Abstract
In the present study, we purified hcbCA I and II from human cord blood erythrocytes using by Sepharose-4B-l tyrosine-sulfanilamide affinity gel chromatography. Also, it was checked the inhibition effects of ampicillin sulfate, ceftriaxone, ceftizoxime and ranitidine on hcbCA I and hcbCA II. IC50 values for ceftriaxone, ceftizoxime and ranitidine were found to be 27.l, 79.4 and 55.5 µM for hcbCA I and of 21.0, 79.1 and 66.1 µM for hcbCA II, respectively. According to these results, Ampicillin sulfate inhibited only hcbCAII and IC50 values of this antibiotic was found to be 56.8 µM. All these substances were found non-competitive inhibitors. It is important to study the inhibition effects of these drugs on hcbCA I and II izoenzymes. Because, pregnant woman is take all of these substance. For this reason, these drugs should be carefully used and the dosage should be very well ordered to minimize side effects.
Keywords::
Introduction
Cord blood remains in the placenta and in the attached umbilical cord after childbirth. Cord blood is obtained from the umbilical cord when the child is born. It is collected because its stem cells contents, including hematopoietic cells, which can be used to treat hematopoietic and genetic disorders. The placenta is a much better source of stem cells since it contains up to ten times more stem cells than cord bloodCitation1–4. On the other hand, enzymes are very important for the life attendance. Carbonic anhydrases is a crucial family in enzymes because they catalyze the reversible reactions of CO2 and waterCitation5–7. CA is of broad interest because it is one of the fastest enzymes known; the turnover number or kcat of some CA isoforms exceeds 1 × 106 s−18. The reactions of CA izoenzymes are essential to several of physiological processes such as calcification, photosynthesis, respiration, ionic, acid-base and fluid balance, metabolism and cell growthCitation9. Therefore, CA appears to be almost ubiquitously expressed in living organismsCitation9,Citation10. Investigation of property of this family is very important. Analyses of inhibition effects of different drugs on CA family are crucial for life.
Antibiotics are widely used to deal with various disorders, but there are few reports of their effects on enzyme activities. Some studies found either increases or decreases in mammalian enzyme activities, and the inhibitor or activator effects of some antibiotics have been investigated. Several investigations are reported, different type of drugs and chemicals have inhibition effects on CAsCitation11–14. Ampicillin is a beta-lactam antibiotic that has been used extensively to treat bacterial infections. Ceftriaxone is a third-generation cephalosporin antibiotic. It has broad spectrum activity against gram-positive and gram-negative bacteria. Ceftizoxime is a parenteral third-generation cephalosporin. Ranitidine is a histamine H2-receptor antagonist that inhibits stomach acid productionCitation15–18. All of these drugs are used during pregnancy.
Most of investigations focus on the property of umbilical cord. Such as the immunologic properties of cord blood differ from mature bone marrow or peripheral blood stem cells. Also, cord blood cells produce increased amounts of anti-inflammatory cytokine interleukin-10, which may downmodulate graft-versushost diseaseCitation1,Citation19. The effects of many of the known antibiotics have not been analyzed on human cord blood CA isozymes yet, which are contained at the highest molar amounts in blood. Usage of these drugs may worsen hcbCA 1 and II activity in cord blood. So, they may be used in pregnant patients; it is important to explore the effect of these on CA I and II activity during pregnancy. The present study was realized on the in vitro effect of ampicillin sulfate, ceftriaxone, ceftizoxime, ranitidine on hbcCA I and II isozymes purified from human cord blood erythrocytes by Sepharose-4B-l tyrosine-sulfanilamide affinity gel chromatography.
Materials and methods
Chemicals
Sepharose 4B, protein assay reagents and 4-nitrophenylacetate were obtained from Sigma-Aldrich Co. (Sigma-Aldrich Chemie GmbH Export Department Eschenstrasse 5, 82024 Taufkirchen, Germany). All other chemicals were analytical grade and obtained from Merck (Merck KGaA Frankfurter strasse 250, D 64293 Darmstadt Germany).
Purification of hbcCA isozymes from human cord blood by affinity chromatography
The purification of the two hcbCA isozymes from human cord blood was performed in a simple single-step method by means of Sepharose-4B-l tyrosine-sulfanilamide affinity gel chromatography. Erythrocytes were purified from fresh human cord blood obtained from the Blood Centre of Erzincan Hospital. From normal pregnant women ad term, following the birth by caesarian section or normal delivery, 2 mL of umbilical cord blood was withdrawn by syringe just before the separation placenta and transferred into K3EDTA anticoagulated blood tubes and mixed. The blood samples were stored at−40°C until analysis. The blood samples were centrifuged at 2250g for 15 min and the plasma and buffy coat were removed. The red cells were isolated and washed twice with 0.9% of NaCl and hemolyzed with 1.5 volumes of ice-cold water. The ghost and intact cells were removed by centrifugation at 14,500g for 30 min at 4°C. The pH of the hemolysate was adjusted to 8.7 with solid Tris. CNBr activated-Sepharose 4B was filtered by Buchner funnel and washing with cold NaHCO3 buffer (0.1 M, pH 10.0). l-Tyrosine by using saturated l-tyrosine solution in the same buffer was coupled to Sepharose 4B-l-tyrosine activated with CNBr. The reaction was completed by stirring with a magnet for 90 min. The affinity gel was obtained by diazotization of sulfanilamide and coupling of this compound to the Sepharose 4B-l-tyrosine. For this purpose sulfanilamide (25 mg) was suspended in 10 mL of ice-cold HCl (1 M), and to the suspension (was added) (75 mg of sodium nitrite 5 mL ice-cold water). After 10 minutes of reaction, the diazotized sulfanilamide was poured to 40 mL of the Sepharose 4B-l-tyrosine suspension. The pH was adjusted to 9.5 with 1 M NaOH. After gentle stirring for 3 h at room temperature, the coupled red Sepharose derivative was washed with 1 L of water and then 200 mL of Tris-sulfate (0.05 M, pH 7.5). The hemolysate was applied to the prepared Sepharose-4B-l tyrosine-sulfanilamide affinity column equilibrated with 25 mM Tris– HCl / 0.1M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22mM Na2SO4 (pH 8.7). The human cord blood carbonic anhydrase (hcbCA I and hcbCA II) isozymes were eluted with 1 M NaCl / 25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa / 0.5 M NaClO4 (pH 5.6), respectively. All procedures were performed at 4°CCitation20–22.
Hydratase activity assay
CA activity was assayed by following the hydration of CO2 according to the method described by Wilbur and AndersonCitation23 described previously by Ozturk Sarikaya (2010Citation24). CO2-hydratase activity as an enzyme unit (EU) was calculated by using the equation (to - tc/tc) where to and tc are the times for pH change of the non-enzymatic and the enzymatic reactions, respectively.
Esterase activity assay
CA activity was assayed according to method of Verpoorte et al.Citation25 described previously by Innocenti et al.Citation26,Citation27 CA activity was determined by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (CHEBIOS UV–VIS). The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL 0.05 M Tris–SO4 buffer (pH 7.4), 1 mL, 3 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution. The inhibitory effects of ampicillin sulfate, ceftriaxone, ceftizoxime, ranitidine were examined. All compounds were tested in triplicate at each concentration used. Different inhibitor concentrations were used. HCA-I enzyme activities were measured for ceftriaxone (0.003–0.033 mM), ceftizoxime (0.017–0.096 mM), ranitidine (0.026–0.053 mM) at cuvette concentrations and HCAII enzyme activities were measured for ampicillin sulfate (0.038–0.095 mM), ceftriaxone (0.003–0.021 mM), ceftizoxime (0.026–0.061 mM) and ranitidine (0.032–0.058 mM) at cuvette concentrations. Control cuvette activity in the absence of inhibitor was taken as 100%. For each inhibitor an Activity (%)-[Inhibitor] graphs were drawn. To determine Ki values, three different inhibitor concentrations were tested. In these experiments, 4-nitrophenylacetate was used as substrate at five different concentrations (0.15–0.75 mM). The Lineweaver-Burk curves were drawnCitation28.
Protein determination
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method using bovine serum albumin as the standardCitation29.
SDS polyacrylamide gel electrophoresis
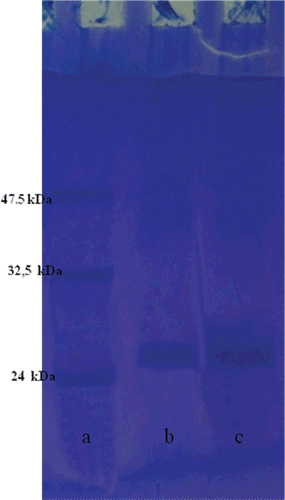
SDS polyacrylamide gel electrophoresis was performed after purification of the cord blood izo enzymes. It was carried out according to Laemmli procedureCitation30. The electrophoretic pattern was photographed ().
Results and discussion
Cord blood is alternative to stem cell source to treat cancer and genetic diseases. Therefore, analyzing enzyme property of cord blood is crucial for organism. Umbilical cord blood contains different enzymes such as glucose 6-phosphate dehydrogenase, glutathione reductase, carbonic anhydrasesCitation31–33. So far, 16 different CA isozymes have been identified in mammals and several novel isozymes have also been identified in non-mammalian vertebrates. Much of the research on the evolution of the structure and function of CA has been focused on the widely distributed CA I and II isozymesCitation33. The purification of the two CA isozymes used here was performed with a simple step method by a Sepharose 4B l-tyrosine sulfanilamide affinity column (). Inhibition effects of drugs derivatives on enzyme activities were tested under in vitro conditions; IC50 values were calculated by Activity (%)-[Inhibitor] graphs and are given in . Ki values were calculated from Lineweaver–Burk graphs and are given in .
Table 1. Purification steps of hcbCA I and II from human cord blood erythrocytes by Sepharose-4B-l tyrosine-sulfanilamide affinity gel chromatography.
Table 2. Inhibitory activities of drugs.
CA catalyzes the conversion of CO2 to H2CO3 at cells and intracellular fluid. Inhibitors of carbonic anhydrases are very important for the treatment of different diseases such as antiglaucoma, diuretics, antiepileptics, in the management of mountain sickness, gastric and duodenal ulcers, neurological disorders or osteoporosisCitation34. CA I and II have been purified many times from different organisms and the effects of various chemicals, pesticides and drugs on its activity have been investigatedCitation11–14. In this study, CA I and II were purified from human cord blood erythrocytes by a simple one step procedure using Sepharose 4B l-tyrosine sulfanilamide affinity column. The activity of the effluents was determined by the hydratase method, with CO2 as substrate and further kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. hcbCA I was purified 142.79-fold with specific activity (1115.22 EU mg/µL) and yield (73%).
Similarly, hcbCA II was purified 730.69-fold with specific activity (5706.67 EU mg/µL) and yield (67%). SDS-PAGE of the enzymes showed a single polypeptide band (). Four inhibitors were prepared and evaluated for the inhibitory effects on hcbCA I and II. The inhibitory effects of drugs were tested in range of 0.001–1000 mM. Among the four inhibitor, three of them (Ceftriaxone, Ceftizoxime, Ranitidine) showed inhibition effects on hcbCA I and all of them on hcbCA II.
Some researchers reported that Ampicillin sulfate showed inhibition on human carbonic anhydrase. IC50 value of this work is 385 µm for HCA I and 774 µm for HCA IICitation35. Other study on rainbow trout erythrocytes pointed out 2160 µm Ki value and 1870 µm IC50 value for carbonic anhydrase. If we check our result, this antibiotic did not prove any inhibition on hcbCA I but showed high inhibition effect on hbcCA II with IC50 value of 56.8 µm and Ki of 83 µm. Ranitidine is H2 blocker and available in prescription strength and over the counter. This drug provides short-term relief as well as over doses of H2 blockersCitation35,Citation36. The studies showed that Ranitidine inhibited cytosolic carbonic anhydrases. IC50 and Ki values determined by that study were 2004 µm and 1770 µmCitation37. IC50 and Ki values of our investigation were 55.5 µm and 54.7 µm for hcbCAI, 66.1 µm and 29.7 µm for hcbCA II. The obtained IC50 values were 1900 µM for hCA-I and 2542 µM for hCA-II for CeftriaxoneCitation38. We found IC50 and Ki values lower than those of Beydemir et alCitation35. For example, IC50’s were 27.1 µm for hcbCAI and 21.0 µm for hcbCA II. Ki’s were 28.5 µm for hcbCAI and 15.8 µm for hcbCA II. Results of Ceftizoxime were found similar to those of Ranitidine.
It is well-known that many chemicals and drugs affect the metabolism by altering enzyme activities and the results may be very dangerousCitation12,Citation13,Citation29,Citation31,Citation35,Citation36. Thus, the drug-enzyme interactions should be well-characterized. Although there is a lack of studies regarding drug-enzyme interactions, the investigations on this issue is increasing day by day. Here, we purified the CA enzymes with simple chromatographic methods and determined inhibitory potentials of commonly used drugs. As seen in , all drugs showed inhibitory action on the enzymes except for ampicillin which had no effect on hcbCA I. Ceftriaxone was the most powerful inhibitor for both enzymes. The reason could be the bulk of the molecule as well as the electronegative groups. The second most potent inhibitor was ranitidine with the Ki values of 54.7 and 29.7 µM for hcbCA I and hcbCA II, respectively. Ceftrizoxime and ampicillin showed rather low inhibitory effect as compared with others. Overall results indicate that these drugs inhibit the enzymes in non-competitive manner. This result is not surprising because molecular structures of the drugs are not similar to physiological substrate of CA enzymes. Also, non-physiological substrates of CA e.g. paranitrophenyl acetate, have quite different molecular structures compared to these drugs investigated here. The drugs are probably interacting with the regions other than the active site, probably with the sulphydril groups as they are quite bulky. As mentioned above, drug-enzyme interaction studies have gained a great interest over the recent years. Our study is thus a contribution to the literature data on the inhibitors or carbonic anhydrase enzymes. Results of our study provide some useful information. First, depending on our data, it is understood that usage of these drugs must be well-determined as they might have serious side effects on CA enzymes which may result in the disruption of acid-base balance and salt transport. Second, our data is very important for design and exploration of novel carbonic anhydrase inhibitors. As known, carbonic anhydrase inhibitors are very important for the treatment of different diseases such as antiglaucoma, diuretics, antiepileptics, mountain sickness, gastric and duodenal ulcers, neurological disorders or osteoporosis, and thus discovery of novel CA inhibitors is very important for medicinal chemists.
Conclusion
HcbCA-I and II isozymes were purified in one-step with high specific activity by the purification method used in this study. Ampicillin sulfate sulfate, Ceftriaxone, Ceftizoxime and Ranitidine at low concentrations showed in vitro inhibitory effects on hcbCA-I (without Ampicillin sulfate) and hcbCA-II activity. Ceftriaxone had the strongest inhibitory effects on hcbCA-I and II, when compared to the Ceftriaxone and others. However, Ceftriaxone and Ranitidine inhibited hCA-I and II at low concentrations. For that reason, uncontrolled usage all of these drugs can cause serious side effects and can be deleterious to health. For this reason, these drugs must be used carefully and the dosage should be closely monitored to decrease side effects.
Declaration of interest
The authors report no conflicts of interest.
References
- Cairo MS, Wagner JE. Placental and/or umbilical cord blood: an alternative source of hematopoietic stem cells for transplantation. Blood 1997;90:4665–4678.
- www.gentlebirth.org
- Hal E, Broxmeyer PD, Franklin O, Smith MD. Cord Blood Hematopoietic Cell Transplantation. Thomas’ Hematopoietic Cell Transplantation, Fourth Edition; 2009.
- http://www.gentlebirth.org/archives/lateClamping.html
- Brinkman R, Margaria R, Meldrum NU, Roughton FJW. The CO2 catalyst present in blood. J Physiol 1932;75 (suppl):3–5.
- Supuran CT. Carbonic anhydrase inhibition/activation: trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr Pharm Des 2010;16:3233–3245.
- Stadie WC, O’Brien H. The catalysis of the hydration of carbon dioxide and dehydration of carbonic acid by an enzyme isolated from red blood cells. J Biol Chem 1933;103:521–529.
- Sentürk M, Gülçin I, Beydemir S, Küfrevioglu OI, Supuran CT. In Vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–499.
- Tashian RE, Hewett-Emmett D, Carter N, Bergenhem NC. Carbonic anhydrase (CA)-related proteins (CA-RPs), and transmembrane proteins with CA or CA-RP domains. Exs 2000;90:105–120.
- Hewett-Emmett D, Tashian RE. Functional diversity, conservation, and convergence in the evolution of the alpha-, beta-, and gamma-carbonic anhydrase gene families. Mol Phylogenet Evol 1996;5:50–77.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Durdagi S, Sentürk M, Ekinci D, Balaydin HT, Göksu S, Küfrevioglu ÖI, Innocenti A, Scozzafava A, Supuran CT. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–1389.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Britta K, Ralf R. β-lactam antibiotics inhibit chloroplast division in a moss (Physcomitrella patens) but not in tomato (Lycopersicon esculentum). J Plant Physiol 1997;150:137–140.
- AHFS Drug Information. American Society of Health-System Pharmacists. 2006.
- Holten KB, Onusko EM. Appropriate prescribing of oral beta-lactam antibiotics. Am Fam Physician 2000;62:611–620.
- Barclay L. CDC issues new treatment recommendations for gonorrhea. Medscape; 2007;56:332–336.
- Laurence B, John L, Keith P. Goodman & Gilman’s The Pharmacological Basis of Therapeutics (11 ed.). McGraw-Hill. 2005.
- Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. j Exp Med 1994;179:493–502.
- Coban TA, Beydemir S, Gülçin I, Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–2261.
- Coban TA, Beydemir S, Gulcin İ, Ekinci D. The inhibitory effect of ethanol on carbonic anhydrase isoenzymes: in vivo and in vitro studies. J Enzyme Inhib Med Chem 2008;23:266–270.
- Abdülkadir Coban T, Beydemir S, Gülcin I, Gücin I, Ekinci D, Innocenti A et al. Sildenafil is a strong activator of mammalian carbonic anhydrase isoforms I-XIV. Bioorg Med Chem 2009;17:5791–5795.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. j Biol Chem 1948;176:147–154.
- Ozturk Sarıkaya SB, Gulcin İ, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des 2010;75:515–520.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. j Biol Chem 1967;242:4221–4229.
- Innocenti A, Beyza Oztürk Sarikaya S, Gülçin I, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–2164.
- Innocenti A, Gülçin I, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–5053.
- Lineweaver H, Burk DJ. The Determination of Enzyme Dissociation Constants Am Chem Soc 1934;56:658–666.
- Bradford M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 1976;72:248.
- Laemmli DK. Clevage of structual proteins during in assembly of the head of bacteriophage T Nature 1970;227:680–683.
- Ceyhun SB, Şentürk M, Yerlikaya E, Erdoğan O, Küfrevioğlu Ö, I Ekinci, D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European Seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharm 2011;32:69–74.
- Ciftçi M, Küfrevioglu OI, Gündogdu M, Ozmen I. Effects of some antibiotics on enzyme activity of glucose-6-phosphate dehydrogenase from human erythrocytes. Pharmacol Res 2000;41:109–113.
- Esbaugh AJ, Tufts BL. The structure and function of carbonic anhydrase isozymes in the respiratory system of vertebrates. Respir Physiol Neurobiol 2006;154:185–198.
- Oztürk Sarikaya SB, Topal F, Sentürk M, Gülçin I, Supuran CT. In vitro inhibition of a-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–4262.
- Ottlecz A, Romero JJ, Lichtenberger LM. Effect of ranitidine bismuth citrate on the phospholipase A2 activity of Naja naja venom and Helicobacter pylori: a biochemical analysis. Aliment Pharmacol Ther 1999;13:875–881.
- Beydemir S, CIftçi M, Ozmen I, Buyukokuroglu ME, Ozdemir H, Küfrevioglu OI. Effects of some medical drugs on enzyme activities of carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. Pharmacol Res 2000;42:187–191.
- Balaydin HT, Soyut H, Ekinci D, Göksu S, Beydemir S, Menzek A, Sahin A. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. j Enzyme Inhib Med Chem 2011; (doi:10.3109/14756366.2011.574131).
- Ekinci D, Beydemir S, Alim Z. Some drugs inhibit in vitro hydratase and esterase activities of human carbonic anhydrase-I and II. Pharmacol Rep 2007;59:580–587.