Abstract
Reaction of 6-/7-hydroxycoumarin with metronidazole afforded conjugates which incorporate two interesting chemotypes which may inhibit carbonic anhydrases (CAs, EC 4.2.1.1) due to the presence of the coumarin moiety and possess radiosensitizing effects due to the presence of the nitroazole. Another dual action compound, which may act both as CA inhibitor as well as monocarboxylate transporter inhibitor, is 3-cyano-7-hydroxy-coumarin. These compounds have been investigated as inhibitors of 11 human CA isoforms. Submicromolar inhibition was observed against hCA VA, hCA VB, hCA VI, hCA VII, hCA IX, hCA XII and hCA XIV, whereas isoforms hCA I, II and XIII were not inhibited by these compounds. These coumarins thus act as isoform-selective CA inhibitors with the possibility to target isoforms involved in pathologies such as obesity (CA VA/VB) or cancer (CA IX and XII) without inhibiting the physiologically dominant, highly abundant hCA I and II.
Introduction
Coumarins are a class of widely spread natural compounds which was only recently revealed to inhibit the zinc enzyme carbonic anhydrase CA (EC 4.2.1.1Citation1). CAs are ubiquitous enzymes in organisms throughout the tree of life, with several genetically distinct families encoding themCitation2–7 in prokaryotes and eukaryotes. CAs are inhibited by metal complexing anions, sulfonamides and their isosteres (sulfamates, sulfamides, etc), phenols and polyaminesCitation2–7, which bind either to the metal ion within the enzyme active site or are anchored to the water molecule coordinated to itCitation2–7. However, the coumarin inhibition mechanism of the α-CAs is totally different and was only recently decipheredCitation1,Citation7. A natural product coumarin, 6-(1S-hydroxy-3-methylbutyl)-7-methoxy-2H-chromen-2-one, isolated from the Australian plant Leionema ellipticum, was shown to possess significant CA inhibitory activityCitation1. By means of X-ray crystallography of its adduct with the human (h) isoform hCA II, it was evidenced the presence of a substituted 2-hydroxy-cinnamic acid in the enzyme active site, which is the hydrolysis product (mediated by the CA) of the original coumarinCitation1. The same situation has been thereafter observed for the simple, unsubstituted coumarinCitation8. The 2-hydroxy-cinnamic acids formed from the original coumarins occlude the entrance to the enzyme active site, a mechanism never evidenced before for CA inhibitionCitation1,Citation8,. Some thiocoumarins (possessing the exocyclic sulfur atom) were also shown to behave in a similar manner to the coumarinsCitation8. Several general facts emerged during these studies of coumarins and thiocoumarins as CA inhibitors (CAIs): (i) they bind in hydrolyzed form at the entrance of the CA-active site and do not interact with the metal ion, constituting an entirely new category of mechanism-based inhibitors (they act as prodrug inhibitorsCitation1,Citation7–10); (ii) the substituted-2-hydroxy-cinnamic acids formed from the original coumarin by hydrolysis were observed bound within the CA-active site either as the cis isomer, as well as as trans isomers (by means of X-ray crystallography of enzyme-inhibitor adducts), depending on the substitution pattern at the original coumarin prodrugCitation1,Citation8; (iii) these inhibitors were observed bound at the entrance of the enzyme active site cavity, plugging its entrance, and blocking thus the catalytic activity of the enzymeCitation1,Citation8. This region of the CA active site is on the other hand the most variable one among the 16 CA isoforms present in mammalsCitation2–7, and this explains the observations that many coumarin/thiocoumarin derivatives show a high selectivity ratio for inhibiting various CA isoforms, many of which have pharmacologic applications for obtaining antiglaucoma, antiobesity, anticonvulsant or antitumor drugs/diagnostic agentsCitation1–7.
In fact recently, some glycosyl-substituted coumarins with potent inhibitory activity and selectivity for the tumor-associated isozyme CA IX and XII, were shown to strongly inhibit the growth of primary tumors and metastases overexpressing these enzymes, in an animal model of breast cancerCitation11,Citation12. For this reason, exploring novel coumarin derivatives as CAIs, or compounds known in the literature but not yet investigated for this activity, is of interest in the design of isoform-selective pharmacological agents of this type.
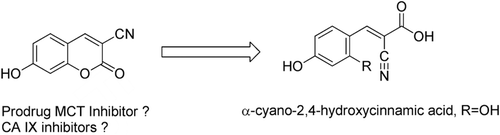
Here we report the synthesis of coumarin derivatives incorporating a nitrozole moiety, the inhibition studies of these compounds against 11 human CA isoforms, and the investigation of a known derivative, 3-cyano-7-hydroxy-coumarin, for its interaction with these enzymes. This small group of compounds is characterized by the fact that they may possess two types of biological activity: CA inhibition due to the presence of the coumarin ring, and (i) either radiosensitizing properties because of the presence of nitroazole moietiesCitation13–15, or (ii) monocarboxylate transporters (MCT) inhibitory propertiesCitation16,Citation17 due to the fact that the hydrolysis product of 3-cyano-7-hydroxy-coumarin could lead to the α-cyano-2,4-hydroxycinnamic acid which is a derivative of α-cyano-4-hydroxycinnamic acid, a well known inhibitor of MCTsCitation18.
Materials and methods
Chemistry: Compounds 1, 2, A and B, as well as solvents and other reagents used for synthesis were commercially available from Sigma-Aldrich (Milan, Italy).
Synthesis of derivatives 1a and 1b
Metronidazole 1 (1 equiv.), 6- or 7-hydroxy-4-methyl coumarin A, B (1 equiv.), and triphenylphospine (1.2 equiv.) were mixed in THF and then diisopropyl azidocarboxylate (DIAD), (1.2 equiv.) was added dropwise. The reaction is stirred 2 days at room temperature. The precipitate is then filtered, washed two times with cold THF and dried under vacuum.
7-O-[2-(2-methyl-5-nitro-imidazol-1-yl)ethyl]-4-methylcoumarine 1a
Yield 48%; Rf: 0.11 (AcOEt 8/ Et2O 2); Mp: 238–240°C; Citation1H NMR (400 MHz, DMSO): δ ppm 1.55 (s, 3H), 1.69 (s, 3H), 3.63 (t, 2H, J = 5.00 Hz), 3.91 (t, 2H, J = 5.00 Hz), 5.38 (d, 1H, J = 1.05Hz), 6.07 (dd, 1H, J = 2.49 Hz, J = 8.81 Hz), 6.15 (d, 1H, J = 2.49 Hz), 6.84 (d, 1H, J = 8.81 Hz), 7.20 (s, 1H); Citation13C NMR (101 MHz, DMSO): δ ppm 14.10, 18.08, 45.00, 67.02, 101.28, 111.38, 112.27, 113.45, 126.54, 132.87, 151.71, 153.25, 154.56, 160.00, 160.66. MS ESI+/ESI−: m/z 330.34 (M+H)+, 328.38 (M-H)−. HRMS: (m/z): [M+H]+ calcd for C16H16N3O5, 330,1090; found 330,1091.
6-O-[2-(2-methyl-5-nitro-imidazol-1-yl)ethyl]-4-methylcoumarine 1b
Yield 42%; Rf: 0.16 (AcOEt 8/ Et2O 2); Mp: 190–191°C; Citation1H NMR (400 MHz, DMSO): δ ppm 1.56 (s, 3H), 1.70 (s, 3H), 3.57 (t, 2H, J = 5.00 Hz), 3.89 (t, 2H, J = 5.00 Hz), 5.53 (s, 1H); 6.32 (m, 2H), 6.46 (d, 1H, J = 9.70 Hz), 7.19 (s, 1H); Citation13C NMR (101 MHz, DMSO): δ ppm 14.15, 18.13, 45.19, 66.97, 108.61, 114.72, 117.58, 120.07, 132.93, 138.31, 147.47, 151.83, 152.94, 154.06, 159.76; MS ESI+/ESI−: m/z 330.34 (M+H)+, 328.38 (M-H)−. HRMS: (m/z): [M+H]+ calcd for C16H16N3O5, 330,1090; found 330,1092.
CA inhibition
An Applied Photophysics stopped-flow instrument has been used for assaying the CA catalysed CO2 hydration activity. Phenol red (at a concentration of 0.2 mM) has been used as an indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constantsCitation19. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with distilled-deionized water. Inhibitor and enzyme solutions were preincubated together for 15 min–72 h at room temperature (15 min) or 4°C (all other incubation times) prior to assay, in order to allow for the formation of the E-I complex or for the eventual active site mediated hydrolysis of the inhibitor. Data reported in show the inhibition after 6 h incubation, which led to the completion of the in situ hydrolysis of the coumarin and formation of the 2-hydroxy-cinnamic acids. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3, as reported earlierCitation1,Citation8–10, and represent the mean from at least three different determinations. CA isofoms were recombinant ones obtained in house as reported earlierCitation1.
Table 1. Inhibition of human (h) CA isoforms hCA I-hCA XIV with compounds 1a, 1b, 2 and the sulfonamide standard CAI acetazolamide AAZ, by a stopped flow, CO2 hydrase techniqueCitation19.
Results and discussion
α-CAs are widespread metalloenzymes in higher vertebrates, including humansCitation2–7. Sixteen such isoforms have been characterized to date in mammals, which differ in their subcellular localization, catalytic activity, and susceptibility to different classes of inhibitors. There are cytosolic isozymes (CA I, CA II, CA III, CA VII and CA XIII), membrane bound ones (CA IV, CA IX, CA XII, CA XIV and CA XV), mitochondrial (CA VA and CA VB) and secreted (CA VI) isoformsCitation2–7. Three acatalytic forms, called CA-related proteins (CARPs), CARP VIII, CARP X and CARP XI, have also been identified. Most CAs are very efficient catalysts for the reversible hydration of carbon dioxide to bicarbonate and protons (CO2 + H2O ↔ HCO3− + H+), a reaction of critical importance in most organismsCitation2–7. The bicarbonate/carbonic acid system is the main buffer in all living cellsCitation4,Citation5. CO2 is generated in high amounts as the final product of the mitochondrial electron transport/oxidative phosphorylation processes, and is immediately converted to bicarbonate by CAs. The uncatalyzed hydration reaction of this gas is too slow to account for the physiological needs of the cell, and this explains why so many CA isoforms have evolvedCitation4,Citation5. In mammals many CAs are involved in vital physiological processes such as respiration and acid-base regulation, electrolyte secretion, bone resorption, calcification and biosynthetic reactions which require bicarbonate as a substrate (lipogenesis, gluconeogenesis and ureagenesis). Two CA isozymes (CA IX and CA XII) are overexpressed in many tumors through the hypoxia inducible transcription factor 1 (HIF-1) pathway, and are associated with cancer progression and response to therapyCitation4,Citation5.
Among the various therapeutic approaches for hypoxic cancers, which are highly non responsive to classical radio- and chemotherapyCitation20 are also those of radiosensitivization, by using nitro-azoles such as misonidazole, metronidazole, benznidazole, desmethylmisonidazole, etanidazole, pimonidazole, nimorazole, ornidazole, etcCitation13,Citation14., which all possess nitroaromatic heterocycles structurally related to metronidazole 1 (). It has been shown that such nitroimidazole are reduced electrolytically or by γ-radiolysis, provoking thereafter DNA damage which is not influenced by the presence of oxygenCitation13. It has been suggested that the protonated one-electron nitro radical anions formed by the radiolysis of these radiosensitizers is the possible candidate for the active damaging species, explaining the cytotoxicity mechanism of these drugs under conditions of hypoxiaCitation13,Citation14. This is the reason why we introduced nitroazole moieties in the molecules of coumarins ().
Scheme 1. Preparation of metronidazole-coumarin conjugates by reaction of metronidazole 1 with 7-hydroxy (A) and 6-hydroxy-coumarin (B).
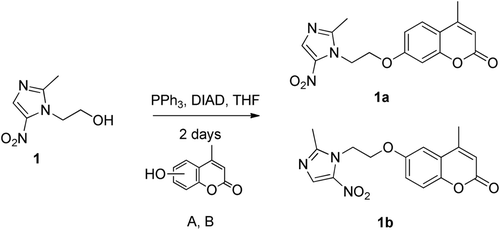
Reaction of metronidazole 1 with the hydroxy-substituted coumarins A or B under a Mitsunobu reaction (in the presence of triphenylphosphine and diisopropyl azodicarboxylate) led to the formation of the ethers 1a or 1b (see Materials and Methods for details). These compounds thus possess two pharmacophores which may be useful for designing CAIs with applications as antitumor agents, the coumarin moiety (leading to isoform-selective CA inhibition) and the nitroazole moiety (which may lead to radiosensitization action of these compounds).
There are at least nine different isoforms of proteins involved in the transport of monocarboxylates out of the cells, with MCT1–4 playing crucial physiological rolesCitation16,Citation17. Due to the Warburg effect, hypoxia and oncogenic mutations favor glycolysis, with the pyruvate to lactate conversion being promoted by increased expression of lactate dehydrogenase A and inactivation of pyruvate dehydrogenase, resulting in high amounts of lactic acid being produced in many solid tumorsCitation5,Citation18. The preferential use of lactate for oxidative metabolism involves the uptake of lactate by bystander, non hypoxic tumor cells, and thus spares glucose for hypoxic tumor cells, which cannot carry out oxidative metabolismCitation5,Citation18. MCTs (mostly MCT1) regulate the entry/exit of lactate in/from tumor cellsCitation5,Citation18 and their inhibition favours the switch from lactate-fuelled respiration to glycolysis, which consecutively kills hypoxic tumor cells through glucose starvationCitation18. MCT overexpression is indeed high in many tumors, with MCT4 being also induced by hypoxia through the HIF-1 cascade, similar to CA IX/XII mentioned aboveCitation5. MCTs inhibitors were shown to have utility as alternative antitumor agents by Sonveaux’s groupCitation18. There are in fact various classes of such inhibitorsCitation5,Citation17, among which α-cyano-4-hydroxycinnamic acid 3. Its 2-hydroxy analogue, is formed by the hydrolysis reaction depicted in from the coumarin 2 investigated here as CAI. This is the rationale why we investigate the coumarin 2, a precursor of α-cyano-4-hydroxycinnamic acid 3 compound family, as a possible CAI in this work.
Scheme 2. Rationale for investigating 3-cyano-7-hydroxy-coumarin 2 as a dual CA/MCT inhibitor. The coumarin 2 may be hydrolyzed through the CA esterase activity to a derivative of α-cyano-4-hydroxycinnamic acid (3, R=H), a known MCT inhibitorCitation5,Citation18.
Coumarins 1a, 1b and 2 were investigated as inhibitors of 11 catalytically active hCA isoforms, hCA I-hCA XIV (). The sulfonamide CAI acetazolamide (AAZ) used clinically was also included in the staudy as standard inhibitor. The following structure-activity reltionship (SAR) can be evidenced from the data of :
Isoforms hCA I, II, IV and XIII were not inhibited significantly by the three coumarin derivatives 1a, 1b and 2 investigated here, which showed inhibition constants > 100 μM, except 2 with hCA XIII (KI of 5.34 μM). Thus, as for other coumarins investigated earlier by our groupCitation8–10, the house-keeping, abundant isoforms hCA I, II and IV are not inhibited by this class of CAIs, which constitutes a highly attractive feature for inhibitors that should target isoforms involved in tumorigenesis (CA IX and XII) or in obesity (CA VA and VB). It should be observed that the sulfonamide inhiibtor AAZ is a highly effective, low nanomolar inhibitor or most of these enzymes, which explains the many side effects observed with sulfonamide drugsCitation2–7.
The mitochondrial isoforms hCA VA and VB were inhibited efficiently by compounds 1a, 1b and 2 investigated here, with KIs in the range of 0.38–2.63 μM (). The best inhibitor was 1a, whereas the least effective one was 2. The sulfonamide AAZ was on the other hand a stronger, nanomolar inhibitor of the two mitochondrial enzymes.
The secreted (CA VI) and transmembrane (CA IX, XII and XIV) isoforms were generlly effectively inhibited by the coumarins investigated here. Thus, the nitroazole coumarin 1a was a subnanomolar inhibitor of all these isoforms, with KIs in the range of 0.37–0.93 μM. Its isomer 1b was an effective, subnanomolar inhibitor of hCA VI, hCA IX and hCA XIV (KIs in the range of 0.40–0.82 μM) but not of hCA XII (KI of 53 μM). Compound 2 was on the other hand a slightly less effective CAI against these isoforms, with KIs in the range of 0.24–7.11 μM. The best inhibited isoform was hCA IX and the last inhibited one was hCA XII.
The only cytosolic isoform inhibited substantially by these coumarins was hCA VII, for which KIs in the range of 0.42–2.98 μM were measured ().
The inhibition profile of these coumarins is quite specific, with small differences in the substitution pattern of the two isomers 1a and 1b, influencing dramatically the enzyme inhibitory activity.
In conclusion, we report here CAIs obtained by reaction of 6-/7-hydroxycoumarin with metronidazole, which afforded conjugates incorporating two interesting chemotypes and which may inhibit CAs due to the presence of the coumarin moiety, but also possess radiosensitizing effects due to the presence of the nitroazole fragment. Another potential dual action compound, which acts both as CA inhibitor as well as potent monocarboxylate transporter inhibitor, is 3-cyano-7-hydroxy-coumarin. These compounds have been investigated as inhibitors of 11 human CA isoforms. Submicromolar inhibition was observed against hCA VA, hCA VB, hCA VI, hCA VII, hCA IX, hCA XII and hCA XIV, whereas isoforms hCA I, II and XIII were not significantly inhibited by these compounds. These coumarins thus act as isoform-selective CA inhibitors with the possibility to target isoforms involved in pathologies such as obesity (CA VA/VB) or cancer (CA IX and XII) without inhibiting the physiologically dominant, highly abundant hCA I and II. Furthermore, by acting as radiosensitizers (due to the presence of the nitroazole moieties) or by also inhibiting MCTs (in addition to the CAs), these hybrid compounds constitute starting points for designing antitumor coumarins with enhanced potency.
Declaration of interest
This work was supported by an EU FP7 research grant (Metoxia project).
References
- Maresca A, Temperini C, Vu H, Pham NB, Poulsen SA, Scozzafava A et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–3062.
- Supuran CT. Carbonic anhydrase inhibition with natural products: novel chemotypes and inhibition mechanisms. Mol Divers 2011;15:305–316.
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–3474.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–777.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Supuran CT. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med Chem 2011;3:1165–1180.
- Maresca A, Temperini C, Pochet L, Masereel B, Scozzafava A, Supuran CT. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–344.
- Maresca A, Supuran CT. Coumarins incorporating hydroxy- and chloro-moieties selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II. Bioorg Med Chem Lett 2010;20:4511–4514.
- Maresca A, Scozzafava A, Supuran CT. 7,8-disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg Med Chem Lett 2010;20:7255–7258.
- Touisni N, Maresca A, McDonald PC, Lou Y, Scozzafava A, Dedhar S et al. Glycosyl Coumarin Carbonic Anhydrase IX and XII Inhibitors Strongly Attenuate the Growth of Primary Breast Tumors. J Med Chem 2011;54:8271–8277.
- Lou Y, McDonald PC, Oloumi A, Chia S, Ostlund C, Ahmadi A et al. Targeting Tumor Hypoxia: Suppression of Breast Tumor Growth and Metastasis by Novel Carbonic Anhydrase IX Inhibitors. Cancer Res 2011;71:3364–3376.
- Zahoor A, Lafleur MV, Knight RC, Loman H, Edwards DI. DNA damage induced by reduced nitroimidazole drugs. Biochem Pharmacol 1987;36:3299–3304.
- Overgaard J. Clinical evaluation of nitroimidazoles as modifiers of hypoxia in solid tumors. Oncol Res 1994;6:509–518.
- Ljungkvist AS, Bussink J, Kaanders JH, van der Kogel AJ. Dynamics of tumor hypoxia measured with bioreductive hypoxic cell markers. Radiat Res 2007;167:127–145.
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J 1999;343 Pt 2:281–299.
- Enerson BE, Drewes LR. Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J Pharm Sci 2003;92:1531–1544.
- Sonveaux P, Végran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 2008;118:3930–3942.
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–2573.
- Ebbesen P, Pettersen EO, Gorr TA, Jobst G, Williams K, Kieninger J et al. Taking advantage of tumor cell adaptations to hypoxia for developing new tumor markers and treatment strategies. J Enzyme Inhib Med Chem 2009;24 Suppl 1:1–39.
