Abstract
Tetrahydrocannabinol and other mixed cannabinoid (CB) receptors CB1/CB2 receptor agonists are well established to elicit antinociceptive effects and psychomimetic actions, however, their potential for abuse have dampened enthusiasm for their therapeutic development. In an effort to refine a semi-rigid structural framework for CB2 receptors binding, we designed novel compounds based on aromatic moiety and flexible linker with various amides mimicking the outlook of the endogenous anandamide which could provide as CB2 receptor ligand. In this direction, we developed and synthesized new aryl or arylidene hexanoic acid amides and aryl alkanoic acid diamide carrying different head groups. These new compounds were tested for their affinities for human recombinant CB receptors CB1 and CB2 and fatty acid amide hydrolase. Although, the preliminary screening of these compounds demonstrated weak binding activity towards CB receptor subtypes at 10 µmole, yet this template still could serve up as probes for further optimization and development of affinity ligand for CB receptors.
Keywords::
Introduction
Since the discovery of the endogenous cannabimimetic (anandamide), many structural ligands were developed for cannabinoid (CB) receptor subtypes. During the last decade, numerous selective ligands for each of the cannabinergic proteins were designed and synthesizedCitation1. Many of these agents serve as important molecular probes, providing structural information about receptor binding sites, as well as serving as pharmacological tools for obtaining information about the role of each of these targets in physiological and disease states. The endocannabinoid system is represented by CB1 and CB2, two well characterized G protein-coupled receptorsCitation2–4. Furthermore, there is evidence for the presence of additional CB receptorsCitation5,Citation6, however, their full characterizations are still incomplete. CB1 receptors in the central nervous system mediate (CNS) the psychomimetic effects of CBs, as well as the potential for abuse and dependence of Tetrahydrocannabinol (THC). Although CB1 is also expressed in lung, liver, and kidney, its role in peripheral tissue is not well understood. On the other hand, CB2 receptors are sparsely expressed in the CNS and are predominately expressed on activated immune cells, including natural killer cells, at higher levels than CB1Citation6. Thus, the CB2 receptor represents a viable target for the development of anti-inflammatory and analgesic agents that lack overt behavioral effects, and has therefore gained much recent attention, as evidenced by a rapidly growing body of researchCitation7.
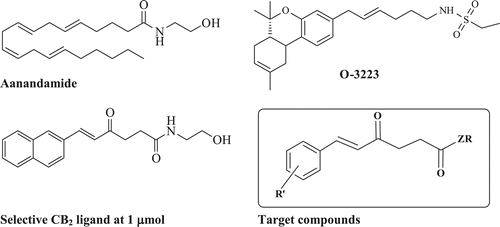
Recently, ethyl sulfonamide THC analogue, O-3223 displayed excellent selectivity and efficacy for the CB2 receptor when evaluated in a variety of murine models of pain and inflammation Citation8.
In the same direction, in our previous work, we designed and synthesized a series of arylidene oxoalkanoic acid amides as CB2 selective ligands. The ethanolamide candidate displayed promising affinity to CB2 receptor at 1 µmolCitation9. This finding motivated us to highlight essential pharmacophoric features necessary for CB2 binding affinity of this scaffold. In continuous effort to optimize this model, we decided to explore and impart certain modifications for this framework through replacing the amide tail with different hydrophilic moieties.
To achieve this goal, we report here the synthesis of several candidates such as 6-arylalkanamide and diamide derivatives. In these compounds, we replaced the aromatic portion of the selective CB2 ligand O-3223 with a variety of oxygenated and non oxygenated aryl moieties and a tail of 6 carbon atoms and different amidic groups.
These compounds were tested for their affinity to CB1/CB2 hoping to obtain new CB2 selective receptor ligands with semi-rigid components.
Material and methods
Chemistry
General procedure for the synthesis of (E)-6-(Substituted-phenyl)-4-oxohex-5-enoic acids (2a–f)
Both the respective aldehyde (30 mmol) and levulinic acid (30 mmol) were dissolved in dry toluene (100 mL) containing acetic acid (3 mL) and piperidine (1 mL). The solution was heated under reflux using Dean–Stark water trap under nitrogen until the theoretical amount of water had been collected (~6–8 h) and TLC analysis (CHCl3: CH3OH 93:7) indicated disappearance of the starting material. The solvent was evaporated under vacuum, cooled and ice cold water (30 mL) was added. The solid formed was collected, washed twice with (10 mL) of diethyl ether and then twice with 2 M HCl (15 mL), dried and crystallized from the benzene/ methanol mixture (10:5 mL).
6-Phenyl-4-oxohex-5-enoic 2aCitation10, 6-(4-methylphenyl)-4-oxohex-5-enoic 2b and 6-(N,N-dimethylaminophenyl)-4-oxohex-5-enoic (2cCitation11) 6-(3,4-dimethoxylphenyl)-4-oxohex-5-enoic (2dCitation12) and 4-(naphthalene-1-ylamino)-4-oxo-butanoic acid (2eCitation13) were prepared as previously reported.
General procedure for the preparation of 6-(substituted-phenyl)-4-oxohex-N-substituted-5-enamide (3a–m)
A mixture of the appropriate acid 2a–f (0.01 mol) and triethylamine (0.072 mol) in dry methylene chloride (10 mL) was cooled in an ice and salt bath to −10°C. Ethyl chloroformate (0.05 mol) was added dropwise, while stirring over a period of 10 min and stirring was continued for 30 min. The amine (0.01 mol) was added gradually within 10 min and stirring was continued overnight at room temperature. The solvent was evaporated under vacuum and after cooling, the residue was extracted twice with (20 mL) ethyl acetate. The ethyl acetate layer was washed with 10% hydrochloric acid twice with (15 mL), then washed with 5% sodium hydroxide twice with (15 mL). The organic layer dried over anhydrous sodium sulphate, filtered and the filtrate was evaporated under reduced pressure and cooled. The crystalline solid was separated, collected and crystallized from chloroform.
The synthesized compounds were tested for their binding affinities for CB receptors CB1 and CB2 receptors adopting reported methodsCitation14,Citation15 and Ki values were calculated by applying the Cheng–Prusoff equation to the IC50 values (obtained by GraghPad) for the displacement of the bound radioligand by increasing concentrations of the test compound. Data are means ± SEM of at least n = 3 experiments and presented in Table S1.
On the other hand, the effect of increasing concentrations of the synthetic compounds on the enzymatic hydrolysis of anandamide was studied using membranes prepared from rat brainCitation16. The results of anadamide hydrolysis assay is tabulated in Table S2.
Discussion
(E)-6-(Substituted-phenyl)-4-oxohex-6-enoic acids (2a–f) were synthesized through condensation of the appropriate aldehyde with levulinic acid using catalytic amounts of piperidine and acetic acid to give the respective arylidene keto acid derivatives according to the general procedure previously reported in the literature (Citation9).The E isomers were obtained as the major products through crystallization of the crude products and this was confirmed with the high coupling constant values of the olefinic protons, which is about 15 Hz, typical for E rather than the Z isomers. Infrared (IR) spectroscopy showed characteristic broad bands at the range of 3300–2400 cm−1 for the O-H stretching of the carboxylic group, and bands at 1690 cm−1 for the carboxylic C=O stretching in addition to bands at 1730–1665 cm−1 for the ketonic C=O stretching.
Scheme 1. Reagents and conditions: (A) piperidine/acetic acid, toluene/reflux, (B) ethyl chloroformate /triethylamine, H-ZR (where Z refer to nitrogen or oxygen and R alkyl or aryl), stirring at rt.
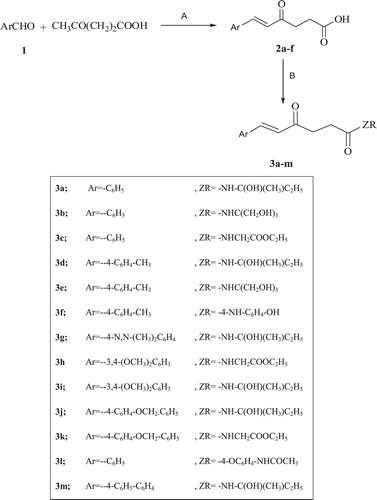
also describes the synthesis of 6-(substituted-phenyl)-4-oxohex-5-enoic acid N-substituted amides (3a–m and 5a–c) through mixed anhydride method using ethyl chloroformate, triethylamine and the appropriate amineCitation17. Secondary amides showed IR bands at 3280 cm−1 of the −NH stretching, and in addition to −C=O stretching of the amide derivatives which showed bands at relatively lower values 1650 cm−1 than the typical carbonyl stretching at 1700 cm−1. The ester amide containing compounds showed an additional −C=O stretching of the ester at 1738 cm−1.
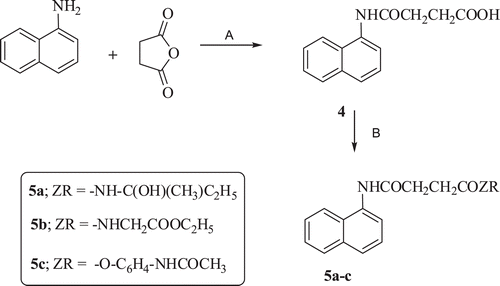
Another series of compounds containing diamides or amide ester was obtained in two step reaction as described in . The first step involved the reaction of 1-aminonaphthalene with succinic anhydride in acetic acid at ambient temperature to afford the amic acid derivative 4. The formed amic acid was subsequently reacted in a similar manner with amines as previously described in to give the diamide 5a–b or amide ester 5c derivatives.
Scheme 2. Reagents and conditions: (A) Acetic acid, stirring at rt, (B) ethyl chloroformate/triethylamine, at 10 °C then H-ZR and stirring at rt.
On the other hand, the results of the binding assay revealed that most of the tested compounds did not exhibit appreciable binding affinity for CB2 at 10 µM except compound 3f which exhibited weak CB2 affinity at 15.9 % and compound 3c which could be weak allosteric modulator (+11.4 %). On the other hand, compounds 5c, 3g, 3m, 5b exhibited maximum displacement percentage of 30.3, 25, 21.8 % and 21.21, respectively, on CB1 at 10 µM. It is worth to mention that, although, the results of anandamide hydrolysis assay for most of tested compounds did not demonstrate appreciable activity at 10 µM, yet, compounds 3b and 3e which possess trihydroxyalkyl amide tail demonstrated stimulation of fatty acid amide hydrolase enzyme rather than inhibition. This could be related to allosteric activation of the enzyme.
Conclusion
These findings could verify that the proposed compounds may lack proper features and/or dimensions of the tail necessary for optimum binding with the target CB2 receptor. Although these compounds demonstrated weak binding affinity yet these finding will be useful as starting points for further structure refinement and for exploring further structural requirements of the CB1/CB2 receptor binding site. This will also serve for further optimization of this proposed template for the development of new ligands by changing the rigid aryl moiety and/or the spacer or the head amide group.
Supplementary Material
Download PDF (97.9 KB)Acknowledgment
The authors are grateful to the staff members micro-analytical unit, Cairo University for performing the spectra data for the synthesized compounds.
Declaration of interest
The authors report no conflict of interest.
References
- Goutopoulos A, Makriyannis A. From cannabis to cannabinergics: new therapeutic opportunities. Pharmacol Ther 2002;95:103–117.
- Goutopoulos A, Fan P, Khanolkar AD, Xie XQ, Lin S, Makriyannis A. Stereochemical selectivity of methanandamides for the CB1 and CB2 cannabinoid receptors and their metabolic stability. Bioorg Med Chem 2001;9:1673–1684.
- Khanolkar AD, Abadji V, Lin S, Hill WA, Taha G, Abouzid K et al. Head group analogs of arachidonylethanolamide, the endogenous cannabinoid ligand. J Med Chem 1996;39:4515–4519.
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993;365:61–65.
- Wiley JL, Martin BR. Cannabinoid pharmacology: implications for additional cannabinoid receptor subtypes. Chem Phys Lipids 2002;121:57–63.
- Joong Y S. Understanding functional residues of the cannabinoid CB1 receptor for drug discovery. Current Topics in Med Chem 2010;10:779–798.
- Pasquini L, Botta T, Semeraro C, Mugnaini A, Ligresti E, Palazzo S, Maione V, Di Marzo F. Three-dimensional quantitative structure selectivity relationships analysis guided rational design of a highly selective ligand for the cannabinoid receptor CB2. J. Med Chem 2008;51:5075–5084.
- Steven G, Kinsey a, Anu Mahadevan b, Bingjun Z, Hang S, Pattipati S, Naidu R, Razdan K, Dana E, Selley M, Imad D, Aron L. The CB2 cannabinoid receptor-selective agonist O-3223 reduces pain and inflammation without apparent cannabinoid behavioral effects. Neuropharm 2011;60:244–251.
- Noha A, Osman A, Mahmoud H, Marco A, Raimund N, Khaled A, Abouzid Vincenzo, M, Ashraf A. Synthesis, binding studies and molecular modeling of novel cannabinoid receptor ligands. Bioorg Med Chem 2010;18:8463–8477.
- Olpp T. Product class 2: 2-oxoaldehydes and heteroatom analogues. Science of Synth 2007;25:423–439.
- Tran Q, Nguyen D. Synthesis and structures of some 6-(2-arylvinyl)-4,5-dihydropyridazin-3(2H)-ones. Tap Chi Hoa Hoc 2004;42:325–328. Through CA 143:347118.
- Passarotti C, Fossati A, Bandi L, Bozzi R, Vimercati G, Galmozzi G, Fabris L, Movalli A, Cerrati A. Synthesis and antiinflammatory activity of some E-ketophenylalkenoic carboxylic acids. Act Toxia Therap 1988;9:289–296.
- Gluzman M, Mil’ner S. Investigation of the acylation process of amines by solid succinic and benzoic anhydrides under isothermal conditions. Khim Khimich Tekhno 1960;3:684–690.
- Giuseppina C, Ernesto F, Orazio S, Giorgio B, Henny A, Dien A, Ligresti V, Di M. Desulfohaplosamate a new phosphate-containing steroid from Dasychalina sp., is a selective cannabinoid CB2 receptor ligand. Steroids 2011;76:998–1002.
- Pasquini S, Botta L, Semeraro T, Mugnaini C, Ligresti A, Palazzo E et al. Investigations on the 4-quinolone-3-carboxylic acid motif. 2. Synthesis and structure-activity relationship of potent and selective cannabinoid-2 receptor agonists endowed with analgesic activity in vivo. J Med Chem 2008;51:5075–5084.
- Brizzi A, Brizzi V, Cascio MG, Bisogno T, Sirianni R, Di Marzo V. Design, synthesis, and binding studies of new potent ligands of cannabinoid receptors. J Med Chem 2005;48:7343–7350.
- Ismail M, Lehmann J, Abou El ella D, Albohy A, Abouzid K.. Lonazolac analogues: molecular modeling, synthesis and in vivo anti-inflammatory activity. Med Chem Res 2009;18:725–744.