Abstract
Carbonic anhydrase inhibitors (CAIs) are a class of pharmaceuticals used as antiglaucoma agents, diuretics and antiepileptics. Thus, discovery of novel CAIs has become of great importance in the recent years. In the current study, in vitro and in vivo inhibition effects of benzodiazepine drugs, diazepam and midazolam, on human erythrocytes carbonic anhydrase I and II isozymes were investigated. After purification of the isoenzymes, in vitro inhibition assays were performed and Ki values were determined to be of 141.5 μM and 40.7 μM for hCA I and of 5.11 μM and 0.58 μM against hCA II by the esterase activity assay, respectively. The drugs showed strong inhibitory effects on hCA II, in the same range as the clinically used sulphonamide acetazolamide. For in vivo studies, five adult male New Zealand White rabbits (3–4.2 kg) were selected for intravenous administrations of the drugs (2 mg/kg and 0.2 mg/kg body weight, respectively). The enzyme was significantly inhibited by 2 mg/kg diazepam (p < 0.05), and 0.2 mg/kg midazolam (p < 0.05) for up to 30 min following intravenous administration.
Introduction
Benzodiazepine drugs have been used for many years as intravenous sedation agents. They are widely used in medicine, for their anxiolytic and amnestic properties. Diazepam, a benzodiazepine, has been commonly used for the last 45 years. An intra-lipid preparation of diazepam reduces the irritant effects of the propylene glycol component in instruments used for injectable diazepam. Midazolam has recently been introduced and has several claimed advantages over diazepam, primarily faster onset and recovery as well as enhanced amnestic qualities. The pharmacology of these drugs is well documentedCitation1,Citation2. Diazepam and midazolam are often administered to patients during general anaesthesia or for sedation of critically ill patientsCitation3,Citation4. Although these benzodiazepines are characterized by relatively minor alterations of hemodynamic variables, they decrease systemic blood pressure in humans and animalsCitation3–5. These actions result from various effects on different target tissues, including the central and autonomic nervous systems, vascular smooth muscle and cardiac muscle. Therefore, the direct effects of benzodiazepines on myocardial contractility are difficult to study in vivo. The negative inotropic effects of diazepam and midazolam have been reported in rat isolated heart preparationsCitation6.
Carbonic anhydrase (EC 4.2.1.1., CA) has been a well-characterized pH regulatory enzyme in most tissues including erythrocytesCitation7. The CA catalyses the reversible hydration of CO2 to HCO3− and H+. At least 16 CA isozymes have so far been described in mammals, the most active ones as catalysts for carbon dioxide hydration being CA II and CA IX8–10. The first one is primarily found in red blood cells but also in many other secretory tissues of the gastrointestinal tract, kidneys, lungs, eye, central nervous system (CNS), etc.Citation8,Citation10, whereas the second one is a tumour-associated isoformCitation10–12. Other CA isoforms are found in a variety of tissues where they participate in several important biological processes such as acid-base balance, respiration, carbon dioxide and ion transport, bone resorption, ureagenesis, gluconeogenesis, lipogenesis and electrolyte secretionCitation9,Citation13.
In this paper, we report that midazolam and diazepam show relevant inhibition properties against human CA I, II isoforms. We also report on an in vivo study conducted on New Zealand White rabbits treated with the drugs. The CA inhibitors are used for several purposes, in particular for the treatment of glaucoma, epilepsy and as diuretics or antitumour agents/diagnostic toolsCitation7,Citation10. Also these benzodiazepine drugs are widely used in anaesthesiology and thus we chose these drugs to test. Therefore, this study demonstrates that midazolam and diazepam are active carbonic anhydrous inhibitors (CAIs) and it is required to perform further studies to determine whether or not they might be used as antiglaucoma, antimalaria and anticancer drugsCitation8,Citation12–16.
Materials and methods
Sepharose 4B, protein assay reagents, 4-nitrophenylacetate were obtained from Sigma-Aldrich Co. All other chemicals were analytical grade and obtained from Merck. The drugs were obtained from the medical faculty of Atatürk University.
Purification of carbonic anhydrase isozymes from human erythrocytes by affinity chromatography
The purification of the enzymes was performed according to the literature methodsCitation10,Citation17–19.
Hydratase activity assay
Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and Anderson. The CO2-hydratase activity as an enzyme unit (EU) was calculated by using the equation (t0−tc/tc), where t0 and tc are the times for pH change of the non-enzymatic and the enzymatic reactions, respectivelyCitation20,Citation21.
Esterase activity assay
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (CHEBIOS UV-VIS) according to the method described by Verpoorte et al. 21. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL 0.05 M Tris-SO4 buffer (pH 7.4), 1 mL 3 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution.
In vitro inhibition study
The inhibitory effects of midazolam and diazepam were examined. All compounds were tested in triplicate at each concentration used. Different inhibitor concentrations were used. The hCA I enzyme activities were measured for midazolam (10–49.5 μM) and diazepam (28–130.4 μM) at cuvette concentrations and hCA II enzyme activities were measured for midazolam (0.15–2.75 μM) and diazepam (0.65–10.33 μM) at cuvette concentrations. Control cuvette activity in the absence of inhibitor was taken as 100%. For each inhibitor, Activity (%)-[Inhibitor] graphs were drawn. To determine Ki values, three different inhibitor concentrations were tested. In these experiments, 4-nitrophenyl acetate was used as substrate at five different concentrations (0.15–0.75 mM)Citation22.
In vivo inhibitory study
Five adult male New Zealand White rabbits (3–4.2 kg) were selected for intravenous administration of diazepam and midazolam (2 and 0.2 mg/kg body weight, respectively). Blood samples (10 × 0.5 mL) were taken from each rabbit prior to diazepam and midazolam administration as well as at 10, 30 min, 1, 2, 4, 6 and 24 h intervals thereafter. The blood was placed into test tubes containing Ethylenediaminetetraacetic acid (EDTA) (2 mM) and subjected to centrifugation at 2500 × g for 15 min at 4°C (HERMLE Z383K). The erythrocyte pellet was washed three times with cold 0.16 M KCl and the supernatant was discarded. One volume of erythrocyte pellet was suspended in five volumes of ice water to give an erythrocyte haemolysate. The CA activity was determined colourimetrically as described aboveCitation19,Citation23.
Statistical analysis
Statistical analysis was performed using the Statistica 6.0 statistical package. For determination of enzyme activities in drug-treated rabbits, 2-way analysis of variance (ANOVA) was used. In twin comparisons, the LSD test was performed and p-values were obtained. Values are presented as numbers (% activity), mean ± SD. A p-value < 0.05 was considered statically significantCitation24.
Protein determination
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as a standardCitation25.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli procedureCitation26.
Results and discussion
There are a few studies existing in the literature on the interactions of anaesthetic drugs and carbonic anhydrase enzymes. The interactions of ceftriaxone, morphine and imipenem with two human isozymes, CA I–II, has only recently been investigatedCitation27, evidencing some low milimolar/micromolar inhibitors as well as the possibility to design isozyme selective CAIs. Indeed, the inhibition profile of various isozymes with this class of agents is very variable, with inhibition constants ranging from the millimolar to the sub-micromolar level for many simple chlorbenzyl-containing moleculesCitation27. Thus, it seemed reasonable to us to extend the previous studiesCitation27, including this study on benzodiazepine drugs with common clinical applications.
We report here the first study on the inhibitory effects of midazolam and diazepam of type on the esterase activity of hCA I and II. The acetazolamide (AZA) CAI acetazolamideCitation14 was used as a negative control in our experiments, and for comparison purposes. The previous studies by Coban et al.18, investigated morphine by using a Verpoorte method, 4-nitrophenyl acetate esterase assay for monitoring CA inhibitionCitation21. The data in show the following regarding inhibition of hCA I and II with midazolam, diazepam and AZA, by an esterase assayCitation21, with 4-nitrophenyl acetate (4-NPA) as the substrate:
Table 1. Summary of purification procedure for human carbonic anhydrase izoenzymes (hCA I and hCA II) by a sepharose-4B-aniline-sulphanilamide affinity column chromatography.
Table 2. The hCA I and II inhibition data with diazepam, midazolam and acetazolamideCitation8,Citation23, by an esterase assay with 4-nitrophenyl acetate as substrate.
Against the slow cytosolic isozyme hCA I, diazepam behaves as a weak inhibitor, with a Ki value of 141.5 μM. Midazolam showed better inhibitory activity when compared to the previously mentioned diazepam, with a Ki value of 40.7 μM (). Thus, the nature of the group in ortho’- to the F moiety strongly influences hCA I inhibitory activity. The AZA is also a medium-weak CAI in this assay and substrate against hCA I (Ki of 36.2 μM). Kinetic investigations (Lineweaver Burk plots, data not shown) indicate that similar to sulphonamides, salicylic acid derivatives, antioxidant phenols, some metal and inorganic anionsCitation10,Citation14,Citation27–35, all the investigated drugs act as non-competitive inhibitors with 4-NPA as substrate, that is they bind in different regions of the active site cavity when compared to the substrate. However, the binding site of 4-NPA is unknown, but it is presumed to be in the same region as that of CO2, the physiological substrate of this enzymeCitation27,Citation33,Citation36.
A better inhibitory activity has been observed with midazolam and diazepam for the inhibition of the rapid cytosolic isozyme hCA II (). Diazepam showed moderate hCA II inhibitory activity with a Ki value of 5.11 μM (), whereas midazolam was quite an effective hCA II inhibitor, with a Ki value of 0.58 μM, (). Structure-activity relationship is thus quite sharp for these drugs, with F moiety in ortho and ortho’ is already a sub-micromolar hCA II inhibitor. It must be stressed that Kis measured using the esterase method are always in the micromolar range because hCA I and II are weak esterasesCitation15–36.
However, it is critically important to explore further classes of potent CAIs in order to detect compounds with a different inhibition profile when compared to the sulphonamides and their bioisosteres and to find novel applications for the inhibitors of these widespread enzymes.
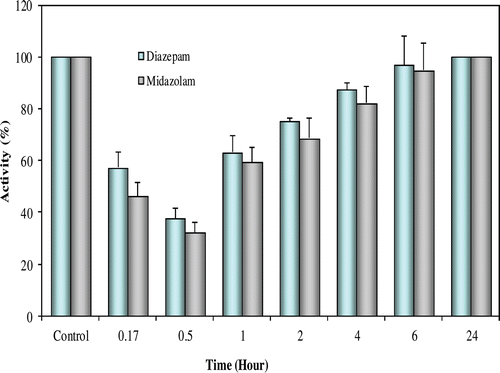
Five adult male New Zealand White rabbits (3–4.2 kg) were used for intravenous administration of the drugs (2 mg/kg and 0.2 mg/kg body weight, respectively) in in vivo studies. The enzyme was significantly inhibited by 2 mg/kg diazepam (p < 0.05), and 0.2 mg/kg midazolam (p < 0.05) for up to 30 min following intravenous administration ().
Figure 1. The in vivo effects of diazepam and midazolam on rabbit erythrocytes CA activity. Five adult male New Zealand white rabbits (3–4.2 kg) were selected for intravenous administration of diazepam and midazolam (2 and 0.2 mg/kg body weight, respectively). Blood samples (10 × 0.5 ml) were taken from each rabbit prior to diazepam and midazolam administration as well as at 0.17, 0.5, 1, 2, 4, 6 and 24 h intervals thereafter.
Conclusions
Midazolam and diazepam drugs affected the activity of CA isozymes due to the presence of the different functional groups (Cl, F and CH3) present in their aromatic scaffold. Our findings here indicate thus another class of possible CAIs of interest, in addition to the well-known sulphonamides/sulphamates/sulphamides, the benzyl, p-chlorobenzyl and o-fluorobenzoyl bearing bulky ortho moieties in their molecules.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Greenblatt DJ, Shader RI, Abernethy DR. Drug therapy. Current status of benzodiazepines. N Engl J Med 1983;309:354–358.
- Kanto JH. Midazolam: the first water-soluble benzodiazepine. Pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Pharmacotherapy 1985;5:138–155.
- Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology 1985;62:310–324.
- Samuelson PN, Reves JG, Kouchoukos NT, Smith LR, Dole KM. Hemodynamic responses to anesthetic induction with midazolam or diazepam in patients with ischemic heart disease. Anesth Analg 1981;60:802–809.
- Jones DJ, Stehling LC, Zauder HL. Cardiovascular responses to diazepam and midazolam maleate in the dog. Anesthesiology 1979;51:430–434.
- Reves JG, Kissin I, Fournier S. Negative inotropic effects of midazolam. Anesthesiology 1984;60:517–518.
- Parui R, Gambhir KK, Mehrotra PP. Changes in carbonic anhydrase may be the initial step of altered metabolism in hypertension. Biochem Int 1991;23:779–789.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–9105.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995;64:375–401.
- Ekinci D, Cavdar H, Talaz O, Sentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–3563.
- Sentürk M, Gülçin I, Dastan A, Küfrevioglu OI, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–3211.
- Sentürk M, Gülçin I, Beydemir S, Küfrevioglu OI, Supuran CT. In Vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–499.
- Durdagi S, Sentürk M, Ekinci D, Balaydin HT, Göksu S, Küfrevioglu ÖI et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–1389.
- Ekinci D, Beydemir S, Küfrevioglu OI. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. j Enzyme Inhib Med Chem 2007;22:745–750.
- Coban TA, Beydemir S, Gülçin I, Ekinci D. The effect of ethanol on erythrocyte carbonic anhydrase isoenzymes activity: an in vitro and in vivo study. J Enzyme Inhib Med Chem 2008;23:266–270.
- Aksakal E, Ceyhun SB, Erdogan O, Ekinci D. Acute and long-term genotoxicity of deltamethrin to insulin-like growth factors and growth hormone in rainbow trout. Comp Biochem Physiol c Toxicol Pharmacol 2010;152:451–455.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–154.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934;57:658–666.
- Ekinci D, Ceyhun SB, Aksakal E, Erdogan O. IGF and GH mRNA levels are suppressed upon exposure to micromolar concentrations of cobalt and zinc in rainbow trout white muscle. Comp Biochem Physiol c Toxicol Pharmacol 2011;153:336–341.
- Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli DK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Renzi G, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: topical sulfonamide antiglaucoma agents incorporating secondary amine moieties. Bioorg Med Chem Lett 2000;10:673–676.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Ceyhun SB, Sentürk M, Ekinci D, Erdogan O, Ciltas A, Kocaman EM. Deltamethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:215–223.
- Sentürk M, Ekinci D, Göksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2011;:.
- Ceyhun SB, Senturk M, Erdogan O, Kufrevioglu OI. In vitro and in vivo effects of some pesticides on carbonic anhydrase enzyme from rainbow trout (oncorhynchus mykiss) gills. Pestic Biochem Phys 2010;97:177–181.
- Cakmak R, Durdagi S, Ekinci D, Sentürk M, Topal G. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett (In Press).
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Oztürk Sarikaya SB, Topal F, Sentürk M, Gülçin I, Supuran CT. In vitro inhibition of a-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–4262.
- Akkemik E, Senturk M, Ozgeris FB, Taser P, Ciftci M. In vitro effects of some drugs on human erythrocyte glutathione reductase. Turkish J Med Sci 2011;41:235–241.
- Alp C, Ekinci D, Gültekin MS, Sentürk M, Sahin E, Küfrevioglu OI. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–4474.
