Abstract
The possible sulfatase activity of several carbonic anhydrase (CA, EC 4.2.1.1) isoforms have been investigated with a series of synthesized methanesulfonate derivatives of phenols. Four α-CA isozymes, i.e. hCA I, hCA II, hCA IV and hCA VI (h = human isoform), were included in the study. We evidenced that the original sulfonate esters are being hydrolyzed effectively to the corresponding phenols which there after act as CA inhibitors. The KI-s of these compounds ranged from 10.24 to 4012 µM against hCA I, 0.10 to 35.42 µM against hCA II, 0.49 to 45.06 µM against hCA IV and 3.27 to 608 µM against CA VI, respectively. The relevant sulfatase activity of CA with these esters is amazing considering the fact that 4-nitrophenyl-sulfate, an activated ester, is not a substrate of these enzymes.
Introduction
The carbonic anhydrases (CA, EC. 4.2.1.1) are a family of metalloenzymes, which participate primarily to the maintenance of pH homeostasis, catalyzing the reversible hydration of CO2 in a two-step reaction, to yield HCO3− and H+ (the physiological reaction in which CAs participate, reaction 1 in Citation1–3). The enzyme plays an important role in many physiological processes, e.g. is coupled with anion exchangers to form metabolons which enhance the chloride and bicarbonate transport across cell membranesCitation1–3. At least 16 CA isozymes have been described up to now in mammalsCitation4. Some of the isozymes are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), two are mitochondrial (CA VA and CA VB), one is secreted (CA VI), and others are membrane bound (CA IV, CA IX, CA XII, CA XIV and CA XVCitation1–8). Many such CA isozymes involved in these processes are important therapeutic targets with the potential to be inhibited/activated for the treatment of a range of disorders such as edema, glaucoma, obesity, cancer, epilepsy and osteoporosisCitation1–8.
Our groups recently investigated the interaction of CA I and II isozymes with several types of phenols, such as the simple phenol, hydroxy-/methoxysubstituted benzoic acids as well as di-/tri-methoxy benzenes, antioxidant bisphenols and several of its substituted derivatives, e.g. salicyclates and some of their derivativesCitation9–11. Here we extend these earlier investigations to a series of phenolic compounds, some of which are widely used as antioxidant food additives or as drugs and which are reported to possess anticancer, anti-carcinogenic, antimutagenic, antibacterial, antiviral or anti-inflammatory activitiesCitation5–11. Phenol 7, phenolic compounds 8–16 are widely used prodrugs or drugs. Vanillin (14) is another phenol derivative which is frequently used in food and drug industry due to its aroma. However, vanillin is used not only as a flavouring ingredient but also to mask undesirable off-flavours developed during storage of products susceptible to oxidative degradationCitation12.
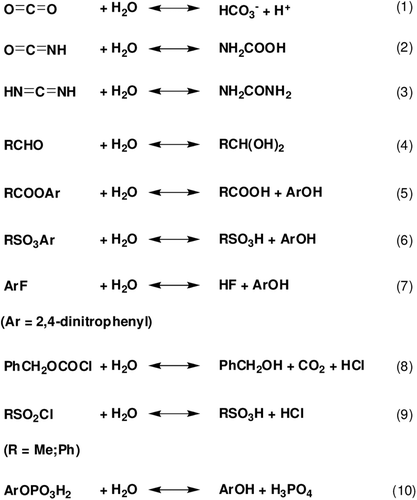
Isoform hCA II, one of the most effective and best studied isoenzyme, is not only a very effective catalyst for the physiological reaction, but also shows some catalytic versatility, participating in other hydrolytic processes which presumably involve non-physiological substrates. Some of these reactions include the hydration of cyanate to carbamic acid (reaction 2, Citation13), or of cyanamide to urea (reaction 3, Citation14), the aldehyde hydration to gem-diols (reaction 4Citation15); the hydrolysis of some carboxylicCitation16,Citation17 or sulfonic acid esters (reactions 5 and 6Citation18–22), as well as other less investigated hydrolytic processes in which aryl halides, chloroformates or sulfonyl chloridesCitation18–22 act as substrates, as described by eq. 7–9 of . A rather controversial issue regards the possible phosphatase activity of CA III, an isozyme with very low CO2 hydration activity, which has originally been reported to be a phosphatase (with 4-nitrophenyl phosphate as substrate, reaction 10 in Citation18–21), but subsequently this has been retractedCitation22, being considered that the phosphatase activity is due to another protein impurity present in the CA III preparations used in the earlier investigationsCitation21.
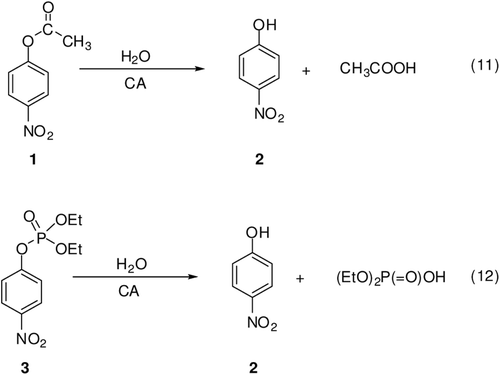
It is known that carboxylate/phosphate esters are hydrolyzed by α-CAs, although 4-nitrophenyl-sulfate was shown not to be a substrate for the cytosolic isoforms hCA I, II and XIIICitation2,Citation18. Recently, one of our groups reported kinetic study on the hydrolysis of 4-nitrophenyl acetate 1 and phsophate in the presence of three cytosolic CA isozymes, hCA I, hCA II and hCA XIIICitation18. In solution, these esters are hydrolyzed by the nucleophilic attack of water (or hydroxide ions) to the central atom (carbonyl CO for acetate 1, phsophorus for phosphate) with formation of a transition state from which the 4-nitrophenoxide is released. Considering the fact that CAs contain the equivalent of a strong base (hydroxide ions, HO− coordinated to the zinc ion) at neutral pH, due to the powerful activation of H2O by the zinc ion from the active site cavity and the hydrophobic environment of the protein, in principle, hydrolytic reactions 11–12 of should have the same mechanism as the hydrolysis catalyzed by bases in solution. In these studies, Innocenti et al.Citation2,Citation18 showed that the hydrolytic processes described by Eqs. 11–12 of , involve the active site Zn2+(OH)− functionality of the enzyme, that is, the same one responsible of the CO2 hydration activity of α-CAs. Probably, compounds 4–6 are hydrolyzed by carbonic anhydrase II in the same way in the current study. It is interesting to note here that the aromatic, methanesulfonate derivatives investigated here, like the aromatic activated one (Ester 1) indeed act as substrates for CAs. The sulfatase activity of this enzyme has been in fact discovered earlier with a cyclic sulfate ester as substrate, by Kaiser and LoCitation19.
Scheme 2. Reactions 11 and 12 catalyzed by α-CAs: 4-nitrophenyl acetate/phosphate hydrolysisCitation18.
We report here an inhibition study of the four catalytically active human isoforms hCA I, II, IV and VI with compounds 4−16. They incorporate methanesulfonate derivatives in their molecules and scaffolds representing thus an interesting starting point for different chemotypes belonging to the CA inhibitors (CAIs). In fact, in an earlier studyCitation23,Citation24 we reported micromolar/submicromolar inhibitors of the cytosolic isoforms hCA I and II with a library of organic nitrates.
Materials and methods
Phenol, biphenyl-4-ol, hydroquinone, pyrogallol, catechol, vanillin, guaiacol, CNBr-activated Sepharose 4B, protein assay reagents, p-aminobenzene sulfonamide L-tyrosine, 4-nitrophenyl acetate and chemicals for electrophoresis were purchased from Sigma-Aldrich Co., Germany. All other chemicals were analytical grade and obtained from Merck.
Protein determination
Protein quantity was determined spectrophotometrically at 595 nm according to the Bradford method during the purification steps, using bovine serum albumin as the standardCitation25.
Sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli procedureCitation26.
General procedure for synthesis of methanesulfonates
Triethylamine (each hydroxyde gruop in phenolic compound 1 eq.) was added to a solution of phenolic compound (1 eq.) and methanesulfonyl chloride (each hydroxyde gruop in phenolic compound 1 eq.) in dichloromethane (10 ml) in an ice bath and the resulting mixture was stirred between 30 minutes and 2 h at room temperature. Water was added to the mixture, which was extracted with dichloromethane. The organic phase was washed with water, brine, dried (Na2SO4), and concentrated. The obtained crude mesylates were crystallized from dichloromethane/hexane (20:1). The corresponding methanesulfonates 4–6, 11, 13, and 15 were obtained in 80–98% yields. Detailed synthetic procedures for the preparation of all derivatives can be found in Ref. Morita et al. (Citation2005)Citation27.
CA purification assay and kinetic studies
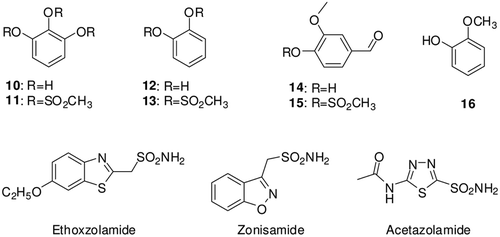
The purification of hCA isozymes was performed with a simple one step method by a Sepharose-4B-aniline-sulfanilamide affinity column, as described earlierCitation7,Citation8. Inhibitory effects of compounds phenyl methanesulfonate (4), biphenyl-4-yl methanesulfonate (5), 1,4-phenylene dimethanesulfonate (6), phenol (7), biphenyl-4-ol (8), hydroquinone (9), pyrogallol (10), benzene-1,2,3-triyl trimethanesulfonate (11), catechol (12), 1,2-phenylene dimethanesulfonate (13), vanillin (14), 4-formyl-2-methoxyphenyl methanesulfonate (15), and guaiacol (16) on these different isoenzymes. The activities of the tested compounds (as KI values) were calculated from Lineweaver–Burk plotsCitation28 and are given in .
Table 1. Inhibition data (KI values, μM) against hCA I, hCA II, hCA IV and hCA VI of compounds 4–16 and standard sulfonamide inhibitors (EZA, ZNS and AZA) by an esterase method with 4-nitrophenyl acetate as substrate.
Enzyme reactions
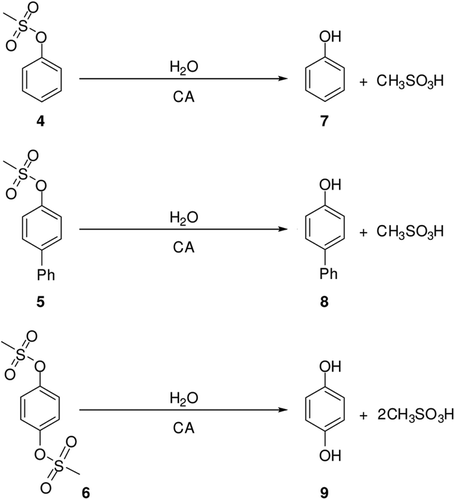
Enzyme mediated synthesis of phenols: The reactions were performed in the presence of hCA II in water at pH 7.5. A 10-fold excess of the starting methanesulfonates 4, 5, 6 was used to limit side reactions. Three reactions were performed with and without enzyme in a sodium phosphate solution at pH 7.4 (20 mM phosphate buffer). Stock solution in dimethyl sulfoxide of three methanesulfonates (10 mM) was added to three aqueous solutions in order to reach the final concentration of 0.08 mM. The clear mixture was incubated at 25°C for 20 minutes for 4, 5 and 6. The sulphate esters yielded the corresponding diols in 100% yield in 20 min. Prior to analysing the products, the mixture was left for 2 h to be separated from CA by decantation. The thermal denaturation of the enzyme (2 min at 80°C) was also tested to ensure the release from casting site of some possible tightly bound ligands.
Results and discussion
The CA isozymes play important roles in different tissuesCitation1–8. It is known that CA has been purified many times from different organisms and the effects of various chemicals, pesticides, anions, metal ions and drugs have been investigated on its activityCitation7–17,Citation29–33. In this study, CA I, II, IV and VI were purified from human erythrocytes and serum by a simple procedure using Sephrarose-4B-aniline-sulfanilamide affinity column. The activity of the effluents was determined by the hydratase method, with CO2 as substrate and further kinetic studies were performed using the esterase activity method, with 4-nitrophenyl acetate (NPA) as substrate. hCA I was purified, 102.4-fold with a specific activity of 873 EUmg−1 and overall yield of 54% and the hCA II enzyme was purified, 724.8-fold with a specific activity of 6193.1 EUmg−1 and overall yield of 44%. hCA IV was purified, 84.7-fold with a specific activity of 742 EUmg−1 and overall yield of 17.5%. Similarly, hCA VI was purified, 69.2-fold with a specific activity of 418 EUmg−1 and overall yield of 13.2%Citation22–37.
We report here a study on the inhibitory effects of methanesulfonate derivatives and some phenolic compounds on the CA esterase activity of isoforms hCA I, II, IV and VI. Data of show the following, regarding inhibition of hCA I, II, IV and VI with compounds 4–16 and with positive controls sulfonamides ethoxzolamide (EZA), zonisamide (ZNA) and acetazolamide (AZA):
Against the slow cytosolic isozyme hCA I, compounds 11–16 behave as weak, micromolar inhibitors, with KI values ranging from 60.23 to 4012 µM. Compound 12 was an ineffective hCA I inhibitor (KI of 4012 µM). A second group of derivative, including 4–6, showed better inhibitory activity as compared to the previously mentioned compounds 11–16, with KI values of 22.14–48.54 μM, (). Data of also show that similarly to phenolic compoundsCitation7–11, most of the investigated methanesulfonates (4, 5 and 6) act as competitive inhibitors with 4-NPA as substrate, i.e. they bind in the same regions of the active site cavity as the substrate. However the binding site of 4-NPA itself is unknown, but it is presumed to be in the same region as that of CO2, the physiological substrate of this enzymeCitation9–11. Similarly to salicylic acid derivatives and phenolic compounds investigated earlier by usCitation8,Citation26–33, the investigated compounds act as competitive inhibitors with 4-NPA as substrate, that is, they bind in same regions of the active site cavity as compared to the substrate.
A better inhibitory activity has been observed with compounds 14 and 16 investigated here for the inhibition of the rapid cytosolic isozyme hCA II (). Six derivatives, i.e. 4–8, 12 and 15 showed moderate hCA II inhibitory activity with KI-s in the range of 4.23–12.24 µM (), whereas the remaining two derivatives were quite effective hCA II inhibitors, with KI-s in the range of 0.1–0.52 µM, (). Structure–activity relationship is thus quite sharp for this small series of methanesulfonates scaffold compounds (4, 5 and 6) are ineffective leads. The best hCA II inhibitor in this series of derivatives 9.
Compound 11 and some of its congener such as compound 13 are also weak inhibitors of CA IV, with KI-s of 41.64 µM. However, again compounds 4–9, 11–13, 15, 16 and ZNA are medium potency inhibitors (KI-s of 3.56–38.45 µM), and compounds 10, 14, EZA and AZA show a higher affinity for this isozyme, with inhibition constant in the range of 0.49–1.84 µM ().
Phenol 12 and some of its congeners such as 4, 5, 7–10, 11–13 and 16 are also weak inhibitors of the secreted isozyme hCA VI, with KI-s of 85.04–608 µMCitation10. However, again the compounds 6 and 14 are medium potent inhibitors (KI of 12.1–28.43 µM), and derivatives 10, 14, EZA, ZNA and AZA show higher affinity for this isozyme, with inhibition constants in the range of 0.34–6.83 µM ().
We hypothesize that CAs (which as we show above, possess esterase activity against several substrates) hydrolyses these methanesulfonates leading to methanesulfonic acid and related phenols, as illustrated in . When (13) giving 12 and (15) giving 14 are compared by hydrolysis, the carbonyl group acting as an electronic withdrawing group might help this hydrolysis sequence through an increase of the electrophilic character of the sulfur atom ().
In recent studies, it was reported that phenols and natural phenolic compounds act as CAIs, and could represent the starting point for a new class of inhibitors that may have advantages for patients with sulfonamide allergies (thioxolone acts as a prodrugCitation7–11,Citation13–17,Citation23,Citation24).
Several multiple sulfated compounds have been found in biologically active compounds and marine organismsCitation38. For instance, sulfated sterols have exhibited effects such as anti-HIV, antiviral activity, and inhibition of protein tyrosine kinasesCitation38–40. Recent studies also showed that sulfo-containing salicylic acid derivatives have inhibitory effects on CA I and II isoenzymesCitation38. However, it is critically important to explore further classes of potent CAIs in order to detect compounds with a different inhibition profiles as compared to the sulfonamides and their bioisosteres and to find novel applications for the inhibitors of these widespread enzymes, in particular tumor-related isoforms. Studies regarding interactions of different proteins and enzymes are of particular interest for toxicologists and medicinal chemistsCitation41–48 and many such investigations exist in the literatureCitation49–55. The discovery of novel inhibitors of CAs has also gained great attention in the recent years.
Phenolic compounds 7, 8 and 9 were synthesized from methanesulfonates 4, 5 and 6 using carbonic anhydrase II isoenzyme. Compounds 7–10, 12, 14, and 16 affect the activity of CA isozymes due to the presence of the functional group (OH) in their aromatic scaffold. Our findings indicate thus another class of possible CAIs of interest, in addition to the well-known sulfonamides/sulfamates/sulfamides, although their mechanism of CA inhibition remains rather elusive at this moment. Indeed, some methanesulfonates investigated here showed moderate CA I, II, IV and VI inhibitory activity in the micromolar range by the esterase assay method. These findings point out that substituted methanesulfonates may be used as leads for generating more potent CAIs eventually targeting other isoforms which have not been assayed yet for their interactions with such agents.
Declaration of interest
This study was financed by Turkish Republic Prime Ministry State Planning Organization (DPT), (Project no: 2010K120440) for (MS). Work from Supuran lab was financed by an FP 7 EU grant (Metoxia project).
References
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Innocenti A, Scozzafava A, Parkkila S, Puccetti L, De Simone G, Supuran CT. Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg Med Chem Lett 2008;18:2267–2271.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–777.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Parkkila S, Parkkila AK. Carbonic anhydrase in the alimentary tract. Roles of the different isozymes and salivary factors in the maintenance of optimal conditions in the gastrointestinal canal. Scand J Gastroenterol 1996;31:305–317.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Mercan L, Okumus A, Sentürk M, Ekinci D. In vitro enzymatic response of Turkish native chicken “Gerze” to heavy metal exposure. J Enzyme Inhib Med Chem 2011. (In Press).
- Cavdar H, Ekinci D, Talaz O, Saraçoglu N, Sentürk M, Supuran CT. a-Carbonic anhydrases are sulfatases with cyclic diol monosulfate esters. J Enzyme Inhib Med Chem 2011. (In Press).
- Nair SK, Ludwig PA, Christianson DW. Two-site binding of phenol in the active site of human carbonic anhydrase II: Structural implications for substrate association. J Am Chem Soc 1994;116:3659–3660.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–1587.
- Durdagi S, Sentürk M, Ekinci D, Balaydin HT, Göksu S, Küfrevioglu ÖI, et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–1389.
- Burri J, Graf M, Lambelet P, Loliger J., Vanillin. More than a flavouring agent-a potent antioxidant. J Sci Food Agric 1989;48:49–56.
- Supuran CT, Conroy CW, Maren TH. Is cyanate a carbonic anhydrase substrate? Proteins 1997;27:272–278.
- Briganti F, Mangani S, Scozzafava A, Vernaglione G, Supuran CT. Carbonic anhydrase catalyzes cyanamide hydration to urea: Is it mimicking the physiological reaction? J Biol Inorg Chem 1999;4:528–536.
- Pocker Y, Dickerson DG. The catalytic versatility of erythrocyte carbonic anhydrase. V. Kinetic studies of enzyme-catalyzed hydrations of aliphatic aldehydes. Biochemistry 1968;7:1995–2004.
- Pocker Y, Meany JE. The catalytic versatility of erythrocyte carbonic anhydrase. II. Kinetic studies of the enzyme-catalyzed hydration of pyridine aldehydes. Biochemistry 1967;6:239–246.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Innocenti A, Supuran CT. Paraoxon, 4-nitrophenyl phosphate and acetate are substrates of a- but not of β-, γ- and ζ-carbonic anhydrases. Bioorg Med Chem Lett 2010;20:6208–6212.
- Kaiser ET, Lo KW. The Carbonic anhydrase catalyzed hydrolysis of 2-hydroxy-5-nitro-α-toluenesuIfonic acid sultone. J Am Chem Soc 1969;91:4912–4918.
- Henkart P, Guidotti G, Edsall JT. Catalysis of the hydrolysis of 1-fluoro-2,4-dinitrobenzene by carbonic anhydrase. J Biol Chem 1968;243:2447–2449.
- Register AM, Koester MK, Noltmann EA. Identification of pseudo-isoenzymic subforms of muscle carbonic anhydrase. J Biol Chem 1978;253:4143–4152.
- Kim G, Selengut J, Levine RL. Carbonic anhydrase III: The phosphatase activity is extrinsic. Arch Biochem Biophys 2000;377:334–340.
- Ekinci D, Cavdar H, Talaz O, Sentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–3563.
- Ekinci D, al-Rashida M, Abbas G, Senturk M, Supuran CT. Chromone containing sulfonamides as potent carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.614607.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli DK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Morita J, Nakatsuji H, Misaki T, Tanabe Y. Green Chem 2005;7:711–715.
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934;56:658–666.
- Ekinci D, Kurbanoglu NI, Salamci E, Senturk M, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of human and bovine isoenzymes by benzenesulfonamides, cyclitols and phenolic compounds. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.621122.
- Alp C, Ekinci D, Gültekin MS, Sentürk M, Sahin E, Küfrevioglu OI. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–4474.
- Ekinci D, Senturk M. Assesment of metal inhibition of antioxidant enzyme glutathione reductase from rainbow trout liver. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.615745.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Wistrand PJ, Carter ND, Conroy CW, Mahieu I. Carbonic anhydrase IV activity is localized on the exterior surface of human erythrocytes. Acta Physiol Scand 1999;165:211–218.
- Whitney PL, Briggle TV. Membrane-associated carbonic anhydrase purified from bovine lung. J Biol Chem 1982;257:12056–12059.
- Kivelä J, Parkkila S, Waheed A, Parkkila AK, Sly WS, Rajaniemi H. Secretory carbonic anhydrase isoenzyme (CA VI) in human serum. Clin Chem 1997;43:2318–2322.
- Karhumaa P, Leinonen J, Parkkila S, Kaunisto K, Tapanainen J, Rajaniemi H. The identification of secreted carbonic anhydrase VI as a constitutive glycoprotein of human and rat milk. Proc Natl Acad Sci USA 2001;98:11604–11608.
- Kornprobst JM, Sallenave C, Barnathan G. Sulfated compounds from marine organisms. Comp Biochem Physiol B, Biochem Mol Biol 1998;119:1–51.
- McKee TC, Cardellina JH 2nd, Riccio R, D’Auria MV, Iorizzi M, Minale L et al. HIV-inhibitory natural products. 11. Comparative studies of sulfated sterols from marine invertebrates. J Med Chem 1994;37:793–797.
- Anderson CJ, Lucas LJH, Widlanski TS. Molecular recognition in biological systems: Phosphate esters vs. sulfate esters and the mechanism of action of steroid sulfatases. J Am Chem Soc 1995;117:3889–3890.
- Senturk M, Ekinci D, Goksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.591290.
- Balaydin HT, Durdagi S, Ekinci D, Senturk M, Goksu S, Menzek A. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, metoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2011. DOI:10.3109/14756366.2011.596836.
- Balaydin HT, Soyut H, Ekinci D, Goksu S, Beydemir S, Menzek A, Sahin E. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.574131.
- Ceyhun SB, Sentürk M, Ekinci D, Erdogan O, Ciltas A, Kocaman EM. Deltamethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:215–223.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Ekinci D, Ceyhun SB, Aksakal E, Erdogan O. IGF and GH mRNA levels are suppressed upon exposure to micromolar concentrations of cobalt and zinc in rainbow trout white muscle. Comp Biochem Physiol C Toxicol Pharmacol 2011;153:336–341.
- Erdogan O, Ceyhun SB, Ekinci D, Aksakal E. Impact of deltamethrin exposure on mRNA expression levels of metallothionein A, B and cytochrome P450 1A in rainbow trout muscles. Gene 2011;484:13–17.
- Cakmak R, Durdagi S, Ekinci D, Sentürk M, Topal G. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett 2011;21:5398–5402.
- Ceyhun SB, Aksakal E, Ekinci D, Erdogan O, Beydemir S. Influence of cobalt and zinc exposure on mRNA expression profiles of metallothionein and cytocrome P450 in rainbow trout. Biol Trace Elem Res 2011. DOI: 10.1007/s12011-011-9068-z.
- Aksakal E, Ekinci D, Erdogan O, Beydemir S, Alim Z, Ceyhun SB. Increasing stocking density causes inhibition of metabolic-antioxidant enzymes and elevates mRNA levels of heat shock protein 70 in rainbow trout. Livest Sci 2011;141:69–75.
- Siktar E, Ekinci D, Siktar E, Beydemir S, Gülçin I, Günay M. Protective role of l-carnitine supplementation against exhaustive exercise induced oxidative stress in rats. Eur J Pharmacol 2011;668:407–413.
- Ekinci D, Beydemir S, Küfrevioglu OI. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 2007;22:745–750.
- Senturk M, Ekinci D, Alici HA, Beydemir S. Paraoxonase-1, an organophosphate detoxifier and cardioprotective enzyme, is inhibited by anesthetics: An in vitro and in vivo insight. Pestic Biochem Physiol 2011. DOI:10.1016/j.pestbp.2011.09.007.
- Sentürk M, Talaz O, Ekinci D, Cavdar H, Küfrevioglu OI. In vitro inhibition of human erythrocyte glutathione reductase by some new organic nitrates. Bioorg Med Chem Lett 2009;19:3661–3663.
- Aksakal E, Ceyhun SB, Erdogan O, Ekinci D. Acute and long-term genotoxicity of deltamethrin to insulin-like growth factors and growth hormone in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:451–455.