Abstract
A series of phenolic and saponin type natural products such as quercetin, rutin, catechin, epicatechin, silymarin, trojanoside H, astragaloside IV, astragaloside VIII and astrasieversianin X, were investigated for their inhibitory effects against the metalloenzyme carbonic anhydrase (CA, EC 4.2.1.1). We here report inhibitory effects of these compounds against five α-CA isozymes (hCA I, hCA II, bCA III, hCA IV and hCA VI). Most of the phenolic and saponin type compounds inhibited the isoenzymes quite effectively at low micromolar KI-s ranging between 0.1 and 4 µM, whereas a few derivatives were ineffective (KI-s > 100 µM). The results were remarkable which might lead to design of novel CAIs with a diverse inhibition mechanism compared to sulfonamide/sulfamate inhibitors.
Introduction
Carbonic anhydrase (EC 4.2.1.1., CA) is a pH regulatory/metabolic enzyme in all life forms. It catalyzes the hydration of carbon dioxide to bicarbonate and the corresponding dehydration of bicarbonate in acidic medium with regeneration of CO2. Sixteen CA isozymes have been described up to now in mammals, of which the most active catalysts are known as CA II and CA IX. The first one is found primarily in red blood cells but also in many other secretory tissues of the gastrointestinal tract, kidneys, lungs, eye, CNS, etc., whereas the second one is a tumour-associated isoformCitation1–9. Other CA isoforms are found in a variety of tissues where they participate in several important biological processes such as acid-base balance, respiration, carbon dioxide and ion transport, bone resorption, ureagenesis, gluconeogenesis, lipogenesis and electrolyte secretionCitation1–3. On this basis, CA isozymes are important therapeutic targets with the potential to be inhibited/activated for the treatment of a range of disorders such as edema, glaucoma, obesity, cancer, epilepsy and osteoporosisCitation1–7.
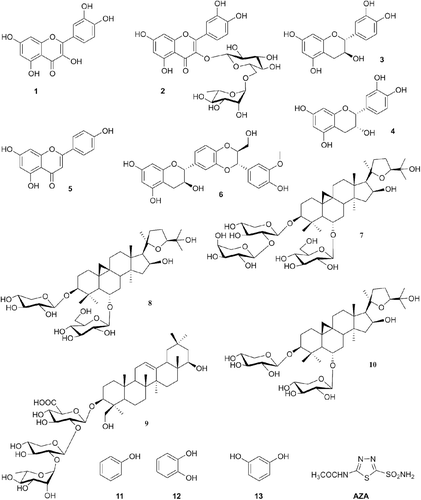
Our groups recently investigated the interaction of hCA I, II, VI isozymes with several types of natural and synthetic compounds, such as catechol, resorcinol, progallol, gallic acid, tannic acid and several of their substituted derivativesCitation10–13. Here we extend our earlier investigations to a series of compounds (1–13), some of which are widely used as antioxidant food additives or as drugs. Among the various natural or synthetic phenolic compounds with antioxidant properties, these compounds are well known for their superior reactive oxygen species quenching abilitiesCitation11,Citation12. They have been reported to possess anticancer, antimutagenic, antibacterial, antiviral or anti-inflammatory activitiesCitation11–13. Quercetin (1), rutin (2), catechin (3), epicatechin (4), apigenin (5), members of the flavonoid family are ubiquitously present in food, including vegetables, fruits, tea, and red wine. Several biological properties of these compounds have been reported to be beneficial to human health, including protection against various diseases, such as osteoporosis, certain forms of cancer, pulmonary and cardiovascular diseases and agingCitation13. Silymarin (6), a plant derived flavolignan, was isolated from the fruits and seeds of the milk thistle (Silybum marianumCitation14), being purportedly reported to be useful for the treatment of cancer, varicose veins, menstrual problems, depression, low breast milk production, liver disorders, cirrhosis and chronic hepatitisCitation14. Compounds 7–10, belonging to the saponin class, were purified from Astragalus pycnocephalus var. pycnocephalus FISCHER by using chromatographic methods. Their structures were determined by means of spectroscopic methodsCitation15–17. Phenol (11), catechol (12), and resorcinol (13) are widely used prodrugs or disinfectantsCitation12.
In the present study, we have purified human CA I, II, IV, VI, bovine CA III, and investigated the in vitro inhibition effects of the compounds 1–13 by using esterase activity of the isoenzymes with 4-nitrophenyl acetate as substrate.
Materials and methods
Chemicals
Quercetin, rutin, catechin, epicatechin, apigenin, silymarin, phenol, resorcinol, catechol, Sepharose 4B, protein assay reagents, 4-nitrophenylacetate (NPA) were obtained from Sigma-Aldrich Co. All other chemicals were analytical grade and obtained from Merck.
Purification of CA isozymes by affinity chromatography
Purification of hCA I and hCA II were previously describedCitation7. Fresh citrated human whole blood obtained from the Blood Center of the Research Hospital at Atatürk University. Cells were washed three times by centrifugation at 1000g at 4 ± 6°C for 20 min in four volumes of 25 mM Na2HPO4 (pH = 7.4) buffer. Supernatant and fluffy coat were removed. The erythrocytes were lysed in 10 volumes of 5 mM Na2HPO4 (pH = 7.4) buffer, containing 1 mM EDTA. After 20 min, the haemolysate was centrifuged at 10000g for 60 min. The particulate fraction was washed four times in the same buffer. The membranes were centrifuged down at 15000g for 60 min. pH was adjusted to 8.3 with solid Tris. Sepharose-4B-aniline-sulfanilamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.3). The affinity gel was washed with 25 mM Tris-HCl/25 mM Na2PO4 (pH 8.3). Finally, human CA IV (hCA IV) isozyme was eluted with 25 mM Tris-HCl/0.5 M NaClO4 (pH 7.4). Fresh non-citrated human whole blood obtained from the Blood Center of the Research Hospital at Atatürk University. The blood samples were centrifuged at 5000 rpm for 15 min and precipitant was removed. The serum was isolated. The pH was adjusted to 8.7 with solid Tris. Sepharose-4B-aniline-sulfanilamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The affinity gel was washed with 25mM Tris-HCl/22 mM Na2SO4 (pH 8.7). The human CA (hCA VI) isozyme was eluted with 0.25 M H2NSO3H/25 mM Na2HPO4 (pH = 6.7)Citation5. All procedures were performed at 4°C. Bovine CA III was obtained from flank steak and purified using a modification of the method of Tu et al. (Citation1986)Citation18. Hundred grams of cubed flank steak was homogenized in small batches in a blender with a total of 150 mL of buffer A (10 mM Tris/H2S04 buffer, pH 8.0, containing 1 mM mercaptoethanol). The resulting product was centrifuged at 4000g for 30 min, and the supernatant pooled. This crude enzyme solution was filtered through Whatman No. 1 filter paper. The clear protein solution was passed through a 2 × 50-cm gel exclusion column using the buffer A. The active fractions were determined and concentrated to lyophilizer. This solution was applied to 2.5 × 50-cm ion exchange column and eluted with the buffer A. The active fractions comprising the major protein peak were pooled and concentrated in 1 mM mercaptoethanol.
Esterase activity assay
CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer according to the method described by Verpoorte et al.Citation19. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL 0.05 M Tris-SO4 buffer (pH 7.4), 1 mL 3 mM 4-NPA, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution.
In vitro inhibition study
The inhibitory effects of compound 1–13, and acetazolamide (AZA) were examined. All compounds were tested in triplicate at each concentration used. Different inhibitor concentrations were used, starting from 1 nM to 1 mM. Control cuvette activity in the absence of inhibitor was taken as 100%. For each inhibitor, Activity (%)–[Inhibitor] graphs were drawn. To determine KI values, three different inhibitor concentrations were tested. In these experiments, 4-NPA was used as substrate at five different concentrations (0.15–0.75 mM). The Lineweaver–Burk curves were drawnCitation20.
Protein determination
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method using bovine serum albumin as a standardCitation21.
Results and discussion
Phenol 11 binds to CA in a different manner from the classical inhibitors of the sulfonamide type, for example AZA, which coordinate to the Zn(II) ion from the enzyme active site by substituting the fourth, non-protein ligand, a water molecule or hydroxide ionCitation10–12,Citation22. The X-ray crystal structure of the adduct of hCA II with phenolCitation22 showed that this compound binds to CA by anchoring its OH moiety to the zinc-bound water/hydroxide ion of the active site through a hydrogen bond, as well as through another hydrogen bond to the NH amide of Thr199, an amino acid conserved in all α-CAs and critically important for the catalytic cycle of these enzymesCitation6–12,Citation22.
We recently investigated the interactions of phenol and some of its substituted derivatives with all mammalian CAsCitation10–12, demonstrating some low micromolar/submicromolar inhibitors as well as the possibility to design isozyme selective CAIs. The inhibition profile of various CA isozymes with this class of agents was very variable with inhibition constants ranging from the millimolar to the submicromolar levels for many simple phenolsCitation10–12. Thus, it seemed reasonable to extend the previous studiesCitation10–12 including, in this investigation, phenolic compounds with clinical and antioxidant applications as food additives, such as compounds 1–6, 12 and 13Citation10–12,Citation15–23. Other structurally related derivatives such as 7–10, were also included in our study ().
The purification of the CA isozymes was performed with a simple one step method by a Sepharose-4B-aniline-sulfanilamide affinity column chromatoghrapyCitation24. hCA I was purified, 111.2-fold with a specific activity of 945.51 EUmg−1 and overall yield of 64.3%. hCA II was purified, 792.6-fold with a specific activity of 7186 EUmg−1 and overall yield of 72.84%. hCA IV was purifed, 85.15-fold with a specific activity of 734.12 EUmg−1 and overall yield of 26.3%. hCA VI was purifed, 71.3-fold with a specific activity of 414.1 EUmg−1 and overall yield of 16.8%. Similarly, bCA III was purifed, 49.2-fold with a specific activity of 462.1 EUmg−1 and overall yield of 48.2%Citation3,Citation19–27. Inhibitory effects of compounds 1–13 on enzyme activities were tested under in vitro conditions; KI values were calculated from Lineweaver–Burk graphs and are given in Citation19,Citation20.
Table 1. Ki values (μM) for compound 1–13 and AZA of some α-carbonic anhydrase isoforms in human (h) and bovine (b).
For the first time, the inhibitory effects of a group of polyphenolic compounds, quercetin (1), rutin (2), catechin (3), epicatechin (4), apigenin (5), silymarin (6), phenol (11), catechol (12), resorcinol (13), and saponins, trojanoside H (7), astragaloside IV (8), astragaloside VIII (9), astrasieversianin X (10), on the esterase activity of hCA I, II, IV, VI and bCA III were investigated. The sulfonamide CAI–AZACitation1–3 was used as negative control. By using esterase assay, the previous reports by Senturk et al.Citation11,Citation12 investigated other phenol derivatives including salicylic acid and gallic acid. Based on the results given in , the following remarks were concluded regarding the inhibition of hCA I, II, IV, VI and bCA III by compounds 1–13 and AZA.
Against the slow cytosolic isozyme hCA I, compound 13 behaved as weak inhibitor, with KI value of 795 µMCitation10,Citation12. Catechol (12) was ineffective with KI of 4003 µM, whereas compound 11 (phenol) and AZA showed better inhibitory activity with KI values of 10.2 and 36.2 µM, respectively (). It was also interesting to note that the polyphenolic compounds 1–6 were stronger hCA I inhibitors as compared to the simple phenolics 11, 12 and 13, with KI-s values in the range of 0.97–2.68 µM. The saponins (7–10) were found to be strong inhibitors for hCA I with KI-s values ranging between 0.94 and 2.21 µM. Kinetic investigations (Lineweaver–Burk plots, data not shown) indicated that all the investigated compounds act as noncompetitive inhibitors with 4-NPA as substrate, similar to sulfonamides, metal ions and inorganic anionsCitation2–7,Citation28–38. It means that they bind in different regions of the active site cavity as compared to the substrate. However, the binding site of 4-NPA itself is unknown, but it is presumed to be in the same region as that of CO2, the physiological substrate of this enzymeCitation23.
Significant inhibitory activities were observed with compounds 5, 7 for the rapid cytosolic isozyme hCA II (). Six derivatives, i.e. 1, 3, 4, 6, 9–13, showed moderate hCA II inhibitory activity with KI-s in the range of 1.13–9.9 µM, whereas the remaining two derivatives 2 and 8 were quite effective hCA II inhibitors with KI-s of 0.83 and 0.97 µM, respectively. The best hCA II inhibitor was found to be 5 with a KI of 0.113 µM, better inhibitor than AZA, a clinically used sulfonamide. It must be stressed that KI-s measured with the esterase method are always in the micromolar range, because hCA I and II are weak esterasesCitation34–38.
Compound 13 was a weak inhibitor of bCA III with KI of 196 µM. While compounds 3 and 4 had medium potency (KI of 8.93 and 9.71 µM, respectively), the phenolic compounds 6, 11 and 12 showed higher affinity for this isozyme with inhibition constants in the range of 4.63–5.79 mM. AZA had KI of 263 µM, whereas the remaining seven derivatives 1, 2, 5, 7–10 were quite effective bCA III inhibitors with KI-s in the range of 1.38–3.73 µM ().
The membrane-anchored isoform hCA IV was poorly inhibited by five of the investigated phenols, (1, 6 and 11–13, Ki-s = 7.89–570 µM). Compounds 2–4 and 7–10 were more effective hCA IV inhibitors, acting similarly to the lead 5 with inhibition constants in the range of 1.12–9.5 µM. These compounds were anyhow weaker inhibitors as compared to the AZA (Ki of 0.578 µM against hCA IV, ).
Phenol (11) and some of its congeners such as 12 and 13 were also weak inhibitors of the secreted isozyme hCA VI, with KI-s of 208–606 µM. Additionally, compounds 1–4 and 6–10 were moderate inhibitors once more (KI of 4.36–9.78 µM), while the derivative 5 showed higher affinity for this isozyme with inhibition constant of 1.49 µM ().
In a recent study, it has been reported that the derivatives of salicylic acid act as a CA I inhibitor, and could represent the starting point for a new class of inhibitors that may have advantages for patients with sulfonamide allergiesCitation10,Citation38. The influences of various substances on different proteins and enzymes have been investigated by our groupCitation39–46. However, further investigations are needed in this area for understanding the behaviour of both enzymes and interacting compounds especially for medicinal chemists. CA inhibitors are of particular interest due to their potency to be used as drugs or prodrugs.
Phenolic compounds assayed in this study 1–6 influence the activity of CA enzyme due to the presence of different functional groups present in their aromatic scaffold. Saponins 7–10 are also quite effective against the isoenzymes. The present data point toward another class of possible CAIs of interest, in addition to the well-known sulfonamides/sulfamates/sulfamides, the phenols/biphenyl diphenols bearing bulky ortho moieties in their molecules. Compound 5 was the most remarkable inhibitor in the esterase method which usually gives KI-s an order of magnitude higher as compared to the CO2 hydratase assayCitation47–49.
Declaration of interest
This study was financed by an FP7 EU grant (Metoxia) to CTS.
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Fernley RT, Wright RD, Coghlan JP. A novel carbonic anhydrase from the ovine parotid gland. FEBS Lett 1979;105:299–302.
- Feldstein JB, Silverman DN. Purification and characterization of carbonic anhydrase from the saliva of the rat. J Biol Chem 1984;259:5447–5453.
- Murakami H, Sly WS. Purification and characterization of human salivary carbonic anhydrase. J Biol Chem 1987;262:1382–1388.
- Kivelä J, Parkkila S, Waheed A, Parkkila AK, Sly WS, Rajaniemi H. Secretory carbonic anhydrase isoenzyme (CA VI) in human serum. Clin Chem 1997;43:2318–2322.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–777.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem 1995;64:375–401.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–1587.
- Sentürk M, Gülçin I, Beydemir S, Küfrevioglu OI, Supuran CT. In vitro inhibition of human carbonic anhydrase i and ii isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–499.
- Cavdar H, Ekinci D, Talaz O, Saracoglu N, Senturk M, Supuran CT.. α-Carbonic anhydrases are sulfatases with cyclic diol monosulfate esters. J Enzyme Inhib Med Chem 2012;27:148–154.
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 2008;585:325–337.
- Huseini HF, Larijani B, Heshmat R, Fakhrzadeh H, Radjabipour B, Toliat T et al. The efficacy of Silybum marianum (L.) Gaertn. (silymarin) in the treatment of type II diabetes: a randomized, double-blind, placebo-controlled, clinical trial. Phytother Res 2006;20:1036–1039.
- Gan L, Han X, Chen Y. Astrasieversianins IX, XI and XV, cycloartane derived saponins from Astragalus sieversianus. Phytochem 1986;25:1437–1441.
- Kitagawa I, Wang HK, Saito M, Yoshikawa M. Chemical constituents of astragali radix, the root of Astragalus membranaceus Bunge. (3). Astragalosides ΙΙΙ, V, and VΙ. Chem Pharm Bull 1983;31:709–715.
- Bedir E, Calis I, Aquino R, Piacente S, Pizza C. Trojanoside H: A Cycloartanetype glycoside from the aerial parts of Astragalus trojanus. Phytochem 1999;51:1017–1020.
- Tu CK, Thomas HG, Wynns GC, Silverman DN. Hydrolysis of 4-nitrophenyl acetate catalyzed by carbonic anhydrase III from bovine skeletal muscle. J Biol Chem 1986;261:10100–10103.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc 1934;56:658–666.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Ekinci D, Beydemir S, Küfrevioglu OI. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 2007;22:745–750.
- Nair SK, Ludwig PA, Christianson DW. Two-site binding of phenol in the active site of human carbonic anhydrase II: structural implications for substrate association. J Am Chem Soc 1994;116:3659–3660.
- Parkkila S, Kaunisto K, Rajaniemi L, Kumpulainen T, Jokinen K, Rajaniemi H. Immunohistochemical localization of carbonic anhydrase isoenzymes VI, II, and I in human parotid and submandibular glands. J Histochem Cytochem 1990;38:941–947.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Ren X, Jonsson BH, Millqvist E, Lindskog S. A comparison of the kinetic properties of native bovine muscle carbonic anhydrase and an activated derivative with modified thiol groups. Biochim Biophys Acta 1988;953:79–85.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Thiry A, Dogné JM, Supuran CT, Masereel B. Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem 2007;7:855–864.
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Expert Opin Ther Pat 2006;16:1627–1664.
- Ekinci D, Cavdar H, Talaz O, Sentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–3563.
- Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Supuran CT, Scozzafava A. Novel aromatic/heterocyclic sulfonamides and their metal complexes as inhibitors of carbonic anhydrase isozymes I, II and IV. J Enzym Inhib 1997;12:37–51.
- Alp C, Ekinci D, Gültekin MS, Sentürk M, Sahin E, Küfrevioglu OI. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–4474.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. Design of zinc binding functions for carbonic anhydrase inhibitors. Curr Pharm Des 2008;14:615–621.
- Abbate F, Winum JY, Potter BV, Casini A, Montero JL, Scozzafava A et al. Carbonic anhydrase inhibitors: X-ray crystallographic structure of the adduct of human isozyme II with EMATE, a dual inhibitor of carbonic anhydrases and steroid sulfatase. Bioorg Med Chem Lett 2004;14:231–234.
- Winum JY, Vullo D, Casini A, Montero JL, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of cytosolic isozymes I and II and transmembrane, tumor-associated isozyme IX with sulfamates including EMATE also acting as steroid sulfatase inhibitors. J Med Chem 2003;46:2197–2204.
- Barrese AA 3rd, Genis C, Fisher SZ, Orwenyo JN, Kumara MT, Dutta SK et al. Inhibition of carbonic anhydrase II by thioxolone: a mechanistic and structural study. Biochemistry 2008;47:3174–3184.
- Durdagi S, Sentürk M, Ekinci D, Balaydin HT, Göksu S, Küfrevioglu ÖI, et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–1389.
- Ceyhun SB, Sentürk M, Ekinci D, Erdogan O, Ciltas A, Kocaman EM. Deltamethrin attenuates antioxidant defense system and induces the expression of heat shock protein 70 in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:215–223.
- Ekinci D, Ceyhun SB, Aksakal E, Erdogan O. IGF and GH mRNA levels are suppressed upon exposure to micromolar concentrations of cobalt and zinc in rainbow trout white muscle. Comp Biochem Physiol C Toxicol Pharmacol 2011;153:336–341.
- Erdogan O, Ceyhun SB, Ekinci D, Aksakal E. Impact of deltamethrin exposure on mRNA expression levels of metallothionein A, B and cytochrome P450 1A in rainbow trout muscles. Gene 2011;484:13–17.
- Cakmak R, Durdagi S, Ekinci D, Sentürk M, Topal G. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett 2011;21:5398–5402.
- Aksakal E, Ekinci D, Erdogan O, Beydemir S, Alim Z, Ceyhun SB. Increasing stocking density causes inhibition of metabolic-antioxidant enzymes and elevates mRNA levels of heat shock protein 70 in rainbow trout. Livest Sci 2011;141:69–75.
- Siktar E, Ekinci D, Siktar E, Beydemir S, Gülçin I, Günay M. Protective role of l-carnitine supplementation against exhaustive exercise induced oxidative stress in rats. Eur J Pharmacol 2011;668:407–413.
- Senturk M, Ekinci D, Alici HA, Beydemir S. Paraoxonase-1, an organophosphate detoxifier and cardioprotective enzyme, is inhibited by anesthetics: an in vitro and in vivo insight. Pestic Biochem Physiol 2011. DOI: 10.1016/j.pestbp.2011.09.007.
- Aksakal E, Ceyhun SB, Erdogan O, Ekinci D. Acute and long-term genotoxicity of deltamethrin to insulin-like growth factors and growth hormone in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:451–455.
- Innocenti A, Beyza Oztürk Sarikaya S, Gülçin I, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–2164.
- Senturk M, Gulcin I, Beydemir S, Kufrevioglu OI, Supuran CT. In Vitro inhibition of human carbonic anhydrase I and II isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–499.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of mammalian isoforms I-XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg Med Chem 2008;16:7424–7428.