (LBD), respectivelyCitation6. The realization that there are two ERs with different tissue distribution raises the possibility of developing subtype selective ligands that can be used in a multitude of clinical states including hormone influenced cancerCitation7. Further ERβ agonists have minimal risk of adverse effects thus differing from selective ER modulators that increase the risk of endometrial cancerCitation8.
Currently, no selective ERβ agonist is available in the market. Hence, to provide a rationale in the design of new anti-cancer agents, quantitative structure-activity relationship (QSAR) studies were employed. Several QSAR models on estrogens were derived in which most of them reported the estimation of different chemical class endocrine disruption potentialCitation9–11 otherwise focused on the classification of ER agonist vs. non-agonist regardless of ER isoformsCitation12. Few authorsCitation13,Citation14 had studied the structural features influencing the binding affinity to ERβ using environmental estrogenic chemicals and phytoestrogens as data set (48 compounds as training set and 12 compounds as test set) in which they concluded that quantum chemical and electrostatic descriptors mainly influenced the ERβ selectivity. However, they concluded that their results on the external test set are not satisfactory in both receptor independent and dependent-based approachCitation13,Citation14. Therefore, we intended to identify the features that will influence the ERβ potency and selectivity. ERα and β isoforms differ in their ligand-binding pocket in two critical amino acids: Leu 384 and Met 421 in ERα, whereas the corresponding residues in ERβ are Met 336 and Ile 373. In addition, ERβ ligand-binding cavity is smaller (390Å3) compared to that of ERα (490Å3)Citation15. These differences in binding site and volume between ERα and β LBD can be capitalized to design ERβ selective ligands. To prove the same rationale, QSAR studies were employed on selective ERβ series.
Materials and methods
Data set and software
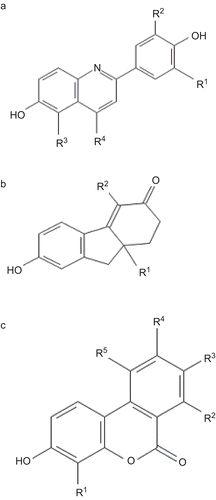
The data used for our analyses were from the reported series of 2-phenylquinolines (21 compounds)Citation16, tetrahydrofluorenones (31 compounds)Citation17, and 3-hydroxy 6H-benzo[c]chromen-6-ones (28 compounds)Citation18 since the series displayed a broad range of reactivity towards ERs (, and ). The same data set of Boriani et al.Citation13 and Spreafico et al.Citation14 was not taken because most of the ligands in their data set are non-selective or ERα selective. The structures of the ligands taken in the present study were constructed in DS Viewer Pro 6.0 (Accelrys Software Inc, 2005). Energy level of the constructed structures and charges were optimized, and QSAR studies were carried out using a commercial package TSAR 3D, version 3.3 (Oxford Molecular Limited, 2000). Binding affinity for ERβ (IC50) and ERβ selectivity (ERα IC50/ERβ IC50 ratio) were considered as the biological activity of ligand–receptor interaction and were converted into the logarithmic scale. For the three series of compounds taken in the present study, a cluster analysis was carried out with TSAR using the complete linkage clustering method (Euclidean distances) with no data standardization for the whole data set comprising of descriptors and activityCitation19,Citation20. Based on the clusters, the data set was divided into training and test sets so that all clusters are properly represented in both training and test sets in the ratio 3:1Citation20.
Table 1. Structural features, observed and predicted ERβ IC50 values and ERα/β fold selectivity for 2-phenylquinoline scaffold.
Table 2. Structural features, observed and predicted ERβ IC50 values and ERα/β fold selectivity for tetrahydrofluorenone scaffold.
Table 3. Structural features, observed and predicted values for ERβ IC50 values and ERα/β fold selectivity for 3-hydroxy- 6H-benzo[c]chromen-6-one scaffold.
Descriptors selection
TSAR includes many structural property parameters like mass, surface area, volume, moments of inertia, molar refractivity, lipophilicity, verloop parameters, dipole, topological indices, connectivity indices, electrotopological state indices (E-state indices), Kier and Hall indices, number of atoms, rings, hydrogen bond acceptors and donors and Vamp electrostatic parameters. From the earlier crystallographic reportCitation15, we hypothesized that size and shape of the ligands mainly influence the ERβ potency and selectivity. To prove the hypothesis, in the current study size and shape parameters like molecular weight, verloop, volume, kappa indices and number of atoms were included. Since atom-type E-state indices code mainly about size, cyclicity, branching and the number of quaternary atoms, which are found out by the rotation of high dimension space in different directions, it has also been included in the studyCitation21. Crystallographic and docking studies revealed that ERβ selective ligands form hydrogen-bonding interactions with Glu 305, Arg 346 and His 475 of ERβCitation22. Hence, the number of hydrogen bond donors and acceptors were taken into account. The descriptors used in the present study are tabulated in .
Table 4. Molecular descriptors selected for the study.
Model construction and validation
Multi-parametric regression analyses between physicochemical descriptors and biological activities were carried out to identify the favourable and adverse parameters for ERβ potency and selectivity. Combinations of predictor variables were selected for further analyses based on low interrelationship between them (|R| < 0.6)Citation23. Correlation matrices between the descriptors were given in the supplementary information. The robustness of the models and their internal predictive ability were evaluated by q2 based on leave-more-out cross-validation (LMOcv). The LMOcv procedure consists of removing three examples from the training set and constructing the model only on the basis of the remaining training data and then testing on the removed examples. In this fashion, all of the training data examples were tested, and q2 was calculated. Compounds were considered as outliers on the basis of their deviation between observed and predicted activities from the model (observed activity− predicted activity >2 S.D., where S.D. is the standard deviation)Citation24. In order to find out any chance correlations associated with the QSAR models recognized in multiple linear regression analysis, each cross-validated model has been put to a randomization test. Randomization was performed in Strike, SchrödingerCitation25 which is a chemically aware statistical tool. In this test, the dependent-variable (ERβ affinity and selectivity) was randomly shuffled and a new QSAR model was developed for each respective series using the unchanged independent variable matrix. This process was repeated for several cycles. It was expected that the resulting QSAR models should have low randomized R2 and high S.D. valuesCitation23. This is a widely used technique to ensure the robustness of a particular QSAR model. Over fitting, due to the excessive number of parameters (which increases the R and S.D values also), can be detected by the examination of Q value. Q is the quality factor (Q = r/S.D.)Citation23. In addition the developed model should be robust enough to be capable of making accurate and reliable predictions of biological activities of new compoundsCitation26. Hence, in the present study, external validation was also carried out which consists of making predictions for an independent set of compounds not used in the model training. The correlation equations, which returned the highest correlation coefficient (R2), Fischer’s value (F) and q2 LMOcv and lowest standard error of the regression model(s) were finally retained for further discussion. Predictability of the generated QSAR equations was externally validated using the test set and is denoted as R2pred.
Docking studies with the target ERβ
Ligand preparation
The most active compound and least active compound of each series were identified and prepared for docking using LigPrepCitation27. LigPrep is a utility of Schrödinger software that generates 3D structures from 2D representation. LigPrep also assigns an appropriate bond order with correct chiralities for each successfully processed input structure and produce a number of structures from each input structure with various ionization states, tautomers, stereoisomers and ring conformations. Subsequently, the structures were optimized by means of OPLS-2005 using a default setting in the LigPrep.
Protein preparation
The X-ray crystal protein structure of ERβ in complex with compound genistein (PDB ID: 1QKM, resolution 1.8Å) obtained from the RCSB Protein Data Bank (PDB) was used in this studyCitation28. Many recent computational studies on ERβ used the crystal structure 1QKM to predict the binding modes of ERβ compoundsCitation18,Citation22. Protein structure was prepared using the Maestro software packageCitation29 and aligned using the protein structure alignment module in PrimeCitation30. Bond orders and formal charges were added for heterogroups, and hydrogens were added to all atoms in the system. Protein was inspected visually for accuracy in the χ2 dihedral angle of Asn and His residues and the χ3 angle of Gln, and rotated by 180° when needed to maximize hydrogen bonding. The proper His tautomer was also manually selected to maximize hydrogen bonding. All Asp, Glu, Arg and Lys residues were left in their charged state. Water molecules of crystallization were removed from the complex except in the active site. A brief relaxation was performed on structure using the Protein Preparation module in Maestro with the “Refinement Only” option. This is a two-part procedure that consists of optimizing hydroxyl and thiol torsions in the first stage followed by an all-atom constrained minimization carried out with the Impact Refinement module (Impref) using the OPLS-2005 force field to alleviate steric clashes that may exist in the original PDB structures. The minimization was terminated when the the root mean square deviation (RMSD) reached a maximum cutoff of 0.30 Å.
Grid generation and ligand docking
Grids were defined by centring them around the ligand in the crystal structure using the default box size setting in Glide: scaling of van der Waals radii of protein atoms partial atomic charge of less then 0.25 by 1.0. Hydrogen bond constraints were not applied. Flips of 5- and 6-member rings were allowed, and non-planar conformation of amide bonds was penalized. van der Waals radii of ligand atoms with partial atomic charge less than 0.15 was scaled by 0.8. The prepared ligands were docked against the ERβ receptor. All docking calculations were performed using the “Extra precision” (XP) mode of Glide programCitation31.
Glide uses a hierarchical series of filters to search for possible locations of the ligand in the active-site region of the receptor. The initial filters test the spatial fit of the ligand to the defined active site, and examine the complementarity of ligand–receptor interactions using a grid-based method. Poses that pass these initial screens enter the final stage of the algorithm, which involves evaluation and minimization of a grid approximation to the OPLS-AA nonbonded ligand–receptor interaction energy. Final scoring is then carried out on the energy-minimized poses. The minimized poses are rescored using Schrödinger’s proprietary GlideScore (GScore) scoring function. GScore is a modified version of ChemScore, but includes a steric-clash term and adds buried polar terms devised by Schrödinger to penalize electrostatic mismatches.
GScore = a × vdW + b × Coul + Lipo + Hbond + Metal + BuryP + RotB + Site
where vdW—Van der Waal energy, Coul—Coulomb energy, Lipo—Lipophilic contact term, Hbond—Hydrogen-bonding term, Metal—Metal-binding term, BuryP—Penalty for buried polar groups, RotB—Penalty for freezing rotatable bonds, Site—Polar interactions at the active site; and the coefficients of vdW and Coul are: a = 0.065, b = 0.130. All docking computations were carried out with the Linux OS (Red Hat Enterprise WS 5.0).
Results and discussion
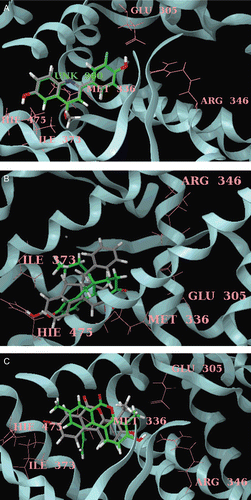
Many structurally diverse compounds have been noted to engage ERs and influence cellular response through them. ER LBD recognizes a wide variety of structurally distinct compounds that act as either receptor agonists, antagonists or have mixed character. In the present study, three structurally different compounds and their derivatives were analyzed for their molecular attributes such as size or shape parameters, lipophilicity and hydrogen-bonding potential. Molecular recognition of estrogenic compounds is achieved through a combination of specific hydrogen bonds with the receptor and hydrophobic interaction between residues that line the receptor cavity and the non-polar region of the ER ligands. Structural plasticity also allows ERs to shrink the cavities by as much as 15% to accommodate the smaller ligands betterCitation32. Thus, the ability of ER to bind a wide range of compounds stems from the flexibility in both the size and shape of the LBD. In the present study, we have considered size and shape indices of the ligands, in addition to their chemical characteristics important in determining ERβ potency and selectivity. Further, earlier report indicates that the differing amino acid at position Met 421 (ERα) and the corresponding Ile 373 (ERβ) would be an important differentiator in determining the specificity of ligand interaction than the other differing amino acid pair, viz Leu 384 (ERα) and Met 336 (ERβ)Citation33. In ERα, Met 421 makes a repulsive interaction with the electronegative and the non-polarisable groupsCitation33, and hence the electrotopological state (E-state index) was considered to be one of the differentiating descriptors to determine ligand selectivity. Before docking study, the original crystal structure of ERβ-genistein complex (PDB ID: 1QKM) was used to validate the Glide-XP docking protocol. This was carried out by removing the co-crystallized genistein ligand outside the active site and then docked back in to the active site. The RMSD was calculated for the best configuration in comparison to the co-crystallized genistein, and it was found to be 1.30 Å. The hydrogen bonding interactions between the genistein and ERβ was found to be in accordance with the crystallographic dataCitation28. Docking results of best active and least active compounds of the phenylquinoline, tetrahydrofluorenone and 3-hydroxy-6H-benzo[c]chromene-6-one series was taken to support our QSAR results.
Phenylquinoline
The chemical structure of 2-phenylquinoline is shown in . Parameters like molecular weight, number of hydrogen bond donors and acceptors, verloop parameters and E-state indices did not show any statistically significant effect on receptor activation and hence not reported here. The parameters showing significant relationship with receptor interaction are shown in . A multi-parameter regression model for the ERβ receptor binding and ERα to ERβ selectivity is shown in Equation 1 and Equation 2 below.
where a = lipophilicity (whole molecule) and b = number of heteroatoms at R4.
where a = ellipsoidal volume (whole molecule) and b = kappa 2 index of substituents at R2.
Table 5. Physicochemical descriptors that significantly influenced the 2-phenylquinoline derivatives binding at ER receptors.
Figure 1. Skeletal structure of estrogenic compounds. The moieties derivatised in each compound is indicated and referred in the , , and .
These equations predict the binding affinity () and ERβ selectivity () using the indicated descriptors. The plot against the observed vs. predicted values depict a good fit, and the statistical parameters are significant which suggests the utility of the model (). The propensity for a ligand to bind with higher affinity to ERβ is correlated with higher LogP (ERα binding affinity decreases with increase in lipophilicity). This is consistent with the ERβ binding pocket where large lipophilic cavities are present. Hence it is not surprising that log P is a modulator for ERβ affinity. Earlier reports also indicate that hydrophobic compounds exhibited better ERβ potencyCitation34 that is consistent with the present findings. Equation 1 suggests that incorporation of heteroatoms at R4 of phenylquinolines will increase the ERβ affinity. From , it can be inferred that a wide variety of functional groups including electronegative, electron-rich, aliphatic, aromatic, and polar substituents were introduced at R4 of phenylquinolines. Among which, compounds that had electronegative substitutions showed increased affinity towards ERβ (). According to Vu et al.Citation16, Met 421 of ERα and Ile 373 of ERβ interacts with the residue at R4 of phenylquinolines. Although the relative contribution of dispersion, electrostatics and exchange repulsion is unclear, it is possible that the electronegativity of the halogens and the methionine sulphur makes an unfavourable electrostatic contribution to the total interaction with ERα thereby increasing the affinity towards ERβ. The repulsion with Met421 of ERα by halides created at this site could permit better moulding with ERβ receptor. In the present study, docking of most active compound 7 in the 2-phenylquinoline series to the binding pocket of the ERβ places the bromo group at R4 in close proximity to the ERβ Ile373 residue () and showed more GScore than the inactive compound 21 in the series. This is in concordance with the earlier report that chemically hard functional group containing electronegative atoms are more attractive synthetic target than others with regard to improved ERβ bindingCitation33. Interestingly, compound 21 containing methoxy group at R4 substitution exhibited a great loss of affinity, presumably due to the unfavourable basicity of the quinoline core induced by the electron-rich methoxy groupCitation16.
Table 6. QSAR statistics of the best multiple linear regression equations.
Figure 2. Plot of predicted vs. observed values of ERβ binding affinity and fold selectivity of the ligands against ER receptors. (A), (C) and (E) represent the ERβ binding affinity of phenylquinoline, tetrahydrofluorenone and 3-hydroxy 6H-benzo[c]chromen-6-one ligands respectively. (B), (D) and (F) represent the ERβ fold selectivity of phenylquinoline, tetrahydrofluorenone and 3-hydroxy 6H-benzo[c]chromen-6-one ligands, respectively.
![Figure 2. Plot of predicted vs. observed values of ERβ binding affinity and fold selectivity of the ligands against ER receptors. (A), (C) and (E) represent the ERβ binding affinity of phenylquinoline, tetrahydrofluorenone and 3-hydroxy 6H-benzo[c]chromen-6-one ligands respectively. (B), (D) and (F) represent the ERβ fold selectivity of phenylquinoline, tetrahydrofluorenone and 3-hydroxy 6H-benzo[c]chromen-6-one ligands, respectively.](/cms/asset/3df81295-0e67-4d90-a1be-b23d905be6db/ienz_a_566219_f0002_b.gif)
Figure 3. (A) Docked pose of most active compound 7 (green) and least active compound 21 (grey) in the phenylquinoline series with ERβ. Only key residues (pink), of the ERβ binding site are shown for simplicity. (B) Docked pose of most active compound 28 (green) and least active compound 7 (grey) in the tetrahydrofluorenone series with ERβ. Only key residues (pink), of the ERβ binding site are shown for simplicity. (C) Docked pose of most active compound 23 (green) and least active compound 7 (grey) in the tetrahydrofluorenone series with ERβ. Only key residues (pink), of the ERβ binding site are shown for simplicity.
In the present study, Equation 2 indicates that decrease in ellipsoidal volume increases the fold selectivity towards ERβ. From , it can be seen that compounds having aliphatic groups such as ethyl, vinyl, alkynyl, and electron-withdrawing cyano group displayed higher ellipsoidal volume and showed only minimal selectivity. Compounds substituted with halides at R4 position displayed low ellipsoidal volume and displayed higher selectivity which is in accordance with the present study results (Equation 2). This may be because the internal volume of the LBD of ERβ is significantly smaller (~20%) than in ERα and hence it may have implications for the selectivity of ligands. Equation 2 also indicates that kappa 2 indices of the substituents at R2 should be less to have increased selectivity in phenylquinolines. The kappa index is the molecular shape index based on the assumption that the shape of a molecule is a function of the number of atoms and their bonding relationship. Kappa 2 index indicates the degree of linearity of bonding patternsCitation35. From , it can be inferred that substitution at R2 decreases the selectivity of the compounds towards ERβ (compare compound 7 vs. 8, 14 vs. 15) which is in accordance with Equation 2.
Tetrahydrofluorenone
The structure of tetrahydrofluorenone is shown in . Among the various descriptors analyzed, the molecular weight, molecular surface area, number of hydrogen bond donors/acceptors, ellipsoidal volume, kappa indices and E-state indices were not significantly correlated with ERβ affinity/selectivity and hence not discussed further. The descriptors showing significant influence on ERβ affinity/selectivity are shown in . The multi-parameter regression analysis is for ERβ receptor binding and ERβ to ERα selectivity is described as in Equation 3 and Equation 4 below.
where a = verloop B2 of substituents at R2 b = lipophilicity (whole molecule) and c = number of halogen atoms at substituent R2.
where a = verloop B1 of substituents at R2 and b = verloop B2 of substituents at R1.
Table 7. Physicochemical descriptors that significantly influenced the tetrahydrofluorenone derivatives binding at ER receptors.
The observed experimental and the values predicted from Equations 3 and 4 are plotted in and . Statistical parameters of Equations 3 and 4 were significant which indicate the robustness of the model (). Equation 3 indicates that increase in the width (verloop B2) of R2 substituents will increase the ERβ affinity of the ligands. From , it can be observed that introduction of methyl group at R2 resulted in increase in ERβ binding (compare compound 2 vs. 8). Further increase in width using substituents like heteroaryl or phenyl groups at R2 increased the binding affinity () which is in accord with the present study results (Equation 3). Like phenylquinolines, in tetrahydrofluorenone series also an increase in affinity for ERβ was observed when the lipophilicity of the ligands increased. Equation 3 also reveals that presence of halogen atoms at R2 increases the affinity of the compounds. From the docking studies of the most active compound 28 in the tetrahydrofluorenone series, it was observed that the functional groups at R2 interacted with Met 336 of ERβ through van der Waal’s force. This observation suggests that presence of halides near Met 336 will enhance ERβ ligand interaction leading to increased affinity. This affinity enhancement for ERβ can be attributed to a favourable overall hydrophobic effect due to the substituent (halogen) itself and additional van der Waals interactions between the halogen and surrounding residues. From , it can be inferred that halogenated analogues at R2 showed more affinity towards ERβ in contrast to heteroaryl and phenyl analogues.
Equation 4 indicates that increase in the width (verloop B1) of substituents at R2 in tetrahydrofluorenones is favourable for increasing selectivity towards ERβ. Verloop B1 is the width parameter and is defined as the smallest width of the substituent in any direction perpendicular to verloop lengthCitation36. As R2 substituents are near to more flexible Met 336 of ERβ, bulkier substituents can be well accommodated when compared to Leu 384 of ERα and display high selectivity. Equation 4 indicates that increase in width (verloop B2) of the substituents at R1 will be detrimental to ERβ selectivity. Verloop B2 is determined by measuring the width of the substituent in the direction opposite to the axis defined by B1. From , it can be inferred that selectivity of tetrahydrofluorenone increases when R1 increases from methyl up to butyl, whereas when the substituents like pentyl, benzyl were introduced the selectivity of the compounds become detrimental towards ERβ which is in accordance with Equation 4. Docking of most active compound 28 in the tetrahydrofluorenone series reveals that selectivity enhancing interaction is the putative favourable hydrophobic interaction between the n-butyl group at R1 which protrudes orthogonally from the plane of the tricyclic core towards Ile373 in ERβ as depicted in . We speculate that Ile373 in ERβ can nicely accommodate the presence of the n-butyl moiety into space which is not available in ERα because the sidechain of the analogous Met 421 fills this spaceCitation17. Docking of most inactive compound 7 in the tetrahydrofluorenone series reveals that substituent like benzyl in R1 is not well accommodated near Ile373 of ERβ and exhibited less GScore when compared with the active compound 7 of the same series.
3-hydroxy-6H- benzo[c]chromene-6-one
The structure of 3-hydroxy-6H-benzo[c]chromene-6-one is shown in . Analogues were obtained by derivatisation at five different positions. The physicochemical properties of the analogues were determined as described in methods of which molecular weight, number of hydrogen bond donors/acceptors, lipophilicity, E-state indices were not found to be significantly influencing ERβ receptor interaction or receptor preference, and hence not further discussed. The values for the other significant parameters are shown in . Multi-parameter regression analysis resulted in the numerical relationship of the descriptors to their reactivity with ER as given below.
where a = molecular surface area (whole molecule) and b = number of heteroatoms of substituents at R3.
n = 16; R2 = 0.780; s = 0.310; F = 14.194; RMSEtraining set = 0.268; q2 = 0.602; R2pred = 0.625; RMSEtest set = 0.373
where a = verloop B2 of substituents at R1, b = verloop B3 of substituents at R5 and c = κ alpha 3 index of substituents at R4.
Table 8. Physicochemical descriptors that significantly influenced the 3-hydroxy 6H-benzo[c]chromen-6-one derivatives binding at ER receptors.
The values predicted by Equations 5 and 6 are plotted against experimentally observed values in and , indicating a good fit between both sets of data and the statistical parameters indicate the utility of the QSAR model (). Equation 5 indicates that increase in molecular surface of chromenones will increase the affinity of the ligands towards ERβ. This affinity enhancement may be attributed to the favourable van der Waals interactions of the chromenone ring and surrounding hydrophobic residues. In , it is noticeable that increasing the number of substituents (thereby molecular surface area) on the phenyl rings gives better ERβ binding affinities. Compound 28 with five substituents on the aromatic rings has excellent binding affinity which is in accord with the results of Equation 5. Equation 5 indicates that increase in heteroatoms at R3 decreases the ERβ affinity remarkably. In the chromenone series, it can be observed that introduction of polar amino groups at R3 decreases the ERβ affinity (). Increasing the acidity of the amino group by substitution with electron-withdrawing groups (compounds 13 and 16) gave poorly active compounds because R3 position is unable to sustain a group much larger than methyl (compounds 3,5,11) which is in accord with the QSAR results (Equation 5).
Equation 6 indicates that decrease in the width of substituents (verloop B2) at R1 is required for increased ERβ selectivity. Increase in verloop B2 (-CH3 to -C2H5) in R1 significantly reduced the selectivity of the molecule (compound 3 vs. 7, ) towards ERβ. This suggests that space availability was constrained to limit the size of the substituents at R1. Increase in width (verloop B3) of the substituents at R5 increases the selectivity of the compounds towards ERβ. Kappa index, another descriptor included in Equation 6, is a molecular shape index based on the assumption that the shape of a molecule is a function of the number of atoms and their bonding relationships. A group of modified indices, kappa alpha indices (κAlpha) are calculated for each atom-type using the ratio of covalent radii of carbon (sp3) and the atomCitation35. Increase in κAlpha 3 index of the substituent at R4 increases the selectivity of the molecules towards ERβ. In 3-hydroxy-6H-benzo[c]chromene-6-ones, compounds 19, 27, 28 displayed higher κAlpha 3 indices and selectivity which is in accord with the QSAR Equation 6. Docking of the most active compound 23 in the 3-hydroxy-6H-benzo[c]chromene-6-one series revealed that the vinyl group in R5 extends into the ERβ Ile373 pocket and sits in a groove formed by Ile373, Ile376 and Phe377 (). The vinyl CH acts as a “hinge” that directs the ethylene moiety into this relatively narrow groove and forces it to be in close proximity to ERβ Ile373 and hence leading to enhanced ERβ selectivity. Similar interactions have been reported earlier with vinyl functional group in aryl diphenolic azolesCitation6 and 7-substituted benzofuran and benzoxazolesCitation33 with ERβ.
In the present study, QSAR analyses indicated that for the three different series of scaffolds, size and shape descriptors were able to explain the ERβ potency and selectivity well. Further, the constructed QSAR models in the present study were more reliable than the previously reportedCitation13,Citation14 as they exhibited values of q2 LMOcv > 0.5; R2pred > 0.6 (except Equation 2) which represents the utility of the QSAR modelsCitation20. Low randomized R2 and high S.D values were exhibited by the QSAR models in randomization test indicates that there is no chance correlation. High Q values () of the obtained QSAR models indicate that there is no over fitting due to more number of descriptorsCitation36. Root mean square error (RMSE) of all active compounds in the training test set for ERβ in the previously reported QSAR model was 0.65, 1.22, respectively and the R2pred = 0.4213. In the present study, all the QSAR models showed less RMSE value which also represents the utility of the QSAR models.
In conclusion, among the descriptors studied, increased lipophilicity, decrease in ellipsoidal volume and width of substituents, presence of halogen atoms were essential for the ligands to have high affinity and selectivity towards ERβ. The present study clearly delineates that the size and shape descriptors are the best modulators of ERβ affinity and selectivity than the quantum chemical and electrostatic descriptors. Information presented here will not only enlarge the areas of their application, but it may also increase our understanding towards the mechanisms of chemical–biological interactions.
Declaration of interest
Authors declare no conflict of interest.
References
- Katzenellenbogen BS. Estrogen receptors: bioactivities and interactions with cell signaling pathways. Biol Reprod 1996;54:287–293.
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA 1996;93:5925–5930.
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett 1996;392:49–53.
- Enmark E, Pelto-Huikko M, Grandien K, Lagercrantz S, Lagercrantz J, Fried G et al. Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern. J Clin Endocrinol Metab 1997;82:4258–4265.
- Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F et al. Cloning, chromosomal localization, and functional analysis of the murine estrogen receptor beta. Mol Endocrinol 1997;11:353–365.
- Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD et al. Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor-beta ligands. J Med Chem 2004;47:5021–5040.
- Imamov O, Shim GJ, Warner M, Gustafsson JA. Estrogen receptor beta in health and disease. Biol Reprod 2005;73:866–871.
- Koehler KF, Helguero LA, Haldosén LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr Rev 2005;26:465–478.
- Vedani A, Smiesko M, Spreafico M, Peristera O, Dobler M. VirtualToxLab - in silico prediction of the toxic (endocrine-disrupting) potential of drugs, chemicals and natural products. Two years and 2,000 compounds of experience: a progress report. Altex 2009;26:167–176.
- Cao Q, Garib V, Yu Q, Connell DW, Campitelli M. Quantitative structure-property relationships (QSPR) for steroidal compounds of environmental importance. Chemosphere 2009;76:453–459.
- Roncaglioni A, Piclin N, Pintore M, Benfenati E. Binary classification models for endocrine disrupter effects mediated through the estrogen receptor. SAR QSAR Environ Res 2008;19:697–733.
- Li H, Ung CY, Yap CW, Xue Y, Li ZR, Chen YZ. Prediction of estrogen receptor agonists and characterization of associated molecular descriptors by statistical learning methods. J Mol Graph Model 2006;25:313–323.
- Boriani E, Spreafico M, Benfenati E, Novic M. Structural features of diverse ligands influencing binding affinities to estrogen alpha and estrogen beta receptors. Part I: Molecular descriptors calculated from minimal energy conformation of isolated ligands. Mol Divers 2007;11:153–169.
- Spreafico M, Boriani E, Benfenati E, Novic M. Structural features of diverse ligands influencing binding affinities to estrogen alpha and estrogen beta receptors. Part II. Molecular descriptors calculated from conformation of the ligands in the complex resulting from previous docking study. Mol Divers 2007;11:171–181.
- Hillisch A, Peters O, Kosemund D, Müller G, Walter A, Schneider B et al. Dissecting physiological roles of estrogen receptor alpha and beta with potent selective ligands from structure-based design. Mol Endocrinol 2004;18:1599–1609.
- Vu AT, Cohn ST, Manas ES, Harris HA, Mewshaw RE. ERbeta ligands. Part 4: Synthesis and structure-activity relationships of a series of 2-phenylquinoline derivatives. Bioorg Med Chem Lett 2005;15:4520–4525.
- Wilkening RR, Ratcliffe RW, Tynebor EC, Wildonger KJ, Fried AK, Hammond ML et al. The discovery of tetrahydrofluorenones as a new class of estrogen receptor beta-subtype selective ligands. Bioorg Med Chem Lett 2006;16:3489–3494.
- Sun W, Cama LD, Birzin ET, Warrier S, Locco L, Mosley R et al. 6H-Benzo[c]chromen-6-one derivatives as selective ERbeta agonists. Bioorg Med Chem Lett 2006;16:1468–1472.
- Trossini GH, Guido RV, Oliva G, Ferreira EI, Andricopulo AD. Quantitative structure-activity relationships for a series of inhibitors of cruzain from Trypanosoma cruzi: molecular modeling, CoMFA and CoMSIA studies. J Mol Graph Model 2009;28:3–11.
- Leonard JT, Roy K. Exploring molecular shape analysis of styrylquinoline derivatives as HIV-1 integrase inhibitors. Eur J Med Chem 2008;43:81–92.
- Hu QN, Liang YZ, Yin H, Peng XL, Fang KT. Structural interpretation of the topological index. 2. The molecular connectivity index, the kappa index, and the atom-type E-state index. J Chem Inf Comput Sci 2004;44:1193–1201.
- Ullrich JW, Unwalla RJ, Singhaus RR Jr, Harris HA, Mewshaw RE. Estrogen receptor beta ligands: design and synthesis of new 2-phenyl-isoindole-1,3-diones. Bioorg Med Chem Lett 2007;17:118–122.
- Umamatheswari S, Balaji B, Ramanathan M, Kabilan S. Synthesis, antimicrobial evaluation and QSAR studies of novel piperidin-4-yl-5-spiro-thiadiazoline derivatives. Bioorg Med Chem Lett 2010;20:6909–6914.
- Hansch C, Verma RP. A QSAR study for the cytotoxic activities of taxoids against macrophage (MPhi)-like cells. Eur J Med Chem 2009;44:274–279.
- Strike, version 1.8, Schrödinger, LLC, New York, NY, 2009.
- Roy K. On some aspects of validation of predictive quantitative structure–activity relationship models. Expert Opin Drug Discov 2007;2:1567–1577.
- LigPrep, version 2.3, Schrödinger, LLC, New York, NY, 2009.
- Pike AC, Brzozowski AM, Hubbard RE, Bonn T, Thorsell AG, Engström O et al. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. Embo J 1999;18:4608–4618.
- Maestro, version 9.0, Schrödinger, LLC, New York, NY, 2009.
- Prime, version 2.1, Schrödinger, LLC, New York, NY, 2009.
- Glide, version 5.5, Schrödinger, LLC, New York, NY, 2009.
- Pike AC. Lessons learnt from structural studies of the oestrogen receptor. Best Pract Res Clin Endocrinol Metab 2006;20:1–14.
- Manas ES, Unwalla RJ, Xu ZB, Malamas MS, Miller CP, Harris HA et al. Structure-based design of estrogen receptor-beta selective ligands. J Am Chem Soc 2004;126:15106–15119.
- Fang H, Tong W, Shi LM, Blair R, Perkins R, Branham W et al. Structure-activity relationships for a large diverse set of natural, synthetic, and environmental estrogens. Chem Res Toxicol 2001;14:280–294.
- Chang HJ, Kim HJ, Chun HS. Quantitative structure-activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci 2007;80:835–841.
- Hansch C, Verma RP, Kurup A, Mekapati SB. The role of QSAR in dopamine interactions. Bioorg Med Chem Lett 2005;15:2149–2157.