Abstract
Few series of novel 4-chloro-2-mercaptobenzenesulfonamides have been synthesized by the reactions of N-(benzenesulfonyl)cyanamide potassium salts 7–15 with corresponding hydrazinecarbodithioic acid esters, 1-substituted carbothioic acid hydrazides, methyl 3-aminothiophene-2-carboxylate, methyl 2-aminobenzoate, 2-aminophenol or 2-aminothiophenol. The synthesized compounds (16–49) were screened in vitro for their antibacterial activity. Some of the tested compounds 16, 17, 23, 24, 31, 32 and 48 showed the promising activity against many of anaerobic Gram-positive bacteria strains.
Introduction
The aryl/heteroaryl sulfonamides constitute an important class of compounds with several types of biological activitiesCitation1. For many years, 2-mercaptobenzenesulfonamide derivatives (MBSAs) are of interest because of the various biological propertiesCitation2–12. Depending on the modification of their chemical structure, they exhibit antitumour activityCitation2–7, HIV-antiviralCitation8–10 or inhibiting carbonic anhydrase CA both cytosolic CA I, II and transmembrane cancer-associated IX and XII isoformsCitation11,Citation12.
Through investigations presented, we have also joined the trend of research on the antibacterial properties of sulfonamides. In particular, the substituted 1,3,4-thiadiazoles have attracted considerable attention due to their wide-range biological activities, including anti-bacterial, anti-tuberculosis, anaesthetic, anti-inflammatory, anti-thrombotic, anti-convulsant, anti-hypertensive, anti-ulcer, anti-viral, anti-HIV-1 or anti-cancer activitiesCitation13–20. Therefore, for the initial chemical studies, we synthesized a series of novel 2-benzylthio-N-(1,3,4-thiadiazol-2-yl)benzenesulfonamide derivatives and determined their anti-bacterial activity. For further investigation, we applied various nucleophiles involving methyl 3-aminothiophene-2-carboxylate, methyl 2-aminobenzoate, 2-aminophenol or 2-aminothiophenol with N-(benzenesulfonyl)cyanamide potassium salts to obtain the corresponding cyclization products and to investigate their anti-bacterial properties.
Materials and methods
Chemistry
The following instruments and parameters were used: melting points Stuart SMP3 apparatus; IR spectra: KBr pellets, 400–4000 cm−1 Thermo Mattson Satellite FTIR spectrometer; 1H NMR: Varian Gemini 200 apparatus at 200 MHz; chemical shifts are expressed at δ values relative to Me4Si as standard. The results of elemental analyses for C, H, and N were in agreement with the calculated values within ± 0.4% range. Compounds 29, 34–36 were obtained in accordance with the previously described proceduresCitation9. The corresponding hydrazinecarbodithioic acid esters were prepared according to the known methodsCitation21,Citation22.
Methyl 3-amino-6-chloro-1,1-dioxo-1,4,2-benzodithiazine-7-carboxylate (3): To a suspension of 1 (7.43 g, 22 mmol) in ethanol (38 mL) 25% aqueous ammonium hydroxide solution (3.46 mL, 22.2 mmol) was added dropwise and the reaction mixture was stirred at room temperature for 44 h. The resulting precipitate was filtered off under diminished pressure, washed with ethanol (2 × 1 mL) and dried, to give 3. Yield: 2.95 g, 73%, mp 260°C–263°C. After crystallization from mixture of ethanol and acetonitrile (v/v 10:1), 3 presented mp 268°C–271°C dec.; IR νmax (KBr)/cm−1 3418, 3382, 3306, 3215 (NH), 2960, 2924 (CH3), 1711 (C=O), 1629 (NH), 1303, 1162 (SO2); 1H NMR δ (DMSO-d6) 3.89 (s, 3H, CH3O), 8.12 (s, 1H, H-5), 9.34 (s, 2H, NH2). Anal. Calcd. for C9H7ClN2O4S2: C, 35.24; H, 2.30; N, 9.13. Found: C, 35.08; H, 2.19; N, 9.20%.
5-Chloro-2-(cyanoaminosulfonyl)-4-(methoxy carbonyl) thiophenolate dipotassium salt (5): A stirred suspension of 3 (3.09 g, 10 mmol) and anhydrous K2CO3 (6.91 g) in dry THF (80 mL) was refluxed for 24 h. After cooling to room temperature the suspension was left in a refrigerator overnight. The resulting precipitate was filtered under diminished pressure and dried. The product was separated from a solid K2CO3 by extraction with boiling ethanol (2 × 80 mL). The extracts were chilled to 0°C, and left to stand in a refrigerator overnight. The precipitate was collected by filtration, washed with cold ethanol (2 × 0.5 mL) and dried at temperatures gradually increasing to 105°C, to give 5. Yield: 1.80 g, 68%, mp 219°C–222°C dec.; IR νmax (KBr)/cm−1 3390 (OH), 2953, 2921, 2836 (CH3), 2173 (C≡N), 1720 (C=O), 1280, 1112 (SO2); 1H NMR δ (DMSO-d6) 3.73 (s, 3H, OCH3), 7.26 (s, 1H, H-3), 8.22 (s, 1H, H-6). Anal. Calcd. for C9H5ClK2N2O4S2: C, 28.23; H, 1.32; N, 7.32. Found: C, 28.31; H, 1.10; N, 6.95%.
N-(2-Benzylthio-4-chloro-5-methoxycarbonylbenze nesulfonyl)cyanamide potassium salt (12) Method A: To a stirred solution of 5 (1.53 g, 4 mmol) in water (7 mL) benzyl chloride (0.46 mL, 4 mmol) was added dropwise. The reaction mixture was stirred at room temperature for 2 h. After cooling to 0°C, the precipitate of crude product was filtered off, then recrystallized from methanol (11 mL), to afford 12. Yield: 1.38 g, 80%, mp 206°C–209°C dec.; IR νmax (KBr)/cm−1 2952, 2836 (CH3, CH2), 2185 (C≡N), 1729, 1711 (CO), 1355, 1141 (SO2); 1H NMR δ (DMSO-d6) 3.86 (s, 3H, OCH3), 4.34 (s, 2H, SCH2), 7.25–7.49 (m, 5H, Ph), 7.54 (s, 1H, H-3), 8.26 (s, 1H, H-6). Anal. Calcd. for C16H12ClKN2O4S2: C, 44.18; H, 2.78; N, 6.44. Found: C, 44.02; H, 2.43; N, 6.29%.
Method B: To a stirred suspension of 3 (1.22 g, 4 mmol) and anhydrous K2CO3 (1.38 g) in dry THF (40 mL), benzyl chloride (0.46 mL, 4 mmol) was added dropwise. The reaction mixture was stirred at reflux for 22 h, then left to stand at room temperature overnight. The precipitate was filtered off and washed twice with dry THF (1 mL). The product was separated from a solid K2CO3 by extraction with boiling ethanol (30 mL) for 10 min. The filtrate was cooled to 0°C, and left to stand in a refrigerator overnight. The precipitate was filtered off, washed with ethanol (2 × 0.5 mL) and dried in air, to give 12. Yield: 1.01 g, 58%, mp 197°C–199°C dec. After crystallization from methanol (7 mL), 12 presented mp 206°C–209°C dec.; IR (KBr) and 1H NMR (DMSO-d6) spectra were identical with authentic sample 12.
General procedure for the preparation of N-[4-chloro-5-methyl-2-(R2-methylthio)benzenesulfonyl]cyanamide potassium salts (13–15): To a suspension of 5-chloro-2-(cyanoaminosulfonyl)-4-methylthiophenolate dipotassium salt (5.08 g, 15 mmol) in water (15 mL) the appropriate alkyl halide (16.5 mmol) was added in portions at 0°C–20°C. The reaction mixture was stirred for 1–2 h and the precipitate was filtered off and dried. Crude product was purified by crystallization from ethanol.
N-(2-Allylthio-4-chloro-5-methylbenzenesulfonyl)cyanamide potassium salt (13): Starting from 6 and allyl bromide (2.06 g), the title compound 13 was obtained. Yield: 4.98 g, 98%, mp 192°C–194°C; IR νmax (KBr)/cm−1 3071 (C=CH), 2921 (CH3), 2173 (C≡N), 1631 (C=C), 1344, 1146 (SO2); 1H NMR δ (DMSO-d6) 2.30 (s, 3H, CH3), 3.65–3.75 (d, 2H, SCH2), 5.10-5.40 (d, 2H, C=CH2), 5.75-5.95 (m, 1H, CH=CH2), 7.35 (s, 1H, H-3), 7.75 (s, 1H, H-6). Anal. Calcd. for C11H10ClKN2O2S2: C, 38.76; H, 2.96; N, 8.22. Found: C, 38.40; H, 2.74; N, 8.28%.
N-(4-Chloro-5-methyl-2-propargylthiobenzenesulfonyl) cyanamide potassium salt (14): Starting from 6 and propargyl bromide (1.96 g) the title compound 14 was obtained. Yield: 4.71 g, 93%, mp 210–212°C; IR νmax (KBr) / cm−1 3311, 3299 (C≡CH), 2946, 2920, 2854 (CH3, CH2), 2172 (C≡N), 2123 (C≡C), 1344, 1142 (SO2); 1H NMR δ (DMSO-d6) 2.31 (s, 3H, CH3), 3.19 (s, 1H, C≡CH), 3.87 (s, 2H, SCH2), 7.44 (s, 1H, H-3), 7.74 (s, 1H, H-6). Anal. Calcd. for C11H8ClKN2O2S2: C, 38.99; H, 2.38; N, 8.26. Found: C, 39.20; H, 2.34; N, 8.49%.
N-[4-Chloro-5-methyl-2-(2-phenylsulfonylethylthio)benzenesulfonyl]cyanamide potassium salt (15): Starting from 6 and 1-chloro-2-phenylsulfonylethan (3.38 g), the title compound 15 was obtained. Yield: 6.14 g, 87%, mp 126°C–129°C; IR νmax (KBr)/cm−1 2924, 2854 (CH3, CH2), 2178 (C≡N), 1284, 1149 (SO2); 1H NMR δ (DMSO-d6) 2.31 (s, 3H, CH3), 3.12-3.19 (m, 2H, SCH2), 3.35-3.62 (m, 2H, SO2CH2), 7.26 (s, 1H, H-3), 7.63–7.81 (m, 4H, arom.), 7.92–7.96 (m, 2H, arom). Anal. Calcd. for C16H14ClKN2O4S3: C, 40.97; H, 3.01; N, 5.97. Found: C, 40.78; H, 2.90; N, 5.50%.
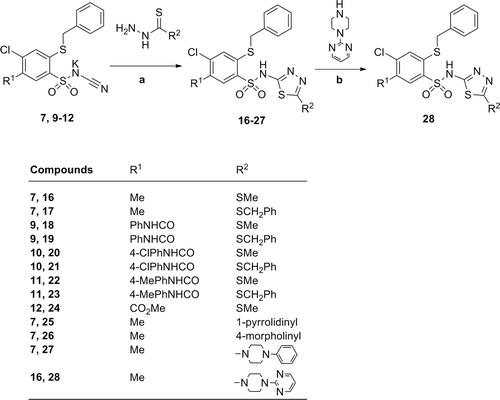
General procedure for the preparation of 2-benzylthio-4-chloro-5-R1-N-(5-R2-1,3,4-thiadiazol-2-yl)benzenesulfonamide derivatives (16–27): To a suspension of corresponding N-(benzenesulfonyl)cyanamide potassium salt 7, 9–12 (2 mmol) in glacial acetic acid (6 mL), the equimolar amount of the appropiate hydrazinecarbodithioic acid esters or 1-substituted carbothioic acid hydrazides was added and then refluxed for 8–40 h. After cooling to 10°C, the precipitate was collected by filtration, washed with cold acetic acid and dried. Crude products were purified by crystallization from proper solvents: 16–18, 22, 23 (ethanol); 19, 25–27 (acetonitrile); 20, 21 (ethanol:acetonitile, v/v 19:1); 24 (ethanol:acetonitile, v/v 5:1).
2-Benzylthio-4-chloro-5-methyl-N-(5-methylthio-1,3,4-thiadiazol-2-yl)benzenesulfonamide (16): Starting from 7 (0.78 g) and methyl hydrazinecarbodithioate (0.24 g) at reflux for 8 h, the title compound 16 was obtained. Yield: 0.55 g, 60%, mp 178°C–180°C dec.; IR νmax (KBr)/cm−1 3127 (NH), 2921, 2855 (CH3, CH2), 1543 (C=N), 1305, 1150 (SO2); 1H NMR δ (DMSO-d6) 2.33 (s, 3H, CH3), 2.62 (s, 3H, SCH3), 4.31 (s, 2H, SCH2), 7.22-7.27 (m, 5H, Ph), 7.58 (s, 1H, H-3), 7.88 (s, 1H, H-6), 14.30 (br s, 1H, SO2NH). Anal. Calcd. for C17H16ClN3O2S4: C, 44.58; H, 3.52; N, 9.17. Found: C, 44.21; H, 3.38; N, 8.90%.
2-Benzylthio-4-chloro-5-methyl-N-(5-benzylthio-1,3,4-thiadiazol-2-yl)benzenesulfonamide (17): Starting from 7 (0.78 g) and benzyl hydrazinecarbodithioate (0.40 g) at reflux for 8 h, the title compound 17 was obtained. Yield: 0.45 g, 42%, mp 194°C–196°C dec.; IR νmax (KBr)/cm−1 3441 (NH), 2921, 2852 (CH3, CH2), 1554 (C=N)), 1310, 1297, 1137 (SO2); 1H NMR δ (DMSO-d6) 2.31 (s, 3H, CH3), 4.26 (s, 2H, SCH2), 4.31 (s, 2H, SCH2), 7.27–7.35 (m, 10H, arom.), 7.42 (s, 1H, H-3), 7.79 (s, 1H, H-6). Anal. Calcd. for C23H20ClN3O2S4: C, 51.72; H, 3.77; N, 7,87. Found: C, 51.40; H, 3.68; N, 7.55%.
4-Benzylthio-2-chloro-5-(5-methylthio-1,3,4-thiadiazol-2-yl-aminosulfonyl)-N-phenylbenzamide (18): Starting from 9 (0.99 g) and methyl hydrazinecarbodithioate (0.24 g) at reflux for 8.5 h, the title compound 18 was obtained. Yield: 0.50 g, 44%, mp 266°C–269°C dec.; IR νmax (KBr)/cm−1 3275 (NH), 2879 (CH3, CH2), 1659 (C=O), 1583, 1544 (C=N), 1322, 1139 (SO2); 1H NMR δ (DMSO-d6) 2.63 (s, 3H, SCH3), 4.45 (s, 2H, SCH2), 7.11–7.38 (m, 8H, arom.), 7.67–7.69 (d, 2H, arom.), 7.73 (s, 1H, H-3), 7.99 (s, 1H, H-6), 10.61 (s, 1H, NHCO), 14,40 (br s, 1H, SO2NH). Anal. Calcd. for C23H19ClN4O3S4: 49.06; H, 3.40; N, 9.95. Found: 49.02; H, 3.44; N, 9.97%.
4-Benzylthio-2-chloro-5-(5-benzylthio-1,3,4-thiadiazol-2-yl-aminosulfonyl)-N-phenylbenzamide (19): Starting from 9 (0.99 g) and benzyl hydrazinecarbodithioate (0.40 g) at reflux for 9 h, the title compound 19 was obtained. Yield: 0.66 g, 51%, mp 231°C–233°C dec.; IR νmax (KBr)/cm−1 3314, 3137 (NH), 2854 (CH3, CH2), 1663 (C=O), 1582 (C=N), 1324, 1151 (SO2); 1H NMR δ (DMSO-d6) 4.41 (s, 4H, 2 × SCH2), 7.08-7.39 (m, 13H, arom.), 7.66–7.71 (m, 3H, H-3 and arom.), 7.96 (s, 1H, H-6), 10.61 (s, 1H, NHCO), 14.45 (br s, 1H, SO2NH). Anal. Calcd. for C29H23ClN4O3S4: 54.49; H, 3.63; N, 8.76. Found: 54.60; H, 3.73; N, 8.87%.
4-Benzylthio-2-chloro-5-(5-methylthio-1,3,4-thiadiazol-2-yl-aminosulfonyl)-N-(4-chlorophenyl)benzamide (20): Starting from 10 (1.06 g) and methyl hydrazinecarbodithioate (0.24 g) at reflux for 8 h, the title compound 20 was obtained. Yield: 0.55 g, 45%, mp 256°C–258°C dec.; IR νmax (KBr)/cm−1 3279 (NH), 2923, 2841 (CH3, CH2), 1659 (C=O), 1581, 1545 (C=N), 1311, 1140 (SO2); 1H NMR δ (DMSO-d6) 2.63 (s, 3H, SCH3), 4.45 (s, 2H, SCH2), 7.27–7.36 (m, 5H, Ph), 7.42 (d, J = 7.8 Hz, 2H, 4-ClC6H4), 7.71 (d, J = 7.8 Hz, 2H, 4-ClC6H4), 7.74 (s, 1H, H-3), 8.01 (s, 1H, H-6), 10.75 (s, 1H, NHCO), 14.40 (br s, 1H, SO2NH). Anal. Calcd. for C23H18Cl2N4O3S4: C, 46.23; H, 3.04; N, 9.38. Found: C, 46.01; H, 2.89; N, 8.71%.
4-Benzylthio-5-(5-benzylthio-1,3,4-thiadiazol-2-yl-aminosulfonyl)-2-chloro-N-(4-chlorophenyl)benzamide (21): Starting from 10 (1.06 g) and benzyl hydrazinecarbodithioate (0.40 g) at reflux for 8.5 h, the title compound 21 was obtained. Yield: 0.63 g, 46%, mp 253°C–256°C dec.; IR νmax (KBr)/cm−1 3342, 3217 (NH), 2923, 2759 (CH3, CH2), 1660 (C=O), 1599, 1578, 1544 (C=N), 1313, 1143 (SO2); 1H NMR δ (DMSO-d6) 4.40 (s, 2H, SCH2), 4.42 (s, 2H, SCH2), 7.20–7.36 (m, 12H, 2 x Ph and 4-ClC6H4), 7.71 (d, J = 7.3 Hz, 2H, 4-ClC6H4), 7.73 (s, 1H, H-3), 7.98 (s, 1H, H-6), 10.75 (s, 1H, NHCO), 14.40 (s, 1H, SO2NH). Anal. Calcd. for C29H22Cl2N4O3S4: C, 51.70; H, 3.29; N, 8.32. Found: C, 51.55; H, 3.19; N, 8.24%.
4-Benzylthio-2-chloro-5-(5-methylthio-1,3,4-thiadiazol-2-yl-aminosulfonyl)-N-(4-methylphenyl)benzamide (22): Starting from 11 (1.02 g) and methyl hydrazinecarbodithioate (0.24 g) at reflux for 9 h, the title compound 22 was obtained. Yield: 0.45 g, 37%, mp 237°C–240°C dec.; IR νmax (KBr)/cm−1 3411, 3278 (NH); 2922 (CH3, CH2), 1652 (C=O), 1580, 1543 (C=N), 1318, 1141 (SO2); 1H NMR δ (DMSO-d6) 2.27 (s, 3H, CH3), 2.62 (s, 3H, SCH3), 4.44 (s, 2H, SCH2), 7.16 (d, J = 8.4 Hz, 2H, 4-MeC6H4), 7.23–7.33 (m, 5H, Ph), 7.56 (d, J = 8.4 Hz, 2H, 4-MeC6H4), 7.72 (s, 1H, H-3), 7.97 (s, 1H, H-6), 10.52 (s, 1H, NHCO), 14.40 (br s, 1H, SO2NH). Anal. Calcd. for C24H21ClN4O3S4: C, 49.94; H, 3.67; N, 9.71. Found: C, 49.75; H, 3.45; N, 9.96%.
4-Benzylthio-5-(5-benzylthio-1,3,4-thiadiazol-2-yl-aminosulfonyl)-2-chloro-N-(4-methylphenyl)benzamide (23): Starting from 11 (1.02 g) and benzyl hydrazinecarbodithioate (0.40 g) at reflux for 8 h, the title compound 23 was obtained. Yield: 0.63 g, 48%, mp 222°C–224°C dec.; IR νmax (KBr)/cm−1 3286, 3137 (NH), 2921, 2847 (CH3, CH2), 1656 (C=O), 1583, 1545, 1518 (C=N), 1322, 1151 (SO2); 1H NMR δ (DMSO-d6) 2.27 (s, 3H, CH3), 4.41 (s, 4H, 2 x SCH2), 7.16 (d, J = 8.3 Hz, 2H, 4-MeC6H4), 7.22–7.36 (m, 10H, 2 × Ph), 7.57 (d, J = 8.3 Hz, 2H, 4-MeC6H4), 7.72 (s, 1H, H-3), 7.95 (s, 1H, H-6), 10.52 (s, 1H, NH), 14,40 (br s, 1H, SO2NH). Anal. Calcd. for C30H25ClN4O3S4: C, 55.16; H, 3.86; N, 8.58. Found: C, 55.10; H, 3.82; N, 8.58%.
Methyl-4-benzylthio-2-chloro-5-(5-methylthio-1,3,4-thiadiazol-2-yl-aminosulfonyl)benzoate (24): Starting from 12 (0.87 g) and methyl hydrazinecarbodithioate (0.24 g) at reflux for 9 h, the title compound 24 was obtained. Yield: 0.43 g, 43%, mp 171°C–173°C dec.; IR νmax (KBr)/cm−1 3360 (NH), 2850 (CH3, CH2), 1731 (C=O), 1577, 1542 (C=N), 1314, 1150 (SO2); 1H NMR δ (DMSO-d6) 2.61 (s, 3H, SCH3), 3.86 (s, 3H, OCH3), 4.41 (s, 2H, SCH2), 7.32 (s, 5H, Ph,), 8.32 (s, 1H, H-3), 8.34 (s, 1H, H-6), 14.40 (br s, 1H, NH). Anal. Calcd. for C18H16ClN3O4S4: C, 43.06; H, 3.21; N, 8.37. Found: C, 42.99; H, 3.19; N, 8.38%.
2-Benzylthio-4-chloro-5-methyl-N-(5-pyrrolidin-1-yl-1,3,4-thiadiazol-2-yl)benzenesulfonamide (25): Starting from 7 (0.78 g) and pyrrolidine-1-carbothiohydrazide (0.29 g) at reflux for 29 h, the title compound 25 was obtained. Yield: 0.56 g, 59%, mp 220°C–223°C dec.; IR νmax (KBr)/cm−1 3247 (NH), 2926, 2859 (CH3, CH2), 1527(C=N), 1342, 1139 (SO2); 1H NMR δ (DMSO-d6) 1.93–1.96 (m, 4H, CH2), 2.33 (s, 3H, CH3), 3.30–3.34 (m, 4H, CH2), 4.31 (s, 2H, SCH2), 7.26–7.34 (m, 5H, Ph), 7.54 (s, 1H, H-3), 7.86 (s, 1H, H-6), 13.23 (s, 1H, NH). Anal. Calcd. for C20H21ClN4O2S3: C, 49.93; H, 4.40; N, 11.65. Found: C, 49.83; H, 4.43; N, 11.73%.
2-Benzylthio-4-chloro-5-methyl-N-(5-morpholin-1-yl-1,3,4-thiadiazol-2-yl)benzenosulfonamide (26): Starting from 7 (0.78 g) and morpholine-4-carbothiohydrazide (0.32 g) at reflux for 11 h, the title compound 26 was obtained. Yield: 0.19 g, 19%, mp 218°C–221°C dec.; IR νmax (KBr)/cm−1 3431, 3236 (NH), 2919, 2854 (CH3, CH2), 1532 (C=N), 1343, 1141 (SO2); 1H NMR δ (DMSO-d6) 2.33 (s, 3H, CH3), 3.24–3.28 (m, 4H, morpholine), 3.64–3.69 (m, 4H, morpholine), 4.31 (s, 2H, SCH2), 7.27–7.31 (m, 5H, Ph), 7.55 (s, 1H, H-3), 7.86 (s, 1H, H-6), 13.36 (s, 1H, NH). Anal. Calcd. for C20H21ClN4O3S3: 48.33; H, 4.26; N, 11.27. Found: C, 48.10; H, 4.02; N, 10.93%.
2-Benzylthio-4-chloro-5-methyl-N-[5-(4-phenylpiperazin-1-yl)-1,3,4-thiadiazol-2-yl]benzenosulfonamide (27): Starting from 7 (0.78 g) 4-phenylpiperazine-1-carbothiohydrazide (0.47 g) at reflux for 40 h, the title compound 27 was obtained. Yield: 0.25 g, 22%, mp 195°C–201°C dec.; IR νmax (KBr)/cm−1 3436 (NH), 2922, 2853 (CH3,CH2), 1537 (C=N), 1345, 1143 (SO2); 1H NMR δ (DMSO-d6) 2.34 (s, 3H, CH3), 3.24 (s, 4H, CH2), 3.43 (s, 4H, CH2), 4.27 (s, 2H, SCH2), 6.83–7.40 (m, 10H, Ph), 7.56 (s, 1H, H-6), 13.36 (s, 1H, NH). Anal. Calcd. for C26H26ClN5O2S3: C, 54.58; H, 4.58; N, 12.24. Found: C, 54.47; H, 4.18; N, 11.71%.
2-Benzylthio-4-chloro-5-methyl-N-{5-[4-(pyrimidin-2-yl)piperazin-1-yl]-1,3,4-thiadiazol-2-yl}benzenosulfonamide (28): To a suspension of 16 (0.46 g, 1 mmol) in dry toluene (14 mL), 2-(piperazin-1-yl)pyrimidine (0.73 g, 4.5 mmol) was added dropwise and then the reaction mixture was stirred at reflux for 56 h. After reaction byproducts were filtered off, the filtrate was evaporated to dryness under reduced pressure. The resulting solid was crystallized from acetonitrile, to give 28. Yield: 0.09 g, 16%, mp 238°C–242°C dec.; IR νmax (KBr)/cm−1 3449, 3160 (NH), 2853 (CH2,CH3), 1534 (C=N), 1345, 1143 (SO2); 1H NMR δ (DMSO-d6) 2.34 (s, 3H, CH3), 3.37–3.39 (t, 4H, CH2), 3.83–3.85 (t, 4H, CH2), 4.32 (s, 1H, SCH2), 6.7–6.72 (t, 1H, pyrimidine), 7.2–7.34 (m, 5H, Ph), 7.56 (s, 1H, H-3), 7.87 (s, 1H, H-6), 8.42-8.43 (d, 2H, pyrimidine), 13.36 (s, 1H, NH). Anal. Calcd. for C24H24ClN7O2S3: C, 50.21; H, 4.21; N, 17.08. Found: C, 49.81; H, 4.00; N, 17.75%.
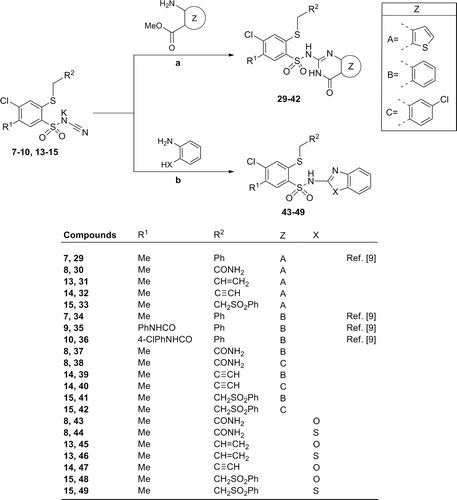
General procedure for the preparation of 2-(R2-methylthio)-4-chloro-N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl)benzenesulfonamide derivatives (30–33): The mixture of N-[4-chloro-2-(R2-methylthio)-5-methyl benzenesulfonyl]cyanamide potassium salt 8, 13–15 (2 mmol) and the equimolar amount of methyl 3-aminothiophene-2-carboxylate (0.317 g, 2 mmol) in glacial acetic acid (7 mL) was stirred at reflux for 15 h and additional 12 h at room temperature. After cooling to 10°C, the precipitate was collected by filtration, washed with cold acetic acid and dried. Crude product was purified by crystallization from ethanol or extraction of contaminations with hot ethanol.
2-Carbamoylmethylthio-4-chloro-N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl)-5-methylbenzenesulfonamide (30): Starting from 8 (0.72 g) compound 30 was obtained. Yield: 0.18 g, 20%, mp 228°C–233°C; IR νmax (KBr)/cm−1 3435 (NH), 2923, 2851 (CH3, CH2), 1655 (C=O), 1555 (C=N), 1334,1130 (SO2); 1H NMR δ (DMSO-d6) 2.35 (s, 3H, CH3), 3.19 (s, 2H, SCH2), 6.75–6.85 (d, 1H, thiophene), 7.15 (s,1H, NH), 7.40 (s, 1H, H-3), 7.60 (s, 1H, NH), 7.75–7.85 (d, 1H, thiophene), 7.95–8.05 (d, 2H, H-6, NH), 10.70–10.80 (s, 1H, NH). Anal. Calcd. for C15H13ClN4O4S3: C, 40.49; H, 2.94; N, 12.59. Found: C, 40.21; H, 2.85; N, 12.39%.
2-Allylthio-4-chloro-N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl)-5-methylbenzenesulfonamide (31): Starting from 13 (0.68 g), compound 31 was obtained. Yield: 0.34 g, 40%, mp 183°C–185°C; IR νmax (KBr)/cm−1 3448, 3305 (NH), 3103 (C=CH), 2922 (CH3, CH2), 1685 (C=O), 1617 (C=N), 1342, 1104 (SO2); 1H NMR δ (DMSO-d6) 2.35 (s, 3H, CH3), 3.75 (d, 2H, SCH2), 4.95–5.25 (dd, 2H, C=CH2), 5.55–5.80 (m, 1H, CH=C), 7.30–7.40 (d, 1H, thiophene), 7.55 (s, 1H, H-3), 7.96 (s, 1H, H-6), 8.14–8.22 (d, 1H, thiophene), 11.70 (s, 1H, NH), 12.20 (s, 1H, NH). Anal. Calcd. for C16H14ClN3O3S3: C, 44.91; H, 3.30; N, 9.82. Found: C, 44.82; H, 3.27; N, 9.62%.
4-Chloro-N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl)-5-methyl-2-propargylthiobenzenesulfonamide (32): Starting from 14 (0.68 g), compound 32 was obtained. Yield: 0.28 g, 34%, mp 222°C–224°C; IR νmax (KBr)/cm−1 3491, 3248 (NH), 3081 (C≡CH), 2953 (CH3, CH2), 1672 (C=O), 1613 (C=N), 1350, 1153 (SO2); 1H NMR δ (DMSO-d6) 2.35 (s, 3H, CH3), 3.15 (s, 1H, C≡CH), 4.95 (d, 2H, SCH2), 7.22–7.32 (d, 1H, thiophene), 7.60 (s, 1H, H-3), 8.00 (s, 1H, H-6), 8.13 (d, 1H, thiophene), 11.60 (s, 1H, NH), 12.20 (s, 1H, NH). Anal. Calcd. for C16H12ClN3O3S3: C, 45.12; H, 2.84; N, 9.87. Found: C, C, 45.22; H, 2.96; N, 10.02%.
4-Chloro-N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl)-5-methyl-2-(2-phenylsulfonylethylthio)benzenesulfonamide (33): Starting from 15 (0.94 g), compound 33 was obtained. Yield: 0.40 g, 36%, mp 233°C–235°C; IR νmax (KBr)/cm−1 3447, 3071 (NH), 2921, 2851 (CH3, CH2), 1654 (C=O), 1553(C=C), 1336, 1152 (SO2); 1H NMR δ (DMSO-d6) 2.35 (s, 3H, CH3), 3.07 (t, 2H, CH2), 3.47 (t, 2H, CH2), 6.71–6.74 (d, 1H, arom.), 7.12 (s, 1H, NH), 7.65 (t, 2H, arom.), 7.74–7.80 (m, 2H, arom.), 7.86 (d, 2H, arom.), 8.00 (s, 1H, H-6), 10.85 (s, 1H, NH). Anal. Calcd. for C21H18ClN3O5S4: C, 45.36; H, 3.26; N, 7.56. Found: C, 45.20; H, 2.99; N, 7.35%.
General procedure for the preparation of 2-(R2-methylthio)-4-chloro-N-(3,4-dihydro-4-oxoquinazolin-2-yl)benzenesulfonamide derivatives (37–42): To a suspension of the corresponding N-[4-chloro-2-(R2-methylthio)-5-methylbenzenesulfonyl]cyanamide potassium salt 8, 14–15 (2 mmol) in glacial acetic acid (7 mL) the appropriate methyl 2-aminobenzoate derivatives (2.2 mmol) was added and refluxed for 14–17 h. After cooling to 10°C, the precipitate was collected by filtration, washed with cold acetic acid and dried. Crude product was purified by crystallization from ethanol or extraction of contaminations with hot ethanol.
2-Carbamoylmethylthio-4-chloro-N-(3,4-dihydro-4-oxoquinazolin-2-yl)-5-methylbenzenesulfonamide (37): Starting from 8 (0.72 g) and methyl 2-aminobenzoate (0.33 g), the title compound 37 was obtained. Yield: 0.36 g, 41%, mp 270°C–272°C; IR νmax (KBr)/cm−1 3383, 3274, 3165 (NH, NH2), 2922 (CH3, CH2), 1720 (C=O), 1707 (C=O), 1642 (C=N), 1361, 1148 (SO2); 1H NMR δ (DMSO-d6) 2.40 (s, 3H, CH3), 3.70 (s, 2H, SCH2), 7.20 (s, 1H, NH2), 7.37 (t, 1H, arom.), 7.55 (d, 2H, arom.), 7.65 (s, 1H, H-3), 7.77 (t, 1H, arom.), 7.95 (s, 1H, H-6), 8.05 (s, 1H, NH2), 11.40 (s, 1H, NH), 11.80 (s, 1H, NH). Anal. Calcd. for C17H15ClN4O4S2: C, 46.52; H, 3.44; N, 12.77. Found: C, 46.44; H, 3.12; N, 12.40%.
2-Carbamoylmethylthio-4-chloro-N-(7-chloro-3,4-dihydro-4-oxoquinazolin-2-yl)-5-methylbenzene sulfonamide (38): Starting from 8 (0.72 g) and methyl 2-amino-4-chlorobenzoate (0.41 g), the title compound 38 was obtained. Yield: 0.37 g, 39%, mp 272°C–274°C; IR νmax (KBr)/cm−1 3391, 3275, 3175 (NH, NH2), 2920 (CH3, CH2), 1719 (C=O), 1691 (C=O), 1643 (C=N), 1360, 1149 (SO2); 1H NMR δ (DMSO-d6) 2.20 (s, 3H, CH3), 3.70 (s, 2H, SCH2), 7.20 (s, 1H, NH), 7.40 (d, 1H, arom.), 7.50 (s, 1H, NH), 7.60 (d, 2H, arom.), 8.00 (s, 2H, arom.), 11.50 (s, 1H, NH), 11.80 (s, 1H, NH). Anal. Calcd. for C17H14Cl2N4O4S2: C, 43.14; H, 2.98; N, 11.84. Found: C, 43.20; H, 2.84; N, 10.97%.
4-Chloro-N-(3,4-dihydro-4-oxoquinazolin-2-yl)-5-methyl-2-propargylthiobenzenesulfonamide (39): Starting from 14 (0.68 g) and methyl 2-aminobenzoate (0.33 g), the title compound 39 was obtained. Yield: 0.45 g, 54%, mp 214°C–216°C; IR νmax (KBr)/cm−1 3275 (C≡CH), 3211, 3091 (NH), 2926 (CH3, CH2), 1696 (C=O), 1633 (C=N), 1381, 1136 (SO2); 1H NMR δ (DMSO-d6) 2.20 (s, 3H, CH3), 3.10 (s, 1H, C≡CH), 4.00 (s, 2H, SCH2), 7.37 (t, 1H, arom.), 7.55 (d, 1H, arom.), 7.65 (s, 1H, arom.), 7.77 (t, 1H, arom.), 8.00 (s, 1H, arom.), 11.40 (s, 1H, NH), 11.85 (s, 1H, NH). Anal. Calcd. for C18H14ClN3O3S2: C, 51.49; H, 3.36; N, 10.01. Found: C, 51.02; H, 3.21; N, 9.21%.
4-Chloro-N-(7-chloro-3,4-dihydro-4-oxoquinazolin-2-yl)-5-methyl-2-propargylthiobenzenesulfonamide (40): Starting from 14 (0.68 g) and methyl 2-amino-4-chlorobenzoate (0.41 g), the title compound 40 was obtained. Yield: 0.17 g, 26%, mp 219°C–221°C; IR νmax (KBr)/cm−1 3441, 3310 (NH), 3191 (C≡CH), 2924, 2853 (CH3, CH2), 1688 (C=O), 1642 (C=N), 1358, 1138 (SO2); 1H NMR δ (DMSO-d6) 2.40 (s, 3H, CH3), 3.15 (s, 1H, C≡CH), 3.95 (s, 2H, SCH2), 7.38 (d, 1H, arom.), 7.65 (d, 2H, arom.), 8.00 (t, 2H, arom.), 11.45 (s, 1H, NH), 11.80 (s, 1H, NH). Anal. Calcd. for C18H13Cl2N3O3S2: C, 47.58; H, 2.88; N, 9.25. Found: C, 47.50; H, 2.82; N, 9.26%.
4-Chloro-N-(3,4-dihydro-4-oxoquinazolin-2-yl)-5-methyl-2-(2-phenylsulfonylethylthio)benzenesulfonamide (41): Starting from 15 (0.94 g) and methyl 2-aminobenzoate (0.33 g), the title compound 41 was obtained. Yield: 0.18 g, 34%, mp 207°C–209°C; IR νmax (KBr)/cm−1 3242, 3165, 3064 (NH), 2924 (CH3, CH2), 1699 (C=O), 1631 (C=N), 1364, 1337, 1149, 1116 (SO2); 1H NMR δ (DMSO-d6) 2.40 (s, 3H, CH3), 3.20 (t, 2H, SCH2), 3.45 (t, 2H, SCH2), 7.20–8.00 (m, 11H, arom.), 11.40 (s, 1H, NH), 11.80 (s, 1H, NH). Anal. Calcd. for C23H20ClN3O5S3: C, 50.22; H, 3.66; N, 7.64. Found: C, 50.01; H, 3.32; N, 6.78%.
4-Chloro-N-(7-chloro-3,4-dihydro-4-oxoquinazolin-2-yl)-5-methyl-2-(2-phenylsulfonylethylthio)benzenesulfonamide (42): Starting from 15 (0.94 g) and methyl 2-amino-4-chlorobenzoate (0.41 g), the title compound 42 was obtained. Yield: 0.30 g, 25%, mp 237°C–240°C; IR νmax (KBr)/cm−1 3436, 3189, 3067 (NH), 2924 (CH3, CH2), 1687 (C=O), 1640 (C=N), 1357, 1148 (SO2); 1H NMR δ (DMSO-d6) 2.40 (s, 3H, CH3), 3.20 (t, 2H, SCH2), 3.45 (t, 2H, SCH2), 7.30–8.10 (m, 11H, arom.), 11.50 (s, 1H, NH), 11.80 (s, 1H, NH). Anal. Calcd. for C23H19Cl2N3O5S3: C, 47.26; H, 3.28; N, 7.19. Found: C, 47.20; H, 3.32; N, 7.30%.
General procedure for the preparation of 2-(R2-methylthio)-N-(2-benzoxazolyl)-4-chloro-5-methylbenzenesulfonamide derivatives (43–49): To a suspension of corresponding N-(benzenesulfonyl)cyanamide potassium salt 8, 13–15 (2 mmol) in glacial acetic acid (6 mL) the equimolar amount of the appropriate 2-aminophenol (2 mmol) or 2-aminothiophenol (2 mmol) was added and the reaction mixture was stirred for 6–7 h at reflux, then 12 h at room temperature. After cooling to 10°C, the precipitate was collected by filtration, washed with cold acetic acid and dried. Crude product was purified by crystallization from ethanol or extraction of contaminations with hot ethanol.
N-(2-Benzoxazolyl)-2-carbamoylmethylthio-4-chloro-5-methylbenzenesulfonamide (43): Starting from 8 (0.72 g) and 2-aminophenol, the title compound 43 was obtained. Yield: 0.38 g, 45%, mp 124°C–125°C; IR νmax (KBr)/cm−1 3430 (NH), 2923 (CH3, CH2), 1642 (C=O), 1624 (C=N), 1344, 1115 (SO2); 1H NMR δ (DMSO-d6) 2.38 (s, 3H, CH3), 3.72 (s, 2H, SCH2), 7.25 (t, 2H: 1H, arom., 1H, NH2), 7.32 (t, 1H, arom.), 7.37 (d, 1H, arom.), 7.55 (t, 2H: 1H, arom., 1H, NH2), 7.63 (s, 1H, arom.), 8.00 (s, 1H, arom.), 12.90 (s, 1H, NH). Anal. Calcd. for C16H14ClN3O4S2: C, 46.66; H, 3.43; N, 10.20. Found: C, 46.33; H, 3.05; N, 9.87%.
N-(2-Benzothiazolyl)-2-carbamoylmethylthio-4-chloro-5-methylbenzenesulfonamide (44): Starting from 8 (0.72 g) and 2-aminothiophenol, the title compound 44 was obtained. Yield: 0.35 g, 41%, mp 212°C–215°C; IR νmax (KBr)/cm−1 3375, 3164 (NH, NH2), 2976, 2920, 2785 (CH3, CH2), 1606 (C=O), 1318, 1145 (SO2); 1H NMR δ (DMSO-d6) 2.40 (s, 3H, CH3), 3.70 (s, 2H, SCH2), 7.20–8.00 (m, 8H: 6H, arom., 2H, NH2), 13.30 (s, 1H, NH). Anal. Calcd. for C16H14ClN3O3S3: C, 44.91; H, 3.30; N, 9.82. Found: C, 44.71; H, 3.20; N, 9.35%.
2-Allylthio-N-(2-benzoxazolyl)-4-chloro-5-methylbenzenesulfonamide (45): Starting from 13 (0.68 g) and 2-aminophenol, the title compound 45 was obtained. Yield: 0.36 g, 47%, mp 140°C–142°C; IR νmax (KBr)/cm−1 3448, 3302 (NH), 3084 (C=CH2), 2923 (CH3, CH2), 1644 (C=N), 1366, 1145 (SO2); 1H NMR δ (DMSO-d6) 2.35 (s, 3H, CH3), 3.77 (d, 2H, SCH2), 5.00–5.05 (d, 1H, CH2), 5.15–5.20 (d, 1H, CH2), 5.65–5.75 (m, 1H, CH), 7.27 (t, 1H, arom.), 7.35 (t, 1H, arom.), 7.39 (d, 1H, arom.), 7.53 (d, 2H, arom.), 8.00 (s, 1H, arom.), 12.80 (br s, 1H, NH). Anal. Calcd. for C17H15ClN2O3S2: C, 51.71; H, 3.83; N, 7.09. Found: C, 51.61; H, 3.74; N, 7.01%.
2-Allylthio-N-(2-benzothiazolyl)-4-chloro-5-methylbenzenesulfonamide (46): Starting from 13 (0.68 g) and 2-aminothiophenol, the title compound 46 was obtained. Yield: 0.41 g, 50%, mp 165°C–168°C; IR νmax (KBr)/cm−1 3449 (NH), 3101 (C=CH2), 2975, 2916 (CH3, CH2), 1314, 1146 (SO2); 1H NMR δ (DMSO-d6) 2.40 (s, 3H, CH3), 3.74 (d, 2H, SCH2), 5.00–5.05 (dd, 2H, C=CH2), 5.62–5.84 (m, 2H, C=CH), 7.20–7.45 (m, 3H, arom.), 7.50 (s, 1H, arom.), 7.81 (d, 1H, arom.), 7.94 (s, 1H, arom.), 13.22 (br s, 1H, NH). Anal. Calcd. for C17H15ClN2O2S3: C, 49.68; H, 3.68; N, 6.82. Found: C, 49.72; H, 3.61; N, 6.78%.
N-(2-Benzoxazolyl)-4-chloro-5-methyl-2-propargyl thiobenzenesulfonamide (47): Starting from 14 (0.68 g) and 2-aminophenol, the title compound 47 was obtained. Yield: 0.37 g, 47%, mp 140°C–142°C; IR νmax (KBr)/cm−1 3374, 3261 (NH), 3091 (C≡CH), 2938 (CH3, CH2), 1648 (C=N), 1303, 1164 (SO2); 1H NMR δ (DMSO-d6) 2.40 (s, 3H, CH3), 3.15 (s, 1H, C≡CH), 3.97 (d, 2H, SCH2), 7.26 (t, 1H, arom.), 7.35 (t, 1H, arom.), 7.37 (d, 1H, arom.), 7.53 (d, 1H, arom.), 7.61 (s, 1H, arom.), 8.02 (s, 1H, arom.), 12.90 (s, 1H, arom.), 12.90 (br s, 1H, NH). Anal. Calcd. for C17H13ClN2O3S2: C, 51.97; H, 3.34; N, 7.13. Found: C, 52.07; H, 3.23; N, 6.96%.
N-(2-Benzoxazolyl)-4-chloro-5-methyl-2-(2-phenylsulfonylethylthio)benzenesulfonamide (48): Starting from 15 (0.94 g) and 2-aminophenol, the title compound 48 was obtained. Yield: 0.39 g, 37%, mp 193°C–195°C; IR νmax (KBr)/cm−1 3436, 3062 (NH), 2928 (CH3, CH2), 1640 (C=N), 1313, 1155 (SO2); 1H NMR δ (DMSO-d6) 2.38 (s, 3H, CH3), 3.21 (t, 2H, CH2), 3.49 (t, 2H, CH2), 7.26 (t, 1H, arom.), 7.32 (t, 1H, arom.), 7.37 (d, 1H, arom.), 7.47 (s, 1H, arom.), 7.52 (d, 1H, arom.), 7.65 (t, 2H, arom.), 7.76 (d, 1H, arom.), 7.86 (d, 2H, arom.), 8.20 (s, 1H, arom.), 12.80 (br s, 1H, NH). Anal. Calcd. for C22H19ClN2O5S3: C, 50.52; H, 3.66; N, 5.36. Found: C, 50.28; H, 3.43; N, 5.18%.
N-(2-Benzothiazolyl)-4-chloro-5-methyl-2-(2-phenylsulfonylethylthio) benzenesulfonamide (49): Starting from 15 (0.94 g) and 2-aminothiophenol, the title compound 49 was obtained. Yield: 0.68 g, 64%, mp 212°C–215°C; IR νmax (KBr)/cm−1 3449, 3102 (NH), 2919 (CH3, CH2), 1546 (C=N), 1320, 1151 (SO2); 1H NMR δ (DMSO-d6) 2.40 (s, 3H, CH3), 3.20 (t, 2H, CH2), 3.50 (t, 2H, CH2), 7.32 (m, 4H, arom.), 7.58–7.90 (m, 6H, arom.), 7.95 (s, 1H, arom.), 13.30 (br s, 1H, NH). Anal. Calcd. for C22H19ClN2O4S4: C, 49.01; H, 3.55; N, 5.20. Found: C, 49.09; H, 3.65; N, 5.37%.
Biology
Anti-bacterial activity
The investigation was carried out on 26 strains of anaerobic bacteria isolated from the oral cavity, respiratory tract and intestinal tract, as well as five standard strains. The anaerobes belonged to the following genera: Peptostreptococcus (2 strains), Finegoldia (2), Micromonas (1), Actinomyces (2), Propionibacterium (3), Prevotella (6), Porphyromonas (2), Fusobacterium (4), Bacteroides (4) and standard strains: Bacteroides fragilis ATCC 25285, Finegoldia magna ATCC 29328, Fusobacterium nucleatum ATCC 25586, Peptostreptococcus anaerobius ATCC 27337 and Propionibacterium acnes ATCC 11827. The susceptibility of the anaerobic bacteria was determinated by means of the plate dilution technique in Brucella agar supplemented with 5% sheep bloodCitation23–25. The compounds were dissolved in 1 mL of DMSO immediately before the experiment. Further dilutions were performed in sterile distilled water. The following concentrations of the compounds were used: 200, 100, 50, 25, 12.5 and 6.2 µg/mL. Metronidazole was applied as a reference substance. The inoculum containing 105 CFU/spot was applied to the agar plates with Steers replicator. The inoculated agar plates and compound-free ones were incubated in anaerobic jars for 48 h at 37°C in 10% CO2, 10% H2 and 80% N2 atmosphere with palladium catalyst and indicator of anaerobiosis.
Aerobic bacteria (25 strains) were isolated from the oral cavity, respiratory tract and intenstinal tract as well as 5 standard strains. The bacteria were as follows: Staphylococcus (6), Enterococcus (3), Corynebacterium (2), Klebsiella (1), Acinetobacter (2), Escherichia (3), Citrobacter (2), Pseudomonas (5), Serratia (2), as well as standard strains: Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Klebsiella pneumoniae ATCC 13883, Acinetobacter baumannii ATCC 19606 and Escherichia coli ATCC 25922. Amikacin was used as a reference compound. The susceptibility of the aerobic bacteria was determined by means of agar dilution technique with Mueller–Hinton agarCitation24–26. Further dilutions were performed in sterile distilled water. The following concentrations of the derivatives were used: 200, 100, 50, 25, 12.5 and 6.2 µg/mL. The inoculum containing 105 CFU/spot was applied to the agar plates with Steers replicator. The inoculated agar plates and compound-free ones were incubated for 24 h at 37°C in aerobic conditions. The minimal inhibitory concentration (MIC) was defined as the lowest compound concentration, which inhibited growth of bacteria.
Results and discussion
Chemistry
The starting 3-methylthiobenzodithiazines 1Citation27, 2Citation28, 3-aminobenzodithiazine 4Citation29, dipotassium salt 6Citation29 and N-(benzenesulfonyl)cyanamide potassium salts 7–11Citation2,Citation4,Citation29 were prepared according to the known methods. The novel substrates 3 and 5 were prepared analogously and the corresponding N-[4-chloro-5-R1-2-(R2-methylthio)benzenesulfonyl]cyanamide potassium salts 12–15 ().
Scheme 1. Synthesis of N-[4-chloro-5-R1-2-(R2-methylthio)benzenesulfonyl]cyanamide potassium salts (12–15). Reagents, conditions and yields: (A) 25% NH4OH / EtOH (1.1 molar equiv.) r.t. 44 h, 73%; (B) anhydrous K2CO3 (excess), dry THF, reflux 24 h, 68%–74%; (C) R2-CH2-X (1.0–1.1 molar equiv.) (X = Br or Cl), water, r.t., 1-2 h, 80%–98%.
![Scheme 1. Synthesis of N-[4-chloro-5-R1-2-(R2-methylthio)benzenesulfonyl]cyanamide potassium salts (12–15). Reagents, conditions and yields: (A) 25% NH4OH / EtOH (1.1 molar equiv.) r.t. 44 h, 73%; (B) anhydrous K2CO3 (excess), dry THF, reflux 24 h, 68%–74%; (C) R2-CH2-X (1.0–1.1 molar equiv.) (X = Br or Cl), water, r.t., 1-2 h, 80%–98%.](/cms/asset/81c9faad-6eaf-4fe1-b783-66065db5834b/ienz_a_625024_f0001_b.gif)
It is well known that, various 2,5-disubstituted 1,3,4-thiadiazole can be prepared in good yields by the reaction of methyl N-[bis(methylthio)methylene]carbamate with either alkyl hydrazinecarbodithioates or 1-substuited thiosemicarbazidesCitation30.
In the present study, we utilized the new method for the synthesis of 2-substituted 1,3,4-thiadiazol-2-yl derivatives 16–24 consisting in the reaction of N-(benzenesulfonyl)cyanamide potassium salts 7, 9–12 with either methyl- or benzyl- hydrazinecarbodithioates in boiling glacial acetic acid (). On the other hand, treatment of 7 with pyrrolidine-, morpholine- or 4-phenylpiperazine-1-carbothiohydrazide afforded the target thiadiazole derivatives 25–27 in 19%–59% yields. As was presented on (route B), compound 28 could be obtained by the direct substitution of methylthio group in position 5 of 16 with 2-(piperazin-1-yl)pyrimidine in boiling dry toluene for 56 h.
Scheme 2. Synthesis of 2-benzylthio-4-chlorobenzenesulfonamide derivatives (16–28). Reagents and conditions: (A) appropriate hydrazinecarbodithioic acid esters or 1-substituted carbothioic acid hydrazides (1 molar equiv.), glacial acetic acid, reflux 8–40 h; (B) 16 (1 mmol), 2-(piperazin-1-yl)pyrimidine (4.5 mmol), dry toluene, reflux 56 h.
The synthesis of 4-chloro-N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl)-5-methylbenzenesulfonamides (30–33) was achieved via cyclization of N-(benzenesulfonyl)cyanamide potassium salt 8, 13–15 with methyl 3-aminothiophene-2-carboxylate, whereas desired 4-chloro-N-(3,4-dihydro-4-oxoquinazolin-2-yl)-5-methylbenzenesulfonamides (37–42) were obtained from potassium salts 8, 14, 15 and methyl 2-aminobenzoate or methyl 2-amino-4-chlorobenzoate (). An analogous reaction of 8, 13–15 with 2-aminophenol or 2-aminothiophenol guided to series of N-(2-benzoxazolyl- or 2-benothiazolyl)-4-chloro-5-methylbenzenesulfonamides (43–49) in 37%–64% yields.
Scheme 3. Synthesis of 4-chloro-2-mercaptobenzenesulfonamide derivatives (29–49). Reagents and conditions: (A) ortho-aminocarboxylate component (1.0–1.1 molar equiv.), glacial acetic acid, reflux 15 h and 12 h at r.t. or reflux 14–17 h; (B) 2-aminophenol or 2-aminothiophenol (1 molar equiv.), glacial acetic acid, reflux 6–7 h, then 12 h r.t.
The structure of the new compounds synthesized was confirmed by elemental analyses (C, H, N) and spectroscopic data presented in the material and methods section.
Biology
Anti-bacterial effect
The susceptibility of anaerobic and aerobic bacteria towards compounds 16–26, 28–33, 38–40 and 43–49 was shown in and . Twenty-six compounds exhibited anti-bacterial activity against 3–23 (12%–88%) strains of anaerobic bacteria at tested concentrations ranged from 6.2 to 100 µg/mL. Compounds 16, 17 and 24 showed high activity towards the tested bacteria and inhibited, respectively, 23, 19, 13 (88%, 73%, 50%) strains at concentrations in the range of 6.2 to 100 µg/mL. On the other hand, among all the compounds, only 16 and 17 were more active against Gram-negative strains then Gram-positive. In series of N-(1,3,4-thiadiazol-2-yl)benzenesulfonamides, relatively low activity presented 20, 22 with inhibition 6 (23%) strains of anaerobic bacteria at 6.2–50 µg/mL range. However, considering the activity at low concentrations, these compounds (20, 22) is interesting because of the inhibition of grows of four strains at concentration 6.2 µg/mL for each of them. It is worth noting that all N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl)benzenesulfonamides (29–33) presented moderate antibacterial activity (23%–46% strains of anaerobic bacteria in the range of 6.2–100 µg/mL) wherein 31 inhibited the growth of six bacteria strains at low concentration 6.2 µg/mL. Among the series of N-(3,4-dihydro-4-oxoquinazolin-2-yl)benzenesulfonamides, only three compounds (38–40) demonstrated activity against Gram-positive bacteria, inhibiting the growth of four to five (15%–19%) strains in range from 6.2 to 100 µg/mL. All N-(2-benzoxazolyl)- and N-(2-benzothiazolyl)benzenesulfonamides (43–49) exhibited influence on anaerobic bacteria that corresponded to three to seven (12%–27%) strains at concentrations 6.2–100 µg/mL. It should be emphasized that compound 48 showed (inhibition up) activity against to seven strains at low concentrations (6.2–12.5 µg/mL), while 45 and 49 suppressed three strains at above conditions.
Table 1. Minimal inhibitory concentration (MIC µg/mL) of test compounds 16–24, 26, 28–33.
Table 2. Minimal inhibitory concentration (MIC µg/mL) of test compounds 38–40, 43–49.
Gram-negative anaerobic bacteria were less susceptible to the tested compounds. Only 16, 17, 23, 24 and 29 inhibited the growth of 2–14 (12–82%) strains of these bacteria at concentrations 25–100 µg/mL (). Compound 16 was the most active derivative and acted on 14 strains at 100 µg/mL, while 17 inhibited 12 strains in the range of 25–100 µg/mL. Other three derivatives (23, 24, 29) had reduced activity on two to six strains in range from 50 to 100 µg/mL.
Some of the tested compounds demonstrated the same or better anti-bacterial activity than reference drugs (, ). Against Actinomyces israelii strains, 16 showed comparable activity to that displayed by metronidazole, whereas 17 demonstrated higher activity than reference drug. The higher activity against Propionibacterium acnes that was displayed by the metronidazole showed 20, 22 and 29, while 29 was also more active than reference substance against Propionibacterium granulosum. Comparably metronidazole activity against Actinomyces viscosus was demonstrated by 22, 30, 31, 33, 44, 48 and 49.
Activity towards aerobes was shown for 11 compounds, which acted against 1-8 (4-32%) strains, at concentrations ranged from 6.2 to 100 µg/mL. In series of N-(1,3,4-thiadiazol-2-yl)benzenesulfonamides, seven compounds (18–24) demonstrated anti-bacterial activity (three to eight strains at tested concentration range). The highest activity was exhibited by 18 with inhibition of eight (30%) strains at tested concentrations. All tested compounds had no anti-bacterial activity against Gram-negative aerobic bacteria.
Summing up, the following structure–activity relationship (SAR) can be drawn by considering data of and :
Anaerobic bacteria strains were susceptible to the compounds (16–26, 28–33, 38–40 and 43–49) in the following order: N-(1,3,4-thiadiazol-2-yl)- (16–24, 26, 28) > N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl)- (29–33) > N-benzoxazol-2-yl- (43, 45, 47, 48) > N-benzothiazol-2-yl- (44, 46, 49) > N-(3,4-dihydro-4-oxoquinazolin-2-yl)- (38–40) benzenesulfonamide derivatives (, ).
The activity towards anaerobic strains depended on both, the nature of substituents at the 5 position of benzenesulfonamide and substitution pattern of heterocyclic ring attached to the nitrogen atom of sulfonamide moiety. Thus, replacement of methylthio (R2 = MeS) group in active 16 by either benzylthio (PhCH2S) group (17), 4-morpholinyl (26) or 4-(pyrimidin-2-yl)piperazinyl (28) groups led to the decrease of activity, whereas introduction of 1-pyrrolidinyl (25) or 4-phenylpiperazinyl (27) moieties in this position resulted in the loss of activity. On the other hand, introducing of methoxycarbonyl group (R1 = CO2Me) (24) instead of methyl group (R1 = Me) in benzene ring (16) resulted in lower activity by 40% ().
The activity against aerobic bacteria strains depended mainly on the electronic nature of substituents at the 5 position of the benzene ring in the N-(1,3,4-thiadiazole)benzenesulfonamide series (16–28). The most active compound 18 has an electron-withdrawing N-phenylcarbamoyl group (R1 = PhNHCO), while other active compounds (20–23) bearing the similar 4-substituted N-phenylcarbamoyl groups inhibited growth of Staphylococcus aureus with MIC in the range of 6.2–12.5 μg/mL (). Most noteworthy is the greater sensitivity of Enterococcus faecalic and Corynebacterium spp strains to the above-mentioned thiadiazole derivatives (18, 20, 21 and 23) in comparison to the reference drug (Amikacin, ).
Relatively broadest spectrum of anti-microbial activity (MIC ≤ 200 μg/mL) revealed compounds: 24, 16 and 17 having 2-benzylthio-4-chloro-N-(5-alkylthio-1,3,4-thiadiazol-2-yl)benzenesulfonamide scaffold.
Compounds that are N-(3,4-dihydro-4-oxoquinazolin-2-yl)benzenesulfonamide derivatives (34–37, 41 and 42) showed no anti-microbial activity in the tested concentration range.
In fact, the molecular mechanism of action of investigated sulfonamides has not been elucidated. However, it seems probably that it is similar to the mechanism of action of other anti-bacterial sulfonamides and is associated with inhibition of bacterial enzymes which synthesize folic acid, essential for the production of purines, pyrimidines and amino acidsCitation31.
Anti-proliferative effect
Compounds 16, 17, 21 and 27 have also been tested in vitro at the National Cancer Institute (Bethesda MD, USA) at a single dose (10 µM) in the full NCI 60 cell panel. None of the compounds tested, however, caused strong inhibition of cancer cell growth. The most susceptible were ovarian (IGROV1) and renal (UO-31) carcinoma cell lines whose growth was inhibited in the range of 28%–31% for compound 27.
Conclusion
We have demonstrated that the reactions of N-(benzenesulfonyl)cyanamide potassium salts with either alkyl hydrazinecarbodithioates or appropriate 1-substituted carbothioic acid hydrazides led to the formation of the corresponding N-(5-substituted-1,3,4-thiadiazol-2-yl)benzenesulfonamide derivatives (16–27). Furthermore, treatment of N-(benzenesulfonyl)cyanamide potassium salts in boiling glacial acetic acid with nucleophiles involving methyl 3-aminothiophene-2-carboxylate, methyl 2-aminobenzoates, 2-aminophenol or 2-aminothiophenol furnished the corresponding N-(3,4-dihydro-4-oxothieno[2,3-e]pyrimidin-2-yl) (30–33), N-(3,4-dihydro-4-oxoquinazolin-2-yl) (37–42) and N-(2-benzoxazolyl- or 2-benothiazolyl)benzenesulfonamide (43–49) derivatives, respectively.
Most of the compounds exhibited anti-bacterial activity. Among them, compounds 16, 17, 23 and 24 (active mainly against anaerobes) seem to be promising lead structures for further investigation in search for better anti-bacterial activity, while others 18, 20, 21, 22, 29 and 32 are interesting due to better anti-bacterial activities against certain strains as compared to reference drugs.
Acknowledgements
The authors are very grateful to Dr. Joel Morris, Ph.D., Chief Drug Synthesis & Chemistry Branch, National Cancer Institute (Bethesda, MD) for the in vitro anticancer screening.
Declaration of interest
The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- Negwer M. Organic-chemical drugs and their synonyms. Berlin: Akademie Verlag, 1994:215–216,375,630–631,2972–2974.
- Sławiński J, Bednarski P, Reszka P. Syntheses and in vitro antitumor activity of 3-amino-N-(4-chlorobenzenesulfonyl)guanidine derivatives containing N’-arylidene moiety. Polish J Chem 2004;78:369–379.
- Sławiński J. Synthesis of a new series of 4-chloro-2-mercapto-5-methylbenzenesulfonamide derivatives with potential antitumor activity. Eur J Med Chem 2004;39:179–188.
- Sławiński J, Gdaniec M. Synthesis, molecular structure, and in vitro antitumor activity of new 4-chloro-2-mercaptobenzenesulfonamide derivatives. Eur J Med Chem 2005;40:377–389.
- Sławiński J, Brzozowski Z. Synthesis and in vitro antitumor activity of novel series 2-benzylthio-4-chlorobenzenesulfonamide derivatives. Eur J Med Chem 2006;41:1180–1189.
- Brzozowski Z, Sączewski F, Sławiński J, Bednarski PJ, Grünert R, Gdaniec M. Synthesis, structural characterization, and in vitro antitumor activity of novel N-(6-chloro-1,1-dioxo-1,4,2-benzodithiazin-3-yl)arylsulfonamides. Bioorg Med Chem 2007;15:2560–2572.
- Sławiński J, Brożewicz K, Fruziński A, Główka ML. Synthesis and antitumor activity of novel N’-(2-benzylthiobenzenesulfonyl)-1H-pyrazole-1-amidine derivatives. Heterocycles 2011;83:1093–1109.
- Kuo ChL, Assefa H, Brzozowski Z, Sławiński J, Sączewski F, Buolamwini IK, Neamati NJ. Application of CoMFA and CoMSIA 3D-QSAR and docking studies in optimization of mercaptobenzenesulfonamides as HIV-1 integrase inhibitors. J Med Chem 2004;47:385–399.
- Brzozowski Z, Sławiński J, Sączewski F, Sanchez T, Neamati N. Synthesis, anti-HIV-1 integrase, and cytotoxic activities of 4-chloro-N-(4-oxopyrimidin-2-yl)-2-mercaptobenzenesulfonamide derivatives. Eur J Med Chem 2008;43:1188–1198.
- Brzozowski Z, Sączewski F, Sławiński J, Sanchez T, Neamati N. Synthesis and anti-HIV-1 integrase activities of 3-aroyl-2,3-dihydro-1,1-dioxo-1,4,2-benzodithiazines. Eur J Med Chem 2009;44:190–196.
- Saczewski F, Innocenti A, Brzozowski Z, Slawinski J, Pomarnacka E, Kornicka A et al. Carbonic anhydrase inhibitors. Selective inhibition of human tumor-associated isozymes IX and XII and cytosolic isozymes I and II with some substituted-2-mercapto-benzenesulfonamides. J Enzyme Inhib Med Chem 2006;21:563–568.
- Sączewski F, Sławiński J, Kornicka A, Brzozowski Z, Pomarnacka E, Innocenti A et al. Carbonic anhydrase inhibitors. Inhibition of the cytosolic human isozymes I and II, and the transmembrane, tumor-associated isozymes IX and XII with substituted aromatic sulfonamides activatable in hypoxic tumors. Bioorg Med Chem Lett 2006;16:4846–4851.
- Foroumadi A, Emami S, Hassanzadeh A, Rajaee M, Sokhanvar K, Moshafi MH et al. Synthesis and antibacterial activity of N-(5-benzylthio-1,3,4-thiadiazol-2-yl) and N-(5-benzylsulfonyl-1,3,4-thiadiazol-2-yl)piperazinyl quinolone derivatives. Bioorg Med Chem Lett 2005;15:4488–4492.
- Kolavi G, Hegde V, Khazi I, Gadad P. Synthesis and evaluation of antitubercular activity of imidazo[2,1-b][1,3,4]thiadiazole derivatives. Bioorg Med Chem 2006;14:3069–3080.
- Hafez HN, Hegab MI, Ahmed-Farag IS, el-Gazzar AB. A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-9′,2-[1,3,4]thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg Med Chem Lett 2008;18:4538–4543.
- Kritsanida M, Mouroutsou A, Marakos P, Pouli N, Papakonstantinou-Garoufalias S, Pannecouque C et al. Synthesis and antiviral activity evaluation of some new 6-substituted 3-(1-adamantyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Farmaco 2002;57:253–257.
- Fujiwara M, Ijichi K, Konno K, Yokota T, Hanasaki Y, Watanabe H, Katsuura K, Shirakawa S, Takayama H, Sakai S, Shigeta S, Baba M. Thiadiazole derivatives as highly potent inhibitor of human immunodeficiency virus type 1 (HIV-1). Antiviral Res 1995;26:A254.
- Li Z, Wang X, Da Y. Synthesis of 2-(5-(2-chlorophenyl)-2-furoylamido)-5-aryloxymethyl-1,3,4-thiadiazoles under microwave irradiation. Synth Commun 2001;31:1829–1836.
- Supuran CT, Briganti F, Tilli S, Chegwidden WR, Scozzafava A. Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorg Med Chem 2001;9:703–714.
- Rzeski W, Matysiak J, Kandefer-Szerszen M. Anticancer, neuroprotective activities and computational studies of 2-amino-1,3,4-thiadiazole based compound. Bioorg Med Chem 2007;15:3201–3207.
- Klayman DL, Bartosevich JF, Griffin TS, Mason CJ, Scovill JP. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J Med Chem 1979;22:855–862.
- Kubota S, Uda M, Mori Y, Kametani F, Terada H. Syntheses and uncoupling activities of alkyl dithiocarbazates and alkyl pyridinecarbonyldithiocarbazates. J Med Chem 1978;21:591–594.
- Clinical Laboratory Standards Institute/ NCCLS: Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standards. 7th ed. CLSI document M11-A7. PA: Wayne, 2007.
- Forbes BA, Sahn DF, Weissfeld AS. Bailey and Scott’s Diagnostics Microbiology. 12th ed. St. Louis: Mosby Elsevier, 2007.
- Murray PR, editor. Manual of Clinical Microbiology. 9th ed. Washington, DC: American Society for Microbiology Press, 2007.
- Clinical Laboratory Standards Institute/ NCCLS: Methods of dilution antimicrobial susceptibility testing for bacteria that grow aerobically. Approved standards. 7th ed. CLSI document M7-A7. PA: Wayne, 2006.
- Brzozowski Z, Sławiński J. [1,1-dioxo-1,4,2-benzodithiazine derivatives. I. Synthesis of various 7-carboxy-3-mercapto-1,1-dioxo-1,4,2-benzodithiazine]. Acta Pol Pharm 1984;41:5–13.
- Brzozowski Z, Sławiński J. Pochodne 1,1-diokso-1,4,2-benzoditiazyny. 2. Syntezy niektórych pochodnych 3-merkapto-1,1-diokso-1,4,2-benzoditiazyny. Acta Polon Pharm 1984;41:133–139.
- Sławiński J. Syntheses and some reactions of 3-amino-6-chloro-7-methyl-1,1-dioxo-1,4,2-benzodithiazine. Pol J Chem 2001;75:1309–1316.
- Evers R, Fischer E, Pulkenat M. Struktur und Reaktionsverhalten aktivierter C-N-Doppelbindungen. Synthese substituierter 1,3,4-Thiadiazole. Z Chem 1980;20:413–414.
- García-Galán MaJ, Díaz-Cruz MS, Barceló D. Identification and determination of metabolites and degradation products of sulfonamide antibiotics. TrAC Trends in Anal Chem 2008;27:1008–1022.
