Abstract
Carbonic anhydrases (CAs, EC 4.2.1.1) were purified from sheep kidney (sCA IV), from the liver of the teleost fish Dicentrarchus labrax (dCA) and from human erythrocytes (hCA I and hCA II). The purification procedure consisted of a single step affinity chromatography on Sepharose 4B-tyrosine-sulfanilamide. The kinetic parameters of these enzymes were determined for their esterase activity with 4-nitrophenyl acetate as substrate. The following metal ions, Pb2+, Co2+, Hg2+, Cd2+, Zn2+, Se2+, Cu2+, Al3+ and Mn3+ showed inhibitory effects on these enzymes. The tested metal ions inhibited these CAs competitively in the low milimolar/submillimolar range. The susceptibility to various cations inhibitors differs significantly between these vertebrate α-CAs and is probably due to their binding to His64 or the histidine cluster.
Introduction
Carbonic anhydrases (CAs; EC 4.2.1.1) are ubiquitous metalloenzymes present in all living organisms that are encoded by five different unrelated gene families. These are the α-CAs (present in vertebrates, bacteria, algae and cytoplasm of green plants), the β-CAs (predominantly in bacteria, algae, chloroplasts of monodicotyledons and dicotyledons), the γ-CAs (mainly in archaea and some bacteria), the δ- and ζ-CAs (present in marine diatomsCitation1–4). Up to date, 16 α-CA isozymes of have been described in mammals, which have various subcellular localizations, a different catalytic activity and susceptibility to different classes of inhibitorsCitation1–4. Some of these isozymes are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), others are membrane bound (CA IV, CA IX, CA XII, CA XIV and CA XV), two are mitochondrial (CA VA and CA VB), and one is secreted in saliva (CA VI). CA XV is an isoform not expressed in humans or other primates, but it is abundant in rodents and other higher vertebratesCitation5–8. These enzymes catalyze the reversible hydration of carbon dioxide in a two-step reaction to yield bicarbonate and protonsCitation1,Citation9. Many tissues contain CAs which have an important role in crucial biological processes, such as acid-base balance (pH homeostasis), respiration, carbon dioxide and ion transport, lipogenesis, gluconeogenesis, ureagenesis and body fluid secretion processesCitation1–4,Citation10–12.
One of the most important problems of environmental toxicology is exposure to heavy metal ions. Most metal ions are toxic to humans, animals, plants and other living organisms. Because of bioaccumulation, man and other organisms are at great risk of health hazards associated with toxic metalsCitation13–22. Several studies shown that people in different regions of the world are being exposed to metal ions by nutrition and respirationCitation22. The meat, vegetables and bakery products may contain lead (Pb) and cadmium (CdCitation22). Another study investigated the content in metal ions, such as copper (Cu) and zinc (Zn) in various fruits and vegetables sold in Egyptian markets, being found that the highest levels of Pb, Cd, Cu and Zn were in strawberries, cucumbers, dates and spinachCitation23. Furthermore, the concentration of heavy metals in commercially important species of fish, shellfish and fish products from the Cochin area showed that different metals (Cd, Pb, Hg, Cr, As, Zn, Cu, Co, Mn, Ni, and Se) were present in the samples at various, worrying levelsCitation24. Determination of these substances in food is important in environmental monitoring for the prevention, control and reduction of pollution. Metal ions are known to act as inhibitors of many enzymes, including the CAsCitation22,Citation25. Indeed, cations such as Pb2+, Co2+ and Hg2+ show inhibitory activity against the human cytosolic isoforms hCA I, II and the fish enzyme dCACitation22,Citation25. Sheep meat is consumed all over the world as an important protein source but few data are reported regarding the metalloenzymes present in itCitation22.
Although there are studies regarding purification of CA from various tissues and different organisms, no reports are available in the literature on the purification and characterization of the enzyme from sheep kidney. In the present study, we purified and characterized one α-CA from sheep kidney, presumably a CA IV-type isoform (sCA IV) for the first time, and investigated hCA I, hCA II, dCA and sCA IV kinetic properties and inhibitory effects with metal ions recognized as pollutants, such as Pb2+, Co2+, Hg2+, Zn2+, Se2+, Cu2+, Al3+ and Mn3+ on these enzymes.
Materials and methods
Pb(NO3)2, CoCl2.6H2O, HgCl2, ZnCl2, SeCl2, CuSO4.5H2O, Al(NO3)3, MnCl3, CNBr-activated Sepharose 4B, protein assay reagents, p-aminobenzene sulfonamide, L-tyrosine, 4-nitrophenyl acetate (NPA) and chemicals for electrophoresis were purchased from Sigma-Aldrich Co, Germany. All other chemicals were of analytical grade and obtained from either Sigma or Merck.
Purification of CA isozymes I, II, Dicentrarchus labrax liver and sheep kidney by affinity chromatography
The purification of hCA I and II isozymes were performed with a simple one step method by a Sepharose-4B-aniline-sulfanilamide affinity column chromatoghrapyCitation8–10. hCA I was purified, 102-fold with a specific activity of 839.6 EU × mg−1 and overall yield of 59.7%; hCA II was purified, 580-fold with a specific activity of 4773.3 EU × mg−1 and overall yield of 53%Citation8–10. The purification of dCA isozyme was performed with a simple one step method by a Sepharose-4B-aniline-sulfanilamide affinity column chromatoghrapy. dCA was purified, 78.8-fold with a specific activity of 751.72 EU·mg−1 and overall yield of 46%Citation22. Fresh sheep kidneys were taken from Erzurum Slaughterhouse under cold conditions (4°C). After the kidney was obtained, it was washed in isotonic saline containing 1 mM EDTA and stored at −20°C before use. One hundred grams of kidney was first cut into small pieces which were thereafter homogenized with 200 mL of buffer A (25 mM triethanolamine sulfate, 60 mM sodium sulfate and 1 mM benzamidine buffer, pH 8.0). The homogenate was centrifuged at 18,000 g for 60 min and the precipitate was removed. Supernatant was centrifuged at 45,000 rpm for 60 min and the precipitate containing sCA IV was separated. This was washed three times, and centrifuged at 45,000 rpm for 60 min. It has been weighted and suspended in 10 mL per g of buffer A and was then dialyzed at 4°C in 50 mM Tris-Sulfate (pH 7.4), for 1 h. The pH of the supernatant was adjusted to 8.7 with solid Tris. The homogenate was applied to the prepared Sepharose 4B-L-tyrosine-sulfanylamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). The sCA IV isozyme was eluted with 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6). All procedures were performed at 4°C.
Hydratase activity assay
CA activity was assayed by following the hydration of CO2 according to the method described by Wilbur and AndersonCitation26. CO2-hydratase activity as an enzyme unit (EU) was calculated by using the equation (tc − tc) where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
Esterase activity and in vitro inhibition assay
CA activity was assayed by following the change in absorbance at 348 nm of NPA to 4-nitrophenoxide ion over a period of 3 min at 25°C using a spectrophotometer according to the method described by Verpoorte et al.Citation27. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL 0.05 M Tris-SO4 buffer (pH 7.4), 1 mL 3 mM NPA, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution. The inhibitory effects of lead, cobalt, mercury, iron, aluminium, zinc, manganese and selenium were examined. All compounds were tested in triplicate at each concentration used. Different inhibitor concentrations were used. hCA I enzyme activities were measured in the presence of cadmium (1–3 mM), zinc (4–6 mM), selenium (0.5–3 mM), copper (0.5–1.5 mM), aluminium (0.5–3 mM) and manganese salts (3–7 mM). hCA II enzyme activities were measured for cadmium (0.5–1 mM), zinc (4–6 mM), selenium (0.5–2 mM), copper (0.05–0.5 mM), aluminium (0.1–1 mM) and manganese salts (3–10 mM); dCA enzyme activities were measured for cadmium (0.05–1 mM) and selenium salts (0.1–2 mM) and sCA IV enzyme activities were measured in the presence of lead (0.5–2 mM), cadmium (0.5–2 mM), zinc (0.5–2 mM), copper (0.1–0.5 mM) and aluminium (1–2.5 mM) salts. Control activity in the absence of inhibitor was taken as 100%. For each inhibitor, an Activity% − [Inhibitor] graph was obtained. To determine Ki values, several different inhibitor concentrations were tested. In these experiments, NPA was used as substrate at five different concentrations (0.15–0.75 mM). The Lineweaver–Burk curves were used to determine kinetic parameters and inhibition constantsCitation28.
Protein determination and sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as the standardCitation29. SDS-PAGE was performed after purification of the enzymes. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to LaemmliCitation30.
Molecular weight determination
Sephadex G-200 gel filtration
The molecular weight of the enzyme was determined according to Andrews’s methodCitation31. At first, for establishing the void volume, Blue Dextran (2000 kDa) was passed through the column; then, bovine erythrocyte CA (29 kDa), bovine serum albumin (66 kDa) and alcohol dehyrogenase (150 kDa) were used as standard proteins (Sigma MW-GF-200).
Results and discussion
At the respiratory epithelium, erythrocytic CA catalyses the rapid dehydration of bicarbonate to molecular carbon dioxide. Moreover, the CO2/HCO3− system constitutes one of the most important physiological buffers for acid-base regulationCitation32–34. Studies regarding influences of various substances on mammalian or organisms CAs have gained a great attention in the recent yearsCitation35–38. For instance, in vitro effect of some heavy metals on enzymes, such as the gill, intestinal and liver ones from various fish species showed that these enzymes were significantly inhibited by heavy metal ionsCitation22,Citation39–41.
In this study, hCA I, hCA II, dCA and sCA IV were purified from human erythrocytes, D. labrax liver and sheep kidney by a simple one step procedure using Sepharose 4B-tyrosine-sulfanilamide affinity column. The activity of the eluents were determined by the hydratase method, with CO2 as substrateCitation26 and further kinetic studies were performed using the esterase method, with NPA as substrateCitation30.
We report here the first study on the inhibitory effects of metal ions on the sCA IV esterase activity. The previous report by Ekinci et al.Citation25 investigated metal ions (including Hg2+, Co2+ and Pb2+) by using esterase activity method, NPA hydrolysis assay for monitoring CA inhibition. Data of show the following regarding inhibition of hCA I, hCA II, dCA and sCA IV with some metal ions:
Table 1. Ki values for the inhibition of hCA I, hCA II, dCA and sCA IV with metal ions by an esterase method with 4-NPA as substrateCitation27.
Against the first cytosolic isozyme hCA I, Co2+, Zn2+ and Mn3+ ions behave as weak, millimolar inhibitors, with Ki-s in the range of 3.91–10.78 mM. A second group of these metal ions, including Cd2+ and Se2+, show better inhibitory activity as compared to the previously mentioned metal ions, with Ki-s in the range of 2.18 and 2.34 mM (). Data of also show that similarly to organic nitrates and inorganic anionsCitation2,Citation9 most of the investigated metal ions act as non-competitive inhibitors with 4-NPA as substrate, i.e. they bind in different regions of the active site cavity as compared to the substrate. However, the binding site of 4-NPA itself is unknown, but it is presumed to be in the same region as that of CO2, the physiological substrate of this enzymeCitation7. It is rather probable that these metal ions interact with the His64 present in many CA II-type enzymes or with the histidine cluster of which His64 is partCitation42,Citation43.
A rather similar activity of these metal ions has been observed also for the inhibition of the hCA II (). Thus, a first groups of these metal ions, including Co2+, Zn2+ and Se2+ showed modest hCA II inhibitory activity with Ki-s in the range of 1.70–2.77 mM, whereas the remaining four metal ions, i.e. the same acting as efficient hCA I inhibitors, showed Ki-s in the range of 0.98–1.42 mM (). Again most of these metal ions act as non-competitive inhibitors with 4-NPA as substrate, except for Pb2+ and Hg2+ which are uncompetitive inhibitors ().
Pb2+, Cd2+, Cu2+ and Al3+ were also strong inhibitors of dCA, with Ki-s in the range of 0.15–0.24 mM. However, again Se2+ and Mn3+ are weak inhibitors (Ki-s of 0.98–3.46 mM). Other metal ions were determined to be weaker inhibitors (Ki-s of 0.53–0.77 mM) ().
Cu2+ and Al3+ were weak inhibitors of sCA IV, with Ki-s of 3.45–4.70 mM. A second group of these metal ions, including Pb2+, Cd2+ and Zn2+, show better inhibitory activity as compared to the previously mentioned metal ions, with Ki-s in the range of 0.963–1.132 mM ().
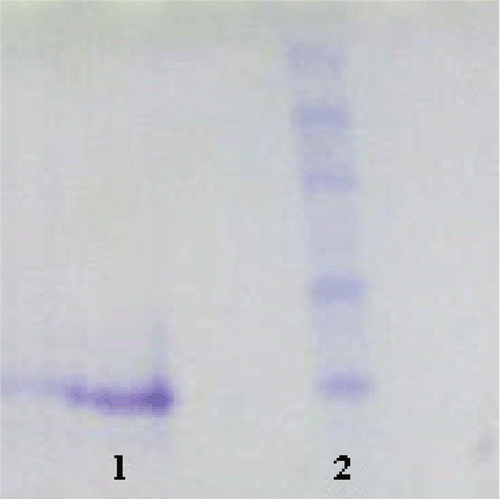
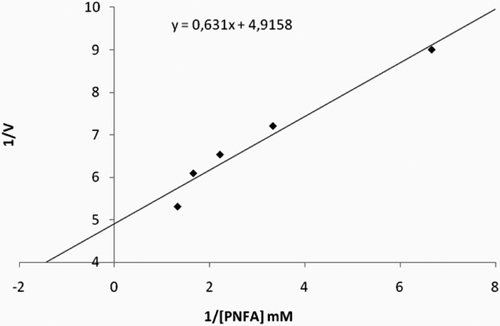
We used a single step chromatographic technique for purifying this new enzyme, sCA IV, employing Sepharose 4B-tyrosine-sulfanilamide affinity chromatography which strongly binds most α-CAsCitation9,Citation10. The optimum pH for the purification of the enzyme was determined to be 7.5. To determine the native molecular weight of the enzyme, gel filtration was carried out. For this purpose, Kav values for the enzyme and standard proteins were calculated, and a Kav − LogMW graph was obtained. The molecular weight was determined to be 29 kDa. Similar results have been observed for the enzyme from different sources. For example, human erythrocyte CA is of 29 kDaCitation9, the teleost fish D. labrax (European seabass) liver CA (dCA) is also of 29 kDaCitation22. The molecular weight was proved to be 28.9 kDa by SDS-PAGE (). We have investigated the esterase activity of sheep CA IV with NPA as substrate (). The KM and Vmax values were calculated for NPA hydrolysis catalyzed by the sheep enzyme by means Lineweaver–Burk graphs (). The Michaelis–Menten constant KM constant was calculated to be of 0.203 mM, and Vmax was 0.128 mmol × min−1 for NPA.
Conclusions
Some metal ions used in this study inhibit the activity of hCA I, hCA II, dCA and sCA IV isozymes. Most of the cations we have investigated here show activity in the low milimolar range, probably due to their binding to His64 or the His cluster of these enzymesCitation42,Citation43. According to Ki values, the best inhibitor for human CA I was the copper(II) cation, for human CA II was the lead(II) one, for the fish liver dCA was aluminum(III) whereas, for sheep kidney sCA was zinc(II). Metal-ligand interactions are stronger especially in aqueous media. The inhibition mechanism of these metal ions against CAs is probably due to the interactions with histidine residues present at the entrance of the active site cavity of these enzymes, which thereafter perturbs the proton shuttling effects in which these amino acid participate and which are essential for the catalytic cycle. Although the inhibitory effects we describe here are weak (submilimolar range), considering the abundance of polluting cations in many environmental niches, they may be significant in such systems due to the bioaacumulation processes which concentrate heavy metal ions in many exposed organismsCitation44.
Declaration of interest
This study was financed by Turkish Republic Prime Ministry State Planning Organization (DPT), (Project no: 2010K120440) for (MS).
References
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–3474.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–777.
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Expert Opin. Ther Pat 2006;16:1627–1664.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 2008;283:27799–27809.
- Supuran CT. Bacterial carbonic anhydrases as drug targets: Toward novel antibiotics? Front Pharmacol 2011;2:34.
- Durdagi S, Sentürk M, Ekinci D, Balaydin HT, Göksu S, Küfrevioglu ÖI et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–1389.
- Ekinci D, Cavdar H, Talaz O, Sentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–3563.
- Senturk M, Ekinci D, Goksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.591290.
- Balaydin HT, Durdagi S, Ekinci D, Senturk M, Goksu S, Menzek A. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, metoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.596836.
- Balaydin HT, Soyut H, Ekinci D, Goksu S, Beydemir S, Menzek A, Sahin E. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzyme Inhib Med Chem 2011. DOI: 10.3109/14756366.2011.574131.
- Ceyhun SB, Sentürk M, Ekinci D, Erdogan O, Ciltas A, Kocaman EM. Deltamethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:215–223.
- Aksakal E, Ceyhun SB, Erdogan O, Ekinci D. Acute and long-term genotoxicity of deltamethrin to insulin-like growth factors and growth hormone in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:451–455.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Ekinci D, Ceyhun SB, Aksakal E, Erdogan O. IGF and GH mRNA levels are suppressed upon exposure to micromolar concentrations of cobalt and zinc in rainbow trout white muscle. Comp Biochem Physiol C Toxicol Pharmacol 2011;153:336–341.
- Erdogan O, Ceyhun SB, Ekinci D, Aksakal E. Impact of deltamethrin exposure on mRNA expression levels of metallothionein A, B and cytochrome P450 1A in rainbow trout muscles. Gene 2011;484:13–17.
- Cakmak R, Durdagi S, Ekinci D, Sentürk M, Topal G. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett 2011;21:5398–5402.
- Ceyhun SB, Aksakal E, Ekinci D, Erdogan O, Beydemir S. Influence of cobalt and zinc exposure on mRNA expression profiles of metallothionein and cytocrome P450 in rainbow trout. Biol Trace Elem Res 2011. DOI: 10.1007/s12011-011-9068-z.
- Aksakal E, Ekinci D, Erdogan O, Beydemir S, Alim Z, Ceyhun SB. Increasing stocking density causes inhibition of metabolic-antioxidant enzymes and elevates mRNA levels of heat shock protein 70 in rainbow trout. Livest Sci 2011;141:69–75.
- Siktar E, Ekinci D, Siktar E, Beydemir S, Gülçin I, Günay M. Protective role of l-carnitine supplementation against exhaustive exercise induced oxidative stress in rats. Eur J Pharmacol 2011;668:407–413.
- Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Radwan MA, Salama AK. Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem Toxicol 2006;44:1273–1278.
- Sivaperumal TV, Sankar PG, Viswanathan Nair PG. Heavy metal concentrations in fish, shellfish and fish products from internal markets of India vis-a-vis international standards. Food Chem 2007;102:612–620.
- Ekinci D, Beydemir S, Küfrevioglu OI. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 2007;22:745–750.
- Wilbur KM, Anderson NG. Electrometric and colorometric determination of carbonic anhydrase. J Biol Chem 1976;176:147–151.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–666.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli DK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J 1965;96:595–606.
- Alp C, Ekinci D, Gültekin MS, Sentürk M, Sahin E, Küfrevioglu OI. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–4474.
- Ceyhun SB, Senturk M, Erdogan O, Kufrevioglu OI. In vitro and in vivo effects of some pesticides on carbonic anhydrase enzyme from rainbow trout (Oncorhynchus mykiss) gills. Pestic Biochem Physiol 2010;97:177–181.
- Senturk M, Ekinci D, Alici HA, Beydemir S. Paraoxonase-1, an organophosphate detoxifier and cardioprotective enzyme, is inhibited by anesthetics: An in vitro and in vivo insight. Pestic Biochem Physiol 2011. DOI: 10.1016/j.pestbp.2011.09.007.
- Thiry A, Dogné JM, Supuran CT, Masereel B. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem 2007;7:855–864.
- Sentürk M, Gülçin I, Beydemir S, Küfrevioglu OI, Supuran CT. In vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–499.
- Sentürk M, Gülçin I, Dastan A, Küfrevioglu OI, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–3211.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Lionetto MG, Giordano ME, Vilella S, Schettino T. Inhibition of eel enzymatic activities by cadmium. Aquat Toxicol 2000;48:561–571.
- Celik I, Camas H, Arslan O, Kufrevioglu OI. The effects of some pesticides on human and bovine erythrocyte carbonic anhydrase enzyme activities in vitro. J Environ Sci Heal A 1996;31:2651–2657.
- Sentürk M, Talaz O, Ekinci D, Cavdar H, Küfrevioglu OI. In vitro inhibition of human erythrocyte glutathione reductase by some new organic nitrates. Bioorg Med Chem Lett 2009;19:3661–3663.
- Briganti F, Mangani S, Orioli P, Scozzafava A, Vernaglione G, Supuran CT. Carbonic anhydrase activators: X-ray crystallographic and spectroscopic investigations for the interaction of isozymes I and II with histamine. Biochemistry 1997;36:10384–10392.
- Scozzafava A, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors. A general approach for the preparation of water-soluble sulfonamides incorporating polyamino-polycarboxylate tails and of their metal complexes possessing long-lasting, topical intraocular pressure-lowering properties. J Med Chem 2002;45:1466–1476.
- Kolayli S, Karahalil F, Sahin H, Dincer B, Supuran CT. Characterization and inhibition studies of an α-carbonic anhydrase from the endangered sturgeon species Acipenser gueldenstaedti. J Enzyme Inhib Med Chem 2011. (In Press).