Abstract
In this study; sheep carbonic anhydrase-II (SCA-II) (E.C: 4.2.1.1) was purified from sheep liver and in vitro effects of heavy metals on the enzyme was examined. SCA-II isozyme was purified with about 203 purification fold, a specific activity of 2320 EU/mg-protein and a yield of 72 by using Sepharose-4B-L tyrosine-sulfanilamide affinity gel chromatography. Purity of the SCA-II enzyme was verified by SDS-PAGE technique and subunit molecular mass of the enzyme was found as 29 kDa. In addition to this, inhibitory effects of some metal ions on the enzyme were examined. In this study, sheep liver tissue was chosen; because the liver is an organ in which metal wastes of air, water and food are collected and it is easy to obtain the liver tissue. Because of the very important duties of CA enzyme on living beings, the effect of metals on the CA enzyme was investigated.
Keywords::
Introduction
CA enzyme, which exists commonly in living organisms, has various izoenzymes according to conditions and necessities of the medium. A new type of isoenzyme has been discovered almost for each year and now it has 16 isoenzymes. It is one of the most studied enzymes, and CA-I and CA-II are the most common isoenzymes. Carbonic anhydrases described in last 5 years has an increasing physiological and biochemical propertiesCitation1. Seven different CA isoenzymes have been characterized from vertebratesCitation2.
The carbonic anhydrases are wide family of zinc, including enzymes, which usually provide maintenance of pH homeostasis in the human body, catalyzing the reversible hydration of carbon dioxide in a two-step reaction to yield bicarbonate and protonsCitation3–5,Citation6. Sixteen isoenzymes have been defined up to now, that differ in their subcellular place, catalytic activity and emotional different types of inhibitors. Some of these enzymes are cytolosolic (CA I, CA II, CA III, CA VII and CA XIII), others are membrane bound (CA IV, CA IX, CA XII and CA XIV), two are mitochondrial (CA VA and CA VB), and one is secreted in saliva (CA VI). It has been clarified that there is no expression of CA XV in human and other primates, but it is plentiful in rodents and other higher vertebratesCitation7,Citation8.
Carbonic anhydrase enzyme can be involved in metaloenzyme family encoded evolutionarily in at least five different gene families in prokaryotes and eukaryotes; α-class CAs (found in bacteria, algae, green plants and vertebrates), β-class CAs (mainly in bacteria, algae), γ-class CAs (in some arkeas and bacteria), δ-class CAs in marinated diatoms (thalassiosira weisflogii) and ϵ class CAs in cyano bacteria and bacteria. Until now, many carbonic anhydrase isoenzymes have been isolated from mammals. There are three different classes including α, β and γ class (in 1994) (and then ϵ class was isolated). These three classes do not have any significant similarity. Despite the structural differences, all three classes share one common property: zinc atom for catalysis in active site. Carbonic anhydrase is a member of zinc metaloenzyme family, and it has a structure that single peptide chain in the active site coordinated with Zn2+ ionCitation9.
Heavy metals can change the activity of enzymes by binding to functional groups or displacing the metal associated with enzymeCitation10. Rapid industrialization, poor emission control, unorganized urbanization, and increased motor traffic cause an increase of heavy metal concentration in the environment. Plants and other organisms in the habitat are affected by the contamination of soil, water, and atmosphere with heavy metalsCitation11,Citation12.
In the current study, we aimed at purifying the carbonic anhydrase from sheep liver and examining in vitro effects of heavy metals (Pb2+, Cu2+, Fe2+, Cr2+, Al3+, Ni2+, Mn2+, Cd2+, Zn2+, Mg2+) on the enzyme activity.
Materials and methods
Chemicals
Sepharose 4B, protein assay reagents and 4-nitrophenylacetate were obtained from Sigma-Aldrich Co (Germany). The other chemicals were of analytical grade and obtained from Merck (Germany).
Hydratase activity assay
Carbonic anhydrase hydratase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and AndersonCitation13. CO2-hydratase activity was calculated as an enzyme unit (EU) by using the equation (to-tc/tc) where t0 and tc are the times for pH change of the non-enzymatic and the enzymatic reactions, respectively.
Esterase activity assay
The esterase activity was assayed by following the change in absorbance of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion at 348 nm over a period of 3 min at 25°C using a spectrophotometer (BECKMAN COULTER UV-VIS) according to the method described by Verpoorte et al.Citation14. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL 0.05 M Tris-SO4 buffer (pH 7.4), 1.0 mL 3 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution.
Protein determination
During each purification step, protein determination was performed spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as the standardCitation15.
Purification of carbonic anhydrase II from sheep liver by affinity chromatography
Sheep liver was obtained from Erzurum Ozbeyli Municipal Slaughterhouse and stored at −80°C until usage. Twenty grams of thawed liver was chopped into small pieces with a knife. The fragments were homogenized with liquid nitrogen and taken into 1.5–2 volumes of buffer solution (50 mM Tris-HCl, pH 7.4). The homogenate was filtered through four layers of cheesecloth and then centrifuged at 18,000 × g for 1 h. The pellet (mitochondria and cell debris) was discarded. The pH of the supernatant was adjusted to 8.7 with solid Tris. The homogenate was applied to the prepared Sepharose 4B L-tyrosine sulfanilamide affinity column equilibrated with 25 mM Tris-HCl/0.1M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH: 8.7). The SCA-II enzyme was eluted with 0.1 M NaCH3COO/0.5 M NaClO4 (pH 5.6). Purified SCA-II enzyme was dialyzed for 3 h against 0.05 M Tris-SO4/1 mM 2-mercaptoethanol (pH 7.4) ().
Table 1. Summary of purification procedure for sheep liver CA II.
SDS polyacrylamide gel electrophoresis
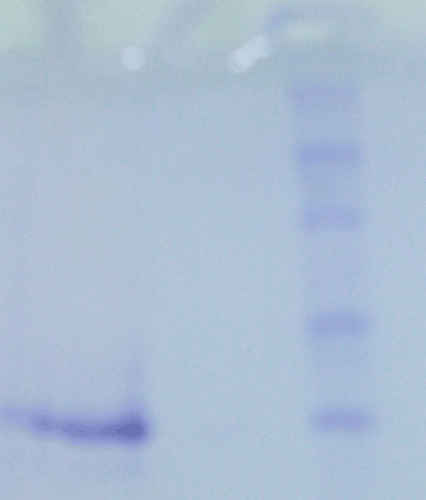
After the purification steps, SDS polyacrylamide gel electrophoresis was performed to verify enzyme purity. It was carried out in 10 and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli procedure. A 20 µg sample was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coommassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then detained with several changes of the same solvent without dyeCitation16 ().
In vitro inhibition effect of heavy metals on the enzyme activity
In this study, SCA-II was purified from sheep liver, and the activities of the effluents were defined by the hydratase method, in which CO2 was used as substrate. Other kinetic studies were performed by using the esterase activity method and 4-nitrophenyl acetate (NPA) as substrate. For Zn2+, Cu2+, Al3+ and Cd2+, inhibition studies were performed by using esterase activity, whereas hydratase activity was used for Zn2+, Cu2+, Co2+, Cd2+ and Ni2+. All compounds were tested in triplicate at each concentration. Five different inhibitor concentrations were treated. In , we reported Ki values at three different inhibitor concentrations by using esterase activity method. In hydratase activity assay, inhibitor concentrations were calculated in and IC50 values were obtained from inhibitor concentration and Activity%—(Inhibitor) graphs.
Table 2. Ki values and inhibition types for four metal ions of SCA II.
Table 3. IC50 values of inhibitors for SCA-II hydratase activity.
Results and discussion
In the current study, we examined the effects of some metals on liver CA II enzyme activity of sheep. And as we know, sheep liver is an important source of nutrient for people. The aim of this study is purifying SCA-II from unstudied tissue of sheep liver and examining the effects of heavy metals on enzyme activity by using both hydratase and esterase activity. In this study, the liver tissue was chosen; because the liver is an organ in which metal wastes of air, water and food are collected and it is easy to obtain the liver tissue. Because of the very important duties of CA enzyme on living beings, the effect of metals on the CA enzyme was investigated.
The enzyme has esterase activity besides its hydratase activity, but physiologically hydratase activity is very important. For instance, it plays an important role in the regulation of acid—base balance in the organisms. Where this balance is impaired, for example, in intraocular tension, therapeutic intervention to carbonic anhydrase enzyme activity is a common applied method. In this respect, compounds of carbonic anhydrase inhibitors gain a clinical importanceCitation17.
Carbonic anhydrase which is a widespread metaloenzyme has previously been purified and characterized from many living organisms including animalsCitation18–21. The isozymes of CA play important roles in different tissuesCitation22,Citation23. The similarities of CAs in various sources have been determined from their crystal structuresCitation24. It is known that carbonic anhydrase has been purified many times from different organisms and the effects of various chemicals, pesticides and drugs on its activity have been investigatedCitation25–27. Hundreds of pollutants in the form of metals, acids, bases and other toxic compounds are being added to rivers, seas and the atmosphere, and this causes a situation which results in destruction of the natural balanceCitation25.
Inhibition and activation effects of many chemicals and drugs on carbonic anhydrase activity have been investigated by scientists and reported in the literatureCitation8,Citation28,Citation29. Today, all living things can inevitably be exposed to the effects of different chemical substances. This situation can be arisen from people’s environment like their work environment. Although there is no connection with the atmosphere of such a work environment, people and other organisms are primarily affected by the waste created by people. In particular, the elimination of waste materials from factories in various countries has been made by burying into the ground or releasing to the air. As a result, many toxic substances such as heavy metals, have been passing into the soil, water, plants, and then from there to animals and people. So today, especially enzyme activity studies made by using these substances remains its popularityCitation25,Citation30–32.
Christensen and TuckerCitation33 found that Ag1+, Cd2+, Cu2+ and Zn2+ ions have inhibitory effect on fish CA enzyme. In another study about enzyme inhibition reported by Ozdemir and colleaguesCitation35, the effects of some active cations on the activities of bovine CA and human (HCA-I and HCA-II) enzymes were investigated in vitro. According to this study, Al3+ inhibited the enzymes competitively, and Mn2+, Sr2+, Hg2+, Ni2+, Ca2+ and Cd2+ ions showed non-competitive inhibition.
In another study with fish liver CA, Al3+, Cu2+, Pb2+, Co3+, Ag1+, Zn2+ and Hg2+ were found to show inhibitory actionCitation33. In addition, Co2+ and Hg2+ ions showed inhibition (Ki 3.91 and 1.42, respectively) effects on human CA enzymesCitation32.
Inhibition effects of many substances such as medical drugs, various metals, anions and pesticides have been reported in the literatureCitation20,Citation22,Citation35,Citation36. Many chemicals affect metabolism by changing normal enzyme activity, particularly inhibition of a specific enzymeCitation36,Citation37 and the effects can be dramatic and systemicCitation38. Hence, heavy metals have various toxicological effects on living organisms. For example, it has been reported that heavy metals, such as mercury and cadmium, exert a toxic action in a synergistic fashion with salinityCitation26,Citation38,Citation39. Also, it has been expressed that the binding of heavy metals with membrane transport ligands can alter their catalytic functionCitation26,Citation40. Pb2+, Cu2+, Fe2+, Cr2+, Al3+, Ni2+, Mn2+, Cd2+, Zn2+, Mg2+ were chosen for investigation of their inhibitory effects on CA in this study, and it was important that heavy metals inhibited the enzyme activity at low concentrations. Ki parameters of these metals were determined, and it was found that the metals were potent inhibitors of CA. Lineweaver-BurkCitation41 plots showed that Zn2+, Cu2+, Al3+ inhibited the enzyme uncompetitively and Cd2+ non-competitively.
Soyut et al. examined the inhibitory effects of cobalt, copper, zinc, silver and cadmium on carbonic anhydrase purified from brains of rainbow trout. They founded that cobalt, silver and cadmium inhibited the enzyme competitively, copper inhibited non-competitively, whereas zinc inhibited the enzyme uncompetitivelyCitation42 In another study, Soyut and Beydemir examined the effects of some metals (cobalt, copper, zinc, silver) on the CA activity of rainbow trout liver. Their results showed that cobalt and zinc inhibited the enzyme in a competitive manner and copper and silver inhibited the enzyme in an uncompetitive mannerCitation43. Besides, Soyut and Beydemir examined the effects of metals (Co2+, Zn2+, Cu2+, Cd2+) on the activity of CA from rainbow trout kidney. They found in vitro inhibition of the metals in the order of Co2+> Zn2+> Cu2+> Cd2+> Ag2+44.
Air, water, soil and other natural sources can be contaminated with metal ions because of technological reasons. These metal ions entering the metabolism through respiration, water and foods show toxic effects in the body. In addition, the metal ions thrown as industrial waste dirt streams and give harm to the plants and animals. Received metal ions affect all living organs in the body, especially in developing children shows itself as mental retardation, learning disabilities and physical growth delay.
In this study, Zn2+, Cu2+, Cd2+ and Al3+ were chosen to investigate their inhibitory effects on SCA II. Ki parameters of these metals were determined. Metal ions inhibited the enzyme activity at low concentrations. It is clear that Cu2+ and Al3+ are the most potent inhibitors for SCA-II enzyme. Ki shows that metals inhibit SCA-II in uncompetitive manner (). According to Ki values, the best inhibitor for SCA-II is aluminium. Metal-ligand interactions are stronger especially in aqueous media. The mechanism of toxicological effects of the metals is probably due to the interactions between negative charged amino acids and the metals or the interaction of the metals with other amino acids around the active siteCitation45,Citation46. We investigated the effects of metals on the SCA-II isoenzyme by measuring hydratase activity occurring in physiological conditions and esterase activity in vitro conditions. We saw that Zn2+, Cu2+, Cd2+ ions inhibited the enzyme either in vitro, or physiological environment. Co2+ and Ni2+ ions inhibited the enzyme only in the physiological environment, Al3+ ion found to inhibit the enzyme in vitro. The majority of heavy metals in this study showed inhibitory effect in both physiological and in vitro conditions.
According to these results, SCA-II enzyme, which is available nearly at all living beings and very important at metabolic events, was purified from sheep liver. And then the effects of some metals were examined, and it was found that the metals inhibited the enzyme even at very low concentrations. As many harmful materials, some metals entrance to the body and their accumulation in it may be the reason of stress factor. The people, especially who work and live at the industrial regions and other living beings, are under the threat of toxic. Because of this, it is possible to see problems at the metabolism of living beings which are exposed to these harmful materials.
Declaration of interest
The authors report no conflicts of interest.
References
- Tripp BC, Smith K, Ferry JG. Carbonic anhydrase: New insights for an ancient enzyme. J Biol Chem 2001;276:48615–48618.
- Tashian RE, Hewett-EmmetD, Venta PJ. Diversity and Evolution in the Carbonic Anhydrase Gene Family. Bèotr F, Gros G, Storey BT (eds.). VCH Verlagsgesellschaft, Weinheim, 1991; pp. 151–161.
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- SupuranCT, Scozzafava A. Applications of carbonic anhydrase inhibitors and activators in therapy. Expert Opin Ther Pat 2002;12:217–242.
- Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995;64:375–401.
- Ozensoy O, Arslan O, Sinan SO. A new method for purification of carbonic anhydrase isozymes by affinity chromatography. Biochemistry Mosc 2004;69:216–219.
- Hilvo M, Tolvanen M, Clark A, Shen B, Shah GN, Waheed A et al. Characterization of CA XV, a new GPI-anchored form of carbonic anhydrase. Biochem J 2005;392:83–92.
- Kayaalp SO (ed.). Rasyonel Tedavi Yönünden Tıbbi Farmakoloji, 10. Baskı, Hacettepe Taş, Ankara. 2002.
- Viarengo A. Biochemical effects of trace metals. Mar Pollut Bull 1985;16:153–8.
- Monaci F, Moni F, Lanciotti E, Grechi D, Bargagli R. Biomonitoring of airborne metals in urban environments: New tracers of vehicle emission, in place of lead. Environ Pollut 2000;107:321–327.
- Onder S, Dursun S. Air borne heavymetal pollution of Cedrus libani (A. Rich.) in the city centre of Konya (Turkey). Atmos Envıron 2006;40:1122–1133.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–154.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- LaemmLi DK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 1970;227:680–683.
- Puscas I, Puscas C, Coltau M, Baican M and Domuta G. The serum of Carcinoma Patients Powerfully Activates Carbonic Anhydrase II. Exp Oncol 2000;22:162–164.
- Krungkrai SR, Suraveratum N, Rochanakij S, Krungkrai J. Characterisation of carbonic anhydrase in Plasmodium falciparum. Int J Parasitol 2001;31:661–668.
- Böttcher K, Waheed A, Sly WS. Membrane-associated carbonic anhydrase from the crab gill: Purification, characterization, and comparison with mammalian CAs. Arch Biochem Biophys 1994;312:429–435.
- Beydemir S, Gülçin I. Effects of melatonin on carbonic anhydrase from human erythrocytes in vitro and from rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2004;19:193–197.
- Zhenyan Y, Liping X, Seunghwan L, Rongqing ZA. Novel carbonic anhydrase fromthe mantle of the pearl oyster (Pinctada fucata). Comp Biochem Physiol B 2006;143:190–194.
- Bülbül M, Hisar O, Beydemir S, Ciftçi M, Küfrevioglu OI. The in vitro and in vivo inhibitory effects of some sulfonamide derivatives on rainbow trout (Oncorhynchus mykiss) erythrocyte carbonic anhydrase activity. J Enzyme Inhib Med Chem 2003;18:371–375.
- Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: Sulfonamides as antitumor agents? Bioorg Med Chem 2001;9:703–714.
- Huang S, Xue Y, Sauer-Eriksson E, Chirica L, Lindskog S, Jonsson BH. Crystal structure of carbonic anhydrase from Neisseria gonorrhoeae and its complex with the inhibitor acetazolamide. J Mol Biol 1998;283:301–310.
- Celik I, Camas H, Arslan O, Kufrevioglu OI. The effects of some pesticides on human and bovine erythrocyte carbonic anhydrase enzyme activities in vitro. J Envıron Scı Heal A 1996;31:2651–2657.
- Vitale AM, Monserrat JM, Castilho P, Rodriguez EM. Inhibitory effects of cadmium on carbonic anhydrase activity and ionic regulation of the estuarine crab Chasmagnathus granulata (Decapoda, Grapsidae). Comp Biochem Physiol C, Pharmacol Toxicol Endocrinol 1999;122:121–129.
- Gervais MR, Tufts BL. Characterization of carbonic anhydrase and anion exchange in the erythrocytes of bowfin (Amia calva), a primitive air-breathing fish. Comp Biochem Phys 1999;23A:343–350.
- Alp C, Ekinci D, Gültekin MS, Sentürk M, Sahin E, Küfrevioglu OI. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–4474.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Norris DO, Camp JM, Maldonado TA, Woodling JD. Some aspects of hepatic function in feral brown trout, Salmo trutta, living in metal contaminated water. Comp Biochem Physiol C Toxicol Pharmacol 2000;127:71–78.
- Blasco J, Puppo J. Effect of heavy metals (Cu, Cd and Pb) on aspartate and alanine aminotransferase in Ruditapes philippinarum (Mollusca: Bivalvia). Comp Biochem Physiol C, Pharmacol Toxicol Endocrinol 1999;122:253–263.
- Ekinci D, Beydemir S, Küfrevioglu OI. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 2007;22:745–750.
- Christensen GM, Tucker JH. Effects of selected water toxicants on the in vitro activity of fish carbonic anhydrase. Chem Biol Interact 1976;13:181–192.
- Ozdemir H, Kufrevioglu OI, Nalbantoglu B, Demir N and Bakan N. The İnhibition Kinetics of Bovine and Human Erythrocyte Carbonic Anhydrase Isoenzymes with Some Active Cations. Turk J Med Sci 1997;27:559–563.
- Innocenti A, Antel J, Wurl M, Vullo D, Firnges MA, Scozzafava A et al. Carbonic anhydrase inhibitors. Inhibition of isozymes I, II, IV, V and IX with complex fluorides, chlorides and cyanides. Bioorg Med Chem Lett 2005;15:1909–1913.
- Casini A, Scozzafava A, Mincione F, Menabuoni L, Supuran CT. Carbonic anhydrase inhibitors: Synthesis of water soluble sulfonamides incorporating a 4-sulfamoylphenylmethylthiourea scaffold, with potent intraocular pressure lowering properties. J Enzyme Inhib Med Chem 2002;17:333–343.
- Casini A, Mincione F, Vullo D, Menabuoni L, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors with strong topical antiglaucoma properties incorporating a 4-(2-aminopyrimidin-4-yl-amino)-benzenesulfonamide scaffold. J Enzyme Inhib Med Chem 2002;17:9–18.
- Christensen GM, Olson D, Riedel B. Chemical effects on the activity of eight enzymes: A review and a discussion relevant to environmental monitoring. Environ Res 1982;29:247–255.
- Hochster RM, Kates M, Quastel JH. Metabolic inhibitors. Vols. 3 and 4. New York: Academic Press 1973; pp. 66–82, 71–89.
- Rainbow PS, Dallinger R. Metal uptake, regulation and excretion in freshwater invertebrates. In: Dallinger R, Rainbow PS (eds.). Ecotoxicology of metals in invertebrates. Boca Raton, FL: Lewis Publishers; 1993. pp. 119–131.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;57:685.
- Soyut H, Beydemir S, Hisar O. Effects of some metals on carbonic anhydrase from brains of rainbow trout. Biol Trace Elem Res 2008;123:179–190.
- Soyut H, Beydemir S. Purification and some kinetic properties of carbonic anhydrase from rainbow trout (Oncorhynchus mykiss) liver and metal inhibition. Protein Pept Lett 2008;15:528–535.
- Soyut H and Beydemir S. The impact of heavy metals on the activity of carbonic anhydrase from rainbow trout (Oncorhynchus mykiss) kidney. Toxicol Ind Health 2011, DOI:10.1177/0748233711410914.
- Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Ozdemir H, Uguz MT. In vitro effects of some anaesthetic drugs on lactoperoxidase enzyme activity. J Enzyme Inhib Med Chem 2005;20:491–495.