Abstract
A series of benzofused sultams and fluorinated benzenesulfonamides were synthesized in superacid HF/SbF5 from simple N-allylic derivatives. Almost all of these original compounds showed micromolar inhibitory activities against carbonic anhydrases I and II. The fluorinated derivatives inhibit better the tumor-associated isoforms IX and XII, and one of the tested compounds showed inhibition in the nanomolar range.
Introduction
Sixteen different α-carbonic anhydrase isoforms have been isolated and characterized in mammals and many of them are well established therapeutic targets to treat a wide range of disordersCitation1–4. Although inhibition of α-carbonic anhydrases (α-CAs) by aromatic and heterocyclic sulfonamides has been largely studied for 50 years with applications in the treatment of various diseasesCitation5–7, this class of compounds remains an attractive chemical family in the design of new inhibitors, as shown by the ongoing research in this fieldCitation8–12. As already reported, small modifications on benzenesulfonamides can induce significant differences in the bioactive profile of this type of compounds. For example, a modification on the aromatic ring can induce very different behavior against human carbonic anhydrases (, ) Citation11,Citation13.
Table 1. Inhibition data of chlorthalidone 1, indapamide 2 and furosemide 3 against isoforms hCA I, II, IX and XII.
Figure 1. Comparison of interactions in which the thiazide/high-celling diuretics chlorthalidone 1 (A), indapamide 2 (B) and furosemide 3 (C) participate when bound to the hCA II active site, as observed in the corresponding X-ray crystal structures (PDB files 3F4X, 3BL1 and 1Z9Y).
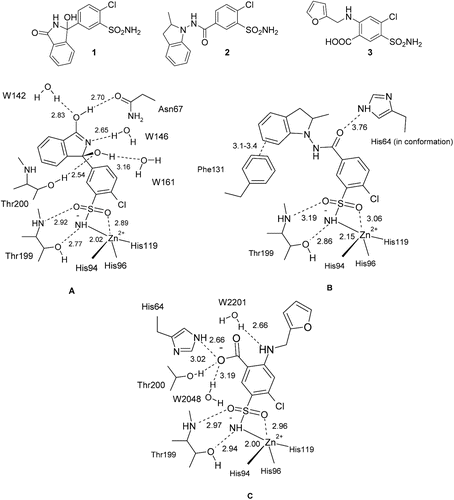
While chlorthalidone 1 and furosemide 3 are efficient inhibitors against CA II, indapamide 2 is a much weaker one. However, this latter one shows good inhibition activity against isozyme IX and XII whereas furosemide 3 is significantly weaker. X-ray crystal structures of their hCA II adducts (), shows several active site water molecules, interactions which may be responsible for this difference of activity. In addition, the variety of aromatic/heterocyclic sulfonamides showing affinity for hCA IX isoform suggesting value in carrying on a drug design campaign of inhibitors in this family. Herein, we report a series of new benzofused sultams, β-fluorinated benzenesulfonamides and 4-aminobenzenesulfonamides, synthesized in superacid HF/SbF5Citation14, which show hCAs inhibition properties.
Material and methods
Chemistry
The authors draw the reader’s attention to the dangerous features of superacidic chemistry. Handling of hydrogen fluoride and antimony pentafluoride must be done by experienced chemists with all the necessary safety arrangements in place.
Reactions performed in superacid were carried out in a sealed Teflon® flask with a magnetic stirrer. No further precautions have to be taken to prevent mixture from moisture (test reaction worked out in anhydrous conditions leads to the same results as expected).
Yields refer to isolated pure products.
1H, 13C and 19F NMR were recorded on a 300 MHz and 400 MHz spectrometers using CDCl3 as solvent. COSY 1H-1H and 1H-13C experiments were used to confirm the NMR peaks assignments.
Melting points were determined in a capillary tube and are uncorrected.
Mass spectra were performed with coupled gas chromatography (electronic impact).
All separations were done under flash-chromatography conditions on silica gel (15–40 µm).
Optimized procedure to synthesize benzofused sultams of type A
To a mixture of HF/SbF5 (6 mL, 4/2 molar ratio) maintained at −20°C, was added nitrogen derivative (1 mmol). The mixture was magnetically stirred at the same temperature for reaction time. The reaction mixture was then neutralized with water-ice-Na2CO3, extracted with dichloromethane (×3). The combined organic phases were dried (MgSO4) and concentrated in vacuo. Products were isolated by column chromatography over silica gel.
For example: Compound A1: 4,6-dimethyl-3,4-dihydro-2H-benzo[e][1,2]thiazine 1,1-dioxide
Optimized procedure (10 min reaction time) was followed, starting from 211 mg of N-tosyl-N-allylamine (1 mmol). Purification by flash column chromatography (98/2: dichloromethane/methanol) afforded 270 mg of the title compound as a colourless oil (64%).
1H NMR (300 MHz, CDCl3, ppm): 1.34 (d, J = 7.2 Hz, 3H, CH3), 2.37 (s, 3H, CH3), 2.98 (m, 1H, H-4), 3.41 (m, 1H, H-3), 3.82 (m, 1H, H-3), 4.89 (t, J = 7.7 Hz, 1H, NH), 7.09 (s, 1H, H-5), 7.14 (d, J = 8.1 Hz, 1H, H-7), 7.62 (d, J = 8.1 Hz, 1H, H-8). 13C NMR (75 MHz, CDCl3, ppm): 19.5 (CH3), 21.6 (CH3), 31.5 (CH, C-4), 48.2 (CH2, C-3), 124.0 (CH, C-7), 128.2 (CH, C-8), 129.0 (C-5), 134.2 (C-8a), 140.2 (C-6), 142.7 (C-4a). MS (GCT, CI+): m/z (relative intensity %) 211 [M]+ (40). HRMS (ESI): Calc for C10H13NO2S: 211.06670, found 211.0664.
Optimized procedure to synthesize β-fluorinated benzenesulfonamides of type B
To a mixture of HF/SbF5 (3 mL, 7/1 molar ratio) maintained at −20°C, was added nitrogen derivative (1 mmol). The mixture was magnetically stirred at the same temperature for reaction time. The reaction mixture was then neutralized with water-ice-Na2CO3, extracted with dichloromethane (×3). The combined organic phases were dried (MgSO4) and concentrated in vacuo. Products were isolated by column chromatography over silica gel.
For example: Compound B4: 4-fluoro-N-(2-fluoro-propyl)-benzenesulfonamide
Optimized procedure (10 min reaction time) was followed; starting from 215 mg of the corresponding allylic starting material (1 mmol), 207 mg of the title compound (88%) was obtained without purification as a solid.
1H NMR (300 MHz, CDCl3, ppm): 1.20 (dd, 3JHF = 23.9 Hz, J = 6.2 Hz, 3H, H-3′), 3.03 (m, 2H, H-1′), 4.62 (dm, 2JHF = 48.8 Hz, 1H, H-2′), 5.47 (bs, 1H, NH), 7.11 (t, 3JHF = 8.6 Hz, 2H, H-3), 7.80 (m, 2H, H-2). 13C NMR (75 MHz, CDCl3, ppm): 18.4 (d, 2JC-F = 22 Hz, CH3, C-3′), 48.5 (d, 2JC-F = 21 Hz, CH2, C-1′), 89.5 (d, 1JC-F = 167 Hz, CH, C-2′), 116.8 (d, 2JC-F = 22 Hz, 2CH, C-3), 130.1 (d, 3JC-F = 9 Hz, 2CH, C-2), 136.3 (d, 4JC-F = 3 Hz, C-1), 165.5 (d, 1JC-F = 253 Hz, C-4). 19F {1H} NMR (282 MHz, CDCl3, ppm): -105.5 (CF) and -180.1 (CHF). MS (GCT, CI+): m/z (relative intensity %) 188 [M-CH3CHF]+ (80). HRMS (ESI): Calc for: (C9H11NO2F2S) 235.04786, found 235.0458. Melting Point (°C): 91.7.
CA inhibition assay
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activityCitation15. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constants. For each inhibitor, at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with distilled-deionized water. Inhibitor and enzyme solutions were preincubated together for 15 min at room temperature prior to assay, in order to allow for the formation of the E-I complex. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3, as reported earlier, and represent the mean from at least three different determinationsCitation16. CA isofoms were recombinant ones obtained in house as reported earlierCitation16,Citation17.
Results and discussion
Chemistry
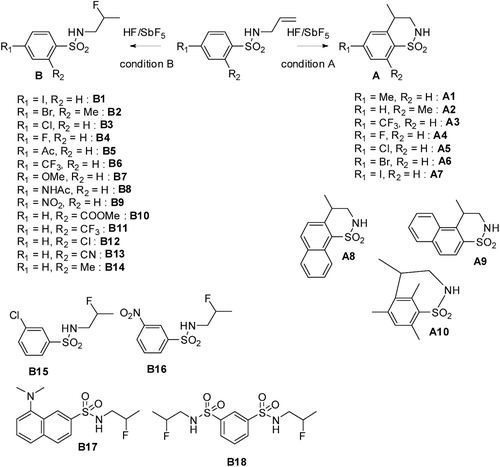
In due course of a part of our work focused on the development of new reactions in superacid to access to fluorinated nitrogen containing compounds, going from high valued building blocksCitation18–20 to bioactive elaborated moleculesCitation21,Citation22, we recently applied this original chemistry toward the synthesis of novel compounds in the benzenesulfonamide familyCitation23. Starting from simple N-allylic benzenesulfonamides, after superelectrophilic activationCitation24, either benzofused sultams of type A are obtained after intramolecular Friedel-Crafts reaction (condition A) or β-fluorinated benzenesulfonamides of type B can be formed after hydrofluorination reaction (condition B). This method allowed the synthesis of a large variety of new benzofused sultams (A1–A10) and β-fluorinated benzene sulfonamides (B1–B18) ().
Scheme 1. Synthesis of benzofuzed sultams A and β-fluorinated benzenesulfonamides B in superacid HF/SbF5. Condition A: 10 min, −20°C, substrate concentration c = 0.16 mol·L−1, % mol SbF5 = 13.6; Condition B: 10 min, −20°C, substrate concentration c = 0.33 mol·L−1, % mol SbF5 = 3.8.
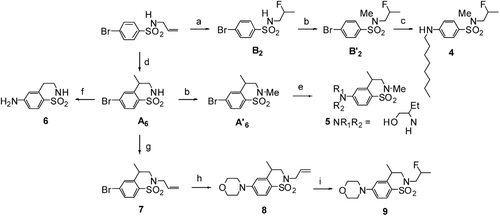
In addition, by following synthetic sequences involving superacid chemistry at various stages, 4-aminobenzenesulfonamides 4–6 and 9 were formed ()Citation25.
Scheme 2. Synthesis of 4-aminobenzofuzed sultams 4, 5, 6 and 9. (a) HF/SbF5 (% mol SbF5 = 3.8), −20°C, 10 min, 42%. (b) CH3I, K2CO3, DMF, rt, 12 h, >95% (c) CuI, L-proline, octylamine, K3PO4, DMSO, 90°C, 24 h, 62%. (d) HF/SbF5 (% mol SbF5 = 13.6), −20°C, 10 min, 62%.(e) CuI, L-proline, 2-aminobutanol, Cs2CO3, DMSO, 90°C, 24 h, 70%. (f) CuI, L-proline, aq. NH3, K3PO4, DMSO, 90°C, 24 h, 81%. (g) allylbromide, K2CO3, DMF, rt, 12 h, 83%. (h) CuI, L-proline, morpholine, K3PO4, DMSO, 90°C, 24 h, 70%. (i) HF/SbF5 (% mol SbF5 = 3.8), −20°C, 10 min, 69%.
Carbonic anhydrase inhibition
Compounds A1–A10 were screened for the inhibition of cytosolic isoforms hCA I and II ()Citation15–17. The benzofused sultams A1–A10 showed inhibition constants in the range of 3–51 µM against the slow cytosolic isoform hCA I.
Table 2. hCA I and II inhibition data with sulfonamides A1–A10, by the CO2 hydrase methodCitation15.
The inhibition constants against the physiologically dominant isoform hCA II were in the range of 4.4–85 µM. Among the tested benzofused sultams, the 4-halogenated substrates A5–A7 showed the best inhibition, and were around one order of magnitude more effective compared to the other substrates. This effect of the halogen on the inhibitor activity against hCA, difficult to be explained at this stage, can be compared to similar reported effects of halogen on sulfanilamide derivativesCitation16,Citation17. However, the 4-fluorinated benzofused sultam A4 did not show a similar activity. In addition, a steric hindrance effect seems to be in favor of hCA II inhibition compared to hCA I, as shown by the obtained results from substrates A8–A10.
The research focused on the involvement of isozymes CA IX and XII in cancer has progressed significantly in recent years. These isozymes are overexpressed in tumor cells in response to the hypoxia inducible factor (HIF) pathwayCitation26. Since the report on the X-ray crystal structure of CA IXCitation27, many classes of compounds have been tested as CA IX inhibitorsCitation26. Among them, fluorine containing sulfonamides showed good affinity for this isoformCitation28. In addition, by testing α-fluoro and α,α-difluoro-benzenemethanesulfonamides, it has been reported that after α-fluorination of sulfonamide, through the electron withdrawing effect of fluorine atom(s)Citation29, a correlation between sulfonamide acidity enhancement and carbonic anhydrase inhibition existsCitation30. Taking into account these data a series of β-fluorinated benzenesulfonamides B1–B18 were tested as inhibitors against hCA I and II, and against tumor-associated isozymes hCA IX and XII ()Citation15–17.
Table 3. CA inhibition data with β-fluorinated derivatives B1–B18 and 4-aminobenzenesulfonamides 4–6, 9 and SA as standard inhibitor, against isozymes I, II, IX and XII, by a stopped-flow CO2 hydrase assay.
All the β-fluorinated benzene sulfonamides are potent hCA inhibitors with very close inhibition constants of 3.1–4.8 µM against the cytosolic form hCA I and of 2.0–4.5 µM against hCA II. Although the previously described benzofused sultams showed low micromolar inhibition constants, the linear β-fluorinated benzene sulfonamides are better inhibitors for hCA I and II. These observations seem to underline a potent fluorine influence on isozyme inhibition, which could be attributed to a modification of the sulfonamide function pKa. However, no significant effect of the aromatic substitution, either on the inhibition or on the selectivity, can be taken out from these results. The tumor-associated transmembrane isoforms hCA IX and XII were also inhibited by all the tested β-fluorinated benzene sulfonamides with inhibition constants in the range of 2.1–5.7 µM against hCA IX and of 1.1–4.6 µM against hCA XII. Again no real SAR emerged from different substitution patterns.
Taking into account the known hCA inhibitor properties of 4-aminobenzene sulfonamides such as sulfanilamide SACitation31, four 4-aminobenzenesulfonamides 4–6 and 9 were also tested and compared to SA (). Unfortunately, N-substituted 4-aminobenzenesulfonamides 4, 5 and 9 were found to be inactive toward hCAs. By considering a potent negative effect of the substitution on the nitrogen atom, corresponding primary amine 6 was tested. This compound was found to be the best carbonic anhydrase inhibitor tested in this project. Compound 6 showed nanomolar inhibition potency against the four tested isoforms and was more active than SA against the tumor-associated isoform IX. The selectivity ratio for the inhibition of the tumor-associated isoforms hCA IX and XII over the physiologically dominant isoform hCA II is of crucial importance in the search of new lead compounds that selectively inhibit hCA IX and XII. Interestingly, even if the inhibitor potency of compound 6 is medium, it can be considered as a selective inhibitor of tumor-associated isoforms over cytosolic ones (selectivity ratio I/IX = 4.7; I/XII = 2.1; II/IX = 3.2; II/XII = 1.4) and a new lead compound for further investigations.
Conclusion
In conclusion, we report here a series of new benzofused sultams, β-fluorinated benzenesulfonamides and 4-aminobenzofused sultams prepared by using superacid chemistry. These compounds have been investigated as inhibitors of cytosolic human carbonic anhydrases I and II and transmembrane tumor-associated carbonic anhydrases isoforms IX and XII. For almost all the tested compounds, inhibition at very low micromolar range has been observed against the tested hCAs. One compound showing inhibition at the nanomolar level has been identified. These results emphasize the potential of using superacid chemistry as a new synthetic tool in the discovery of new carbonic anhydrase inhibitors with potential use as antitumor agentsCitation31,Citation32.
Acknowledgement
We thank the region Poitou-Charentes (grant to F.Liu.), the CNRS and the University of Poitiers for financial support and an EU FP7 grant (Metoxia, to CTS).
Declaration of interest
Financial support and EU FP7 grant (Metoxia, to CTS) were provided by the region Poitou-Charentes (grant to F.Liu.), the CNRS and the University of Poitierregion. The authors report no declarations of interest.
References
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Supuran CT. Carbonic Anhydrases as Drug Targets—General Presentation. In: Supuran CT, Winum JY, (eds). Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications. Hoboken, NJ: Wiley; 2009. pp 15–38.
- Winum JY, Rami M, Scozzafava A, Montero JL, Supuran C. Carbonic anhydrase IX: A new druggable target for the design of antitumor agents. Med Res Rev 2008;28:445–463.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Thiry A, Dogné JM, Masereel B, Supuran CT. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 2006;27:566–573.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Supuran CT. Carbonic anhydrase inhibition/activation: Trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr Pharm Des 2010;16:3233–3245.
- Rami M, Maresca A, Smaine FZ, Montero JL, Scozzafava A, Winum JY et al. Sulfonamides incorporating boroxazolidone moieties are potent inhibitors of the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII. Bioorg Med Chem Lett 2011;21:2975–2979.
- Capkauskaite E, Baranauskiene L, Golovenko D, Manakova E, Gražulis S, Tumkevicius S et al. Indapamide-like benzenesulfonamides as inhibitors of carbonic anhydrases I, II, VII, and XIII. Bioorg Med Chem 2010;18:7357–7364.
- Mader P, Brynda J, Gitto R, Agnello S, Pachl P, Supuran CT et al. Structural basis for the interaction between carbonic anhydrase and 1,2,3,4-tetrahydroisoquinolin-2-ylsulfonamides. J Med Chem 2011;54:2522–2526.
- Mincione F, Benedini F, Biondi S, Cecchi A, Temperini C, Formicola G et al. Synthesis and crystallographic analysis of new sulfonamides incorporating NO-donating moieties with potent antiglaucoma action. Bioorg Med Chem Lett 2011;21:3216–3221.
- Bertucci A, Innocenti A, Scozzafava A, Tambutté S, Zoccola D, Supuran CT. Carbonic anhydrase inhibitors. Inhibition studies with anions and sulfonamides of a new cytosolic enzyme from the scleractinian coral Stylophora pistillata. Bioorg Med Chem Lett 2011;21:710–714.
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–3474.
- Olah GA, Prakash GKS, Molnar A, Sommer J. Superacids, 2nd Edition. New York: Wiley; 2009.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–2573.
- Ilies MA, Vullo D, Pastorek J, Scozzafava A, Ilies M, Caproiu MT et al. Carbonic anhydrase inhibitors. Inhibition of tumor-associated isozyme IX by halogenosulfanilamide and halogenophenylaminobenzolamide derivatives. J Med Chem 2003;46:2187–2196.
- Biswas S, Aggarwal M, Güzel Ö, Scozzafava A, McKenna R, Supuran CT. Conformational variability of different sulfonamide inhibitors with thienyl-acetamido moieties attributes to differential binding in the active site of cytosolic human carbonic anhydrase isoforms. Bioorg Med Chem 2011;19:3732–3738.
- Jacquesy J-C. Organic Synthesis in Superacid. In: Olah GA, (ed). Carbocation Chemistry. New York: Wiley; 2009. pp 359–376.
- Thibaudeau S, Martin-Mingot A, Jouannetaud M-P, Karam O, Zunino F. A novel, facile route to β-fluoroamines by hydrofluorination reaction in superacid HF-SbF5. Chem Commun 2007;3198–3200.
- Vardelle E, Gamba-Sanchez D, Martin-Mingot A, Jouannetaud M-P, Thibaudeau S, Marrot J. Cyclisation/fluoriantion of nitrogen containing dienes in superacid HF-SbF5: A new route to 3- and 4-fluoropiperidines. Chem Commun 2008;1473–1475.
- Fahy J, Duflos A, Ribert J-P, Jacquesy J-C, Berrier C, Jouannetaud M-P, Zunino F. Vinca alkaloids in superacidic media: A method for creating a new family of antitumor derivatives. J Am Chem Soc 1997;119:8576–8577.
- Debarge S, Thibaudeau S, Violeau B, Martin-Minfot A, Jouannetaud M-P, Jacquesy J-C, Cousson A. Rearrangement or gem-difluorination of quinine and 9-epiquinine and their acetates in superacid. Tetrahedron 2005;61:2065–2073.
- Liu F, Martin-Mingot A, Jouannetaud MP, Zunino F, Thibaudeau S. Superelectrophilic activation in superacid HF/SbF(5) and synthesis of benzofused sultams. Org Lett 2010;12:868–871.
- Olah GA, Klumpp D. Superelectrophiles and their chemistry. New York: Wiley; 2008.
- Liu F, Musadji NY, Lecornué F, Jouannetaud M-P, Thibaudeau S. Superacid mediated reactions applied to 4-aminobenzofused sultams and fluorinated 4-aminobenzene sulfonamides synthesis. Tetrahedron 2010;66:7112–7118.
- Thiry A, Supuran CT, Masereel B, Dogné JM. Recent developments of carbonic anhydrase inhibitors as potential anticancer drugs. J Med Chem 2008;51:3051–3056.
- Alterio V, Hilvo M, Di Fiore A, Supuran CT, Pan P, Parkkila S et al. Crystal structure of the catalytic domain of the tumor-associated human carbonic anhydrase IX. Proc Natl Acad Sci USA 2009;106:16233–16238.
- Vullo D, Scozzafava A, Pastorekova S, Pastorek J, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of the tumor-associated isozyme IX with fluorine-containing sulfonamides. The first subnanomolar CA IX inhibitor discovered. Bioorg Med Chem Lett 2004;14:2351–2356.
- O’Hagan D. Understanding organofluorine chemistry. An introduction to the C-F bond. Chem Soc Rev 2008;37:308–319.
- Blackburn GM, Türkmen H. Synthesis of alpha-fluoro- and alpha, alpha-difluoro-benzenemethanesulfonamides: New inhibitors of carbonic anhydrase. Org Biomol Chem 2005;3:225–226.
- Alterio V, Di Fiore A, D’Ambrosio K, Supuran CT, De Simone G. X-ray crystallography of ca inhibitors and its importance in drug design. In: Supuran CT, Winum JY ed,. Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications. Hoboken, NJ: Wiley; 2009. pp 73–138.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–777.