Abstract
In the present study, four Pt(II) complexes with 2-ethyl (1)/or benzyl (2)/or p-chlorobenzyl (3)/or 2-phenoxymethyl (4) benzimidazole carrier ligands were evaluated for their in vitro cytotoxic activities against the human HeLa cervix, oestrogen receptor-positive MCF-7 breast, and oestrogen receptor-negative MDA-MB 231 breast cancer cell lines. The plasmid DNA interactions and inhibition of the BamHI restriction enzyme activities of the complexes were also studied. Complex 3 was found to be more active than carboplatin for all examined cell lines and comparable with cisplatin, except for the HeLa cell line.
Introduction
Cisplatin [cis-diamminedichloroplatinum(II)] and its analogues, carboplatin [cis-diammine-1,1-cyclobutanedicarboxylate platinum(II)] and oxaliplatin ([(1R,2R)-cyclohexane-1,2-diamine](ethanedioato-O,O′)platinum(II)), are among the most commonly used antitumour drugs. The clinical efficacy of these anticancer drugs is diminished by intrinsic and acquired tumour resistance and side effects. Therefore, much attention has been focused on designing new platinum compounds with improved pharmacological properties and a broader range of antitumour activityCitation1.
The biochemical mechanism of cisplatin cytotoxicity involves the binding of the drug to DNA and non-DNA targets and the subsequent induction of cell death through apoptosis, necrosis, or bothCitation2.
It is generally believed that the biological activity of cisplatin is associated with the recognition of its DNA adducts by cellular proteins such as repair enzymes, transcription factors, histones, and high-mobility group (HMG) domain proteinsCitation3. New evidence suggests that proteins and other biomolecules can also be the targets of platinum complexes and may be important in the cytotoxicity mechanismCitation4.
It is known that the modification of the carrier ligands in antitumour platinum(II) complexes can alter their efficacy and the spectrum of activityCitation1. The carrier ligand of the platinum(II) DNA-damaging agents may affect biodistribution, the rates and types of DNA adduct formation, and recognition of damaged DNA by repair enzymes or regulatory/binding proteinsCitation5. The use of sterically demanding diamines as carrier ligands for an alternative compound to cisplatin can slow or block repair enzymesCitation6. In addition, platinum complexes with distinctively different DNA-binding modes from that of cisplatin may provide higher antitumour activity against cisplatin-resistant cancer cellsCitation7.
In previous studies, with the consideration that variations in the chemical structure of the amine groups of cisplatin might have a significant effect on the cytotoxic activity and toxicity of platinum complexes and with the aim of determining the role of the substituents on position 2 of the benzimidazole carrier ligands of platinum(II) complexes on cytotoxic properties, we synthesized some platinum(II) complexes with 2-substituted benzimidazole ligandsCitation8–16.
It was determined that some of these platinum complexes have in vitro cytotoxic activities on RDCitation9, HeLaCitation12,Citation14–16, MCF-7Citation10,Citation12,Citation14–16, and HEp-2Citation16 cell lines.
In the present study, as an extension of the investigation on the probable anticancer activity of platinum complexes with 2-substituted benzimidazole ligands, four Pt(II) complexes with bulky, near-planar, hydrophobic amine ligands, including 2-ethyl (1), benzyl (2), p-chlorobenzyl (3), and phenoxymethyl (4) benzimidazoles, were evaluated for their in vitro cytotoxic activities against the human HeLa cervix, oestrogen receptor-positive MCF-7 breast, and oestrogen receptor-negative MDA-MB 231 breast cancer cell lines. The plasmid DNA interactions and inhibition of the BamHI restriction enzyme activity of the complexes were also studied.
Experimental
Synthesis
The synthesis and structural analyses of platinum(II) complex 3 were reported in our previous workCitation11. Complexes 1, 2, and 4 were synthesized and characterized as described previouslyCitation13 ().
In vitro cytotoxicity studies
Cell lines and growth conditions
Cisplatin (CAS 15663-27-1) and carboplatin (CAS 41575-94-4), used as reference compounds, were obtained from Sigma (St. Louis, Missouri, USA). The human HeLa cervix, oestrogen receptor-positive MCF-7 breast, and oestrogen receptor-negative MDA-MB 231 breast cancer cell lines used in this study were obtained from the Cell Culture Collection (HUKUK numbers 90061901, 00092502, and 02031201, respectively) of the Institute for Foot and Mouth Disease (IFMD), Turkey. The cells were grown as monolayer cultures in T75 flasks, subcultured three times at 37°C in an atmosphere of 5% CO2 in air and 100% relative humidity, and maintained in third passage. Three cell suspensions were prepared at the concentration of 8.7 × 103 cell/mL and dispensed onto 96-well cell culture plates (100 µL per well). The multiwell plates were incubated at 37°C and 5% CO2 in air for 24 h. After 24 h, the culture medium was removed from the wells and equal volumes (100 µL) of serial dilutions (80, 40, 20, 10, and 5 µM) of the test compounds were added to each well. After 72 h incubation periods, cytotoxicity was evaluated colorimetrically by MTT assayCitation17. Three experiments were carried out for each compound.
Studies of interaction with pBR322 plasmid DNA
The cisplatin and carboplatin used as reference compounds, plasmid DNA pBR322, ethidium bromide, agarose, and enzyme BamHI were purchased from Sigma.
The interaction of platinum(II) complexes 1–4, carboplatin, and cisplatin with pBR322 plasmid DNA was studied by agarose gel electrophoresisCitation18. Stock solutions of the tested compounds in dimethylformamide (DMF) were prepared and used within 1 h. The final amount of DMF never exceeded 0.1%. In brief, 40 μL aliquots of increasing concentrations of the compounds, ranging from 0 to 160 μM, were added to 1 μL of plasmid DNA (concentration of 0.5 μg/mL) in a buffer solution containing TE (10 mM Tris–HCl, 0.1 mM EDTA, pH = 7.4). The samples were incubated at 37°C for 24 h in the dark, and then 10 μL aliquots of drug–DNA mixtures were mixed with loading buffer (0.1% bromophenol blue, 0.1% xylene cyanol) and loaded onto 1% agarose gel with or without ethidium bromide. Electrophoresis was carried out under TAE buffer (0.05 M Tris base, 0.05 M glacial acetic acid, 1 mM EDTA, pH = 8.0) for 5 h at 40 V. At the end of the electrophoresis, the gel without ethidium bromide was stained in the same buffer containing ethidium bromide (0.5 μg/mL). The gels were then viewed with a transilluminator and the image was captured with a video camera (GelDoc-It Imaging System, UVP) as a TIFF file. The experiments were repeated three times.
BamHI restriction enzyme digestion
In this series of experiments, the drug–DNA mixtures were first incubated for 24 h and then subjected to BamHI digestion for 1 h at 37°C. To each 8 μL of incubated drug–DNA mixture was added 1 μL of 10× digestion buffer and then 0.1 μL of BamHI (1 unit). The mixtures were left in a shaking water bath for 1 h at 37°C. The digestion was terminated by rapid cooling. The restricted DNA was run in 1% agarose gel electrophoresis for 3 h at 40 V in TAE buffer. The gel was stained with ethidium bromide; the gels were then viewed with a transilluminator and the images were captured by video camera as TIFF files.
Results and discussion
Cytotoxicity
As an extension of our previous studies on benzimidazole–Pt(II) complexes, the present study evaluates the in vitro cytotoxic activities of Pt(II) complexes with 2-ethyl (1)/or benzyl (2)/or p-chlorobenzyl (3)/or 2-phenoxymethyl (4) benzimidazole carrier ligands on HeLa, MCF-7, and MDA-MB-231 cell lines.
The growth inhibitory effects of platinum(II) complexes 1–4, cisplatin, and carboplatin against the cell lines was measured by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. The cytotoxic activity values of platinum complexes 1–4 and the reference compounds, expressed as Inh.%, are presented in .
Table 1. Cytotoxicity of benzimidazole–platinum(II) complexes 1–4, cisplatin, and carboplatin on the HeLa, MCF-7, and MDA-MB-231 cell lines.
The cytotoxicity results obtained for complexes 1–4 showed that the cytotoxicity of complex 3, with p-chlorobenzyl groups on position 2 of the benzimidazole carrier ligands, was approximately 3-fold greater than those of compounds 1 and 2, with ethyl and benzyl groups on their carrier ligands, respectively, and carboplatin, and comparable with that of cisplatin against the three cell lines used.
The result of the preliminary cytotoxic activity studies indicated that, in general, the following orders of the relative in vitro cytotoxic activity of the compounds tested could be considered: cisplatin > 3 > 4, carboplatin > 1, 2 against the HeLa cell line; cisplatin, 3 > carboplatin > 4 > 1, 2 against the MCF-7 cell line; and cisplatin > 3 > carboplatin > 4 > 1 > 2 against the MDA-MB 231 cell line.
Interaction with pBR322 plasmid DNA
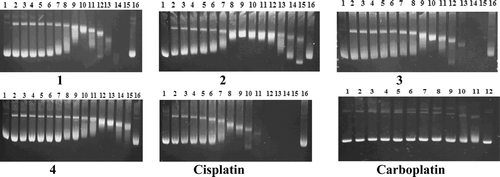
In order to detect whether compounds 1–4 induced conformational changes on the DNA helix and whether there was a relationship between the plasmid DNA binding affinity and the cytotoxicity of the compounds, we investigated their capacity to remove and reverse the supercoiling of closed circular pBR322 plasmid DNA as assessed by electrophoretic mobility measurements on agarose gels. shows the electrophoretic mobility pattern of the covalently closed circular Form I and open circular Form II bands of pBR322 plasmid DNA after incubation in a range of concentrations from 0 to 160 µM at 37°C for 24 h. In the electrophoretograms, the untreated pBR322 plasmid DNA was used as a control.
Figure 1. Modification of gel electrophoretic mobility of pBR322 plasmid DNA when incubated with various concentrations of complexes 1–4, cisplatin, and carboplatin. Concentrations (in μM) are as follows. For complexes 1–4 and cisplatin: lines 1 and 16, for carboplatin: lines 1 and 12, untreated pBR322 plasmid DNA; (line 2) 0.019; (line 3) 0.039; (line 4) 0.078; (line 5) 0.156; (line 6) 0.312; (line 7) 0.625; (line 8) 1.25; (line 9) 2.5; (line 10) 5; (line 11) 10; (line 12) 20; (line 13) 40; (line 14) 80; (line 15) 160. For carboplatin: (line 2) 0.312; (line 3) 0.625; (line 4) 1.25; (line 5) 2.5; (line 6) 5; (line 7) 10; (line 8) 20; (line 9) 40; (line 10) 80; (line 11) 160. The top and the bottom bands correspond to Form II (open circular) and Form I (covalently closed circular) plasmids, respectively.
Nuclear DNA is an important molecular target for platinum anticancer compounds, which bind purine bases at the N7 position. The resulting Pt-DNA damage triggers downstream effects, including inhibition of replication and transcription. If the cell cannot remove the damage, then it dies by one of several pathwaysCitation19.
It is widely documented that platinum-based complexes can untwist and/or bend plasmid DNA, depending on the kind of specific adducts formed on the DNACitation20. Monofunctional or intercalating adducts may untwist dsDNA, whereas bifunctional adducts and intra- and interstrand cross-links, in addition, bend DNA.
A decrease in the rate of migration of Form I bands is the result of untwisting the DNA, as this reduces the number of supercoils. It can be seen in that complexes 1–4 and cisplatin caused a significant decrease in the rate of migration of the Form I band of the pBR322 plasmid DNA. In contrast, carboplatin did not untwist the DNA significantly.
All of the compounds tested affected the mobilization of the oc form of the plasmid DNA. These differences in plasmid migration are consistent with DNA condensation, which may be caused by bending. Compounds 1–3 are evidently more efficient than 4 and carboplatin in inducing changes in DNA secondary structure. By comparing the mobilization of the oc forms of the plasmid, it is obvious that the bending induced by carboplatin was least pronounced.
The concentrations required to achieve total unwinding of the pBR322 plasmid DNA for compounds 1, 2, 3, and 4 and cisplatin were 5, 2.5, 5, 40, and 2.5 µM, respectively. The presence of a coalescence point indicates a strong unwinding of the supercoiled DNA.
BamHI digestion of drug–pBR322 plasmid DNA
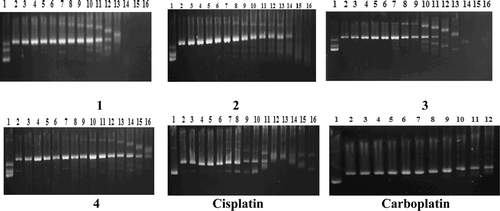
In order to assess whether the benzimidazole–Pt(II) complexes showed affinity towards the guanine–guanine (GG) region, we carried out restriction endonuclease analysis of the compound–pBR322 plasmid DNA adducts digested by BamHI enzyme (). The BamHI enzyme binds at the recognition sequence 5′-G/GATCC-3′ and cleaves these sequences just after the 5′-guanine site, converting, as a result, Form I and Form II DNA to linear Form III DNACitation21.
Figure 2. Electrophoretograms for the BamHI-digested mixtures of pBR322 plasmid DNA after their treatment with various concentrations of complexes 1–4, cisplatin, and carboplatin. Concentrations (in μM) are as follows: (lines 1 and 2) untreated pBR322 plasmid DNA and pBR322 DNA linearized by BamHI, respectively. For complexes 1–4 and cisplatin: (line 3) 0.019; (line 4) 0.039; (line 5) 0.078; (line 6) 0.156; (line 7) 0.312; (line 8) 0.625; (line 9) 1.25; (line 10) 2.5; (line 11) 5; (line 12) 10; (line 13) 20; (line 14) 40; (line 15) 80; (line 16) 160. For carboplatin: (line 3) 0.312; (line 4) 0.625; (line 5) 1.25; (line 6) 2.5; (line 7) 5; (line 8) 10; (line 9) 20; (line 10) 40; (line 11) 80; (line 12) 160. The top, bottom, and middle bands indicate Form I (covalently closed circular), Form II (open circular), and Form III (linear) plasmids, respectively.
The major DNA lesion formed by cisplatin is a 1,2-intrastrand cross-link between adjacent purinesCitation22. Although it is not a proven fact, it is generally accepted that the 1,2-intrastrand DNA adduct is responsible for the anticancer activity of cisplatinCitation23. The cisplatin–DNA adduct structures examined using NMR spectroscopyCitation24 and X-ray crystallographyCitation25 showed a dominant form (>90%) of the drug intrastrand cross-link with two adjacent GG regions on position N7 of the guanine bases.
As the concentrations of complexes 1–4 and the reference compounds were increased, it was seen that BamHI digestion was increasingly prevented. This was probably due to their affinity for the GG regions of the plasmid DNA and the conformational change in the plasmid DNA brought about by the covalent binding of the compounds with the DNA.
The following order of relative decreased prevention of BamHI digestion by the complexes was observed: cisplatin > 1, 3 > 2 > 4 > carboplatin.
To determine whether the hydrophobicity of the Pt(II) complexes influenced their cytotoxicities on the cell lines tested and their plasmid DNA interactions, theoretical calculations of the hydrophobicity of the 2-ethyl (L1), benzyl (L2), p-chlorobenzyl (L3), and phenoxymethyl (L4) benzimidazole carrier ligands as Log P were performed using ACD/Log P software. It was reported in a systematic study of the hydrophobicity of cis-diam(m)ine platinum(II) complexes that when the leaving groups are unchanged, the hydrophobicity of a platinum complex is linearly related to that of the am(m)ine ligandCitation26. The Log P values of carrier ligands L1–L4 were 2.11 ± 0.22, 3.93 ± 0.26, 4.52 ± 0.27, and 3.12 ± 0.27, respectively.
If complexes 1–4 are compared in order of decreasing cytotoxicity against the three cell lines used (3 > 4 > 1, 2) with the order of the Log P values of the corresponding ligands (L3 > L2 > L4 > L1), it is difficult to establish a correlation, except for complex 3.
In general, there was no clear correlation between the plasmid DNA-binding affinity and the Log P or the cytotoxic activity of the complexes tested.
Interestingly, although the interaction of complex 3 with pBR322 plasmid DNA was observed at an approximately similar effective concentration as complexes 1 and 2, complex 3 was found to be three times more cytotoxic than complexes 1 and 2. It has been reported in the literatureCitation27 that halogen-bonding interactions seem to become comparable in magnitude to the well-studied hydrogen bonds and are responsible for the different conformation of the molecules in the active site. It has also been reported that halogen-bonding interactions are one of the important interactions leading to the overall protein–ligand-binding affinity. At this stage of the study, it is not possible to explain clearly why complex 3 was the most cytotoxic compound in the cell lines used, compared with the other benzimidazole–Pt(II) complexes tested. However, considering the literature data mentioned above and other data reporting that DNA platination is a necessary condition for the cytotoxic activity of platinum complexes but may not be the sole mechanism that determines their cytotoxicityCitation28, it may be concluded with great caution that chloro substituents on the benzimidazole carrier ligands of complex 3 may play a role in its cytotoxicity. Further experiments are required to verify this conclusion.
Conclusion
The preliminary data obtained in this study lead us to conclude that the complex with a p-chlorobenzyl group on position 2 of the benzimidazole carrier ligand (complex 3) produced appreciable activity against the cell lines used and must be taken into consideration for further studies. It is interesting to note that complexes 1 and 2, having sterically less bulky substituents than complexes 3 and 4, were found to be less active against the cell lines used. The results of the plasmid DNA interaction and the restriction studies suggest that, in general, there is no clear correlation between the plasmid DNA-binding affinity and the cytotoxic activity of complexes 1–4. The possible role of the halogen atoms on the cytotoxic activity of the 2-substituted benzimidazole–Pt(II) complexes will also be taken into consideration for future studies.
Acknowledgement
We would like to thank the Research Foundation of Gazi University (02/2007-16) for financial support.
Declaration of interest
Financial support of this work (02/2007-16) by the Research Foundation of Gazi University is gratefully acknowledged.
References
- Zhang CX, Lippard SJ. New metal complexes as potential therapeutics. Curr Opin Chem Biol 2003;7:481–489.
- Fuertes MA, Alonso C, Pérez JM. Biochemical modulation of cisplatin mechanisms of action: enhancement of antitumor activity and circumvention of drug resistance. Chem Rev 2003;103:645–662.
- Brabec V, Kasparkova J. Molecular aspects of resistance to antitumor platinum drugs. Drug Resist Updat 2002;5:147–161.
- Bose RN, Maurmann L, Mishur RJ, Yasui L, Gupta S, Grayburn WS et al. Non-DNA-binding platinum anticancer agents: cytotoxic activities of platinum–phosphato complexes towards human ovarian cancer cells. Proc Natl Acad Sci USA 2008;105:18314–18319.
- Reardon JT, Vaisman A, Chaney SG, Sancar A. Efficient nucleotide excision repair of cisplatin, oxaliplatin, and bis-aceto-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216) platinum intrastrand DNA diadducts. Cancer Res 1999;59:3968–3971.
- Reedijk J. Why does cisplatin reach guanine-n7 with competing s-donor ligands available in the cell? Chem Rev 1999;99:2499–2510.
- Kostova I. Platinum complexes as anticancer agents. Recent Pat Anticancer Drug Discov 2006;1:1–22.
- Gümüş F, İzgü F, Algül Ö. Synthesis and structural characterization of some 5(6)-substituted-2-hydroxymethylbenzimidazole derivatives and their platinum(II) complexes and determination of their in vitro antitumor activities by “Rec-Assay Test”. FABAD Farm Bil Der 1996;21:7–15.
- Gümüs F, Pamuk I, Ozden T, Yildiz S, Diril N, Oksüzoglu E et al. Synthesis, characterization and in vitro cytotoxic, mutagenic and antimicrobial activity of platinum(II) complexes with substituted benzimidazole ligands. J Inorg Biochem 2003;94:255–262.
- Gümüs F, Algül O, Eren G, Eroglu H, Diril N, Gür S et al. Synthesis, cytotoxic activity on MCF-7 cell line and mutagenic activity of platinum(II) complexes with 2-substituted benzimidazole ligands. Eur J Med Chem 2003;38:473–480.
- Gümüs F, Demirci AB, Ozden T, Eroglu H, Diril N. Synthesis, characterization and mutagenicity of new cis-[Pt(2-substituted-benzimidazole)2Cl2] complexes. Pharmazie 2003;58:303–307.
- Gökçe M, Utku S, Gür S, Ozkul A, Gümüs F. Synthesis, in vitro cytotoxic and antiviral activity of cis-[Pt(R(−) and S(+)-2-alpha-hydroxybenzylbenzimidazole)2Cl2] complexes. Eur J Med Chem 2005;40:135–141.
- Algül Ö, Özçelik B, Abbasoğlu U, Gümüş F. Synthesis, characterization and genotoxicity of platinum(II) complexes with substituted benzimidazole ligands. Turk J Chem 2005;29:607–615.
- Utku S, Gümüş F, Gür S, Özkul A. Synthesis and cytotoxic activity of platinum(II) and platinum(IV) complexes with 2-hydroxymethylbenzimidazole or 5(6)-chloro-2-hydroxymethylbenzimidazole ligands against MCF-7 and HeLa cell line. Turk J Chem 2007;31:503–514.
- Gümüs F, Eren G, Açik L, Celebi A, Oztürk F, Yilmaz S et al. Synthesis, cytotoxicity, and DNA interactions of new cisplatin analogues containing substituted benzimidazole ligands. J Med Chem 2009;52:1345–1357.
- Utku S, Gumus F, Tezcan S, Serin MS, Ozkul A. Synthesis, characterization, cytotoxicity, and DNA binding of some new platinum(II) and platinum(IV) complexes with benzimidazole ligands. J Enzyme Inhib Med Chem 2010;25:502–508.
- Holst-Hansen C, Brünner N. Cell and tissue culture and associated techniques. Cell Biol 1998;1:16–18.
- Maniatis T, Fristsh EF, Sambrook J. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, New York, 1989.
- Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 2005;4:307–320.
- Bierbach U, Qu Y, Hambley TW, Peroutka J, Nguyen HL, Doedee M et al. Synthesis, structure, biological activity, and DNA binding of platinum(II) complexes of the type trans-[PtCl(2)(NH(3))L] (L = planar nitrogen base). Effect of L and cis/trans isomerism on sequence specificity and unwinding properties observed in globally platinated DNA. Inorg Chem 1999;38:3535–3542.
- Roberts RJ, Wilson GA, Young FE. Recognition sequence of specific endonuclease BamHI from Bacillus amyloliquefaciens H. Nature 1977;265:82–84.
- Brabec V, Kasparkova J. Modifications of DNA by platinum complexes. Relation to resistance of tumors to platinum antitumor drugs. Drug Resist Updat 2005;8:131–146.
- Fuertes MA, Castilla J, Alonso C, Pérez JM. Cisplatin biochemical mechanism of action: from cytotoxicity to induction of cell death through interconnections between apoptotic and necrotic pathways. Curr Med Chem 2003;10:257–266.
- Yang D, van Boom SS, Reedijk J, van Boom JH, Wang AH. Structure and isomerization of an intrastrand cisplatin–cross-linked octamer DNA duplex by NMR analysis. Biochemistry 1995;34:12912–12920.
- Takahara PM, Rosenzweig AC, Frederick CA, Lippard SJ. Crystal structure of double-stranded DNA containing the major adduct of the anticancer drug cisplatin. Nature 1995;377:649–652.
- Souchard JP, Ha TT, Cros S, Johnson NP. Hydrophobicity parameters for platinum complexes. J Med Chem 1991;34:863–864.
- Lu Y, Shi T, Wang Y, Yang H, Yan X, Luo X et al. Halogen bonding—a novel interaction for rational drug design? J Med Chem 2009;52:2854–2862.
- Khazanov E, Barenholz Y, Gibson D, Najajreh Y. Novel apoptosis-inducing trans-platinum piperidine derivatives: synthesis and biological characterization. J Med Chem 2002;45:5196–5204.