Abstract
In the present study, some thiazole derivatives were synthesized via the ring closure reaction of 1-[2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetyl]thiosemicarbazide with various phenacyl bromides. The chemical structures of the compounds were elucidated by 1H NMR, 13C NMR and mass spectral data and elemental analyses. Each derivative was evaluated for its ability to inhibit acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) using a modification of Ellman’s spectrophotometric method. The compounds were also investigated for their cytotoxic properties using MTT assay. The most potent AChE inhibitor was found as compound 4e (IC50 = 25.5 ± 2.12 µg/mL) followed by compounds 4i (IC50 = 38.50 ± 2.12 µg/mL), 4c (IC50 = 58.42 ± 3.14 µg/mL) and 4g (IC50 = 68 ± 2.12 µg/mL) when compared with eserine (IC50 = 0.025 ± 0.01 µg/mL). Effective compounds on AChE exhibited weak inhibition on BuChE (IC50 > 80 µg/mL). MTT assay indicated that the cytotoxic dose (IC50 = 71.67 ± 7.63 µg/mL) of compound 4e was higher than its effective dose.
Introduction
Drug design through enzyme inhibition has gained great importance as a promising approach for the rational discovery of new drugs due to the increasing need to discover more selective and potent inhibitors in an effort to increase the therapeutic benefit to patientsCitation1,Citation2.
Cholinesterase inhibitors (ChEIs) have attracted a great deal of interest among researchers owing to their importance in the treatment of myasthenia gravis, glaucoma and Alzheimer’s diseaseCitation1–3.
Two cholinesterases are present in humans: acetylcholinesterase (AChE), which selectively hydrolyses acetylcholine, and butyrylcholinesterase (BuChE), which is a non-specific cholinesterase. The main difference between two types of cholinesterase is the respective preferences for substrates: the former hydrolyses acetylcholine more quickly; the latter hydrolyses butyrylcholine more quickly. The main function of AChE is the termination of cholinergic neurotransmission, but the function of BuChE is not so clearCitation1,Citation4.
Acetylcholinesterase inhibitors (AChEIs) exert their therapeutic action by inhibiting AChE, which results in the enhancement of cholinergic actionCitation2. In particular, AChEIs play a leading role in the first-line treatment for symptoms of Alzheimer’s disease, which is the most common age-related neurodegenerative disorderCitation5–9.
In the last few decades, the chemistry of thiazoles and their fused heterocyclic derivatives has received considerable attention due to their synthetic and biological importance. Compounds bearing thiazole moiety have been reported to exhibit a wide spectrum of biological effects including anticholinesterase activityCitation10–16.
Acotiamide hydrochloride (acotiamide; N-[2-[bis(1-methylethyl)amino]ethyl]-2-[(2-hydroxy-4, 5-dimethoxybenzoyl)amino]thiazole-4-carboxamide monohydrochloride trihydrate, Z-338) has been reported to be a new selective AChEI for the treatment of functional dyspepsia in clinical studiesCitation15.
In a previous work, the inhibition of AChE by thiamine and its derivatives was investigated and structure-activity relationship study was also carried out to identify structural features that are associated with the inhibitory potency of these compoundsCitation16.
Some researchers also carried out considerable research for novel cholinesterase inhibitors bearing amide and hydrazide moieties previouslyCitation17.
On the basis of these findings, we became interested in biological evaluation of thiazoles as anticholinesterase agents. Herein, we described the synthesis of novel thiazole derivatives bearing hydrazide moiety and focused on their anticholinesterase effects on AChE and BuChE. The compounds were also investigated for their cytotoxic properties.
Methods
Chemistry
All reagents were purchased from commercial suppliers and used without further purification. Melting points (m.p.) were determined on a Electrothermal 9100 melting point apparatus (Weiss-Gallenkamp, Loughborough, UK) and are uncorrected. Proton nuclear magnetic resonance (1H NMR) spectra were recorded on Bruker 400 MHz spectrometer (Bruker, Billerica, MA, USA). Carbon nuclear magnetic resonance (13C NMR) spectra were recorded on Bruker 100 MHz spectrometer (Bruker, Billerica, MA, USA). Chemical shifts were expressed in parts per million (ppm) and tetramethylsilane was used as an internal standard. Mass spectra were recorded on a VG Quattro Mass spectrometer (Agilent, Minnesota, USA). Elemental analyses were performed on a Perkin-Elmer EAL 240 elemental analyser (Perkin-Elmer, Norwalk, CT, USA).
General procedure for the synthesis of the compounds
Ethyl 2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetate (1)
A mixture of benzo[d]thiazol-2(3H)-one (0.1 mol) and ethyl chloroacetate (0.1 mol) in the presence of potassium carbonate (0.1 mol) in acetone (50 mL) was refluxed for 10 h. The reaction mixture was poured into 100 mL of ice–water mixture, filtered and washed with waterCitation18.
2-(2-Oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (2)
A mixture of the ester (1) (0.09 mol) and hydrazine hydrate (0.18 mol) in ethanol (50 mL) was stirred at room temperature for 3 h and then filteredCitation18.
1-[2-(2-Oxobenzo[d]thiazol-3(2H)-yl)acetyl]thiosemicarbazide (3)
A mixture of the hydrazide (2) (0.05 mol), potassium thiocyanate (0.1 mol), conc. HCl (20 mL) in water (40 mL) was refluxed for 3 h. After cooling, the resulting solid was collected by filtration, washed with water, dried, and recrystallized from ethanol.
2-(2-Oxobenzo[d]thiazol-3(2H)-yl)-N′-(4-phenylthiazol-2-yl)acetohydrazide derivatives (4a–j)
A mixture of 1-[2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetyl]thiosemicarbazide (3) (0.001 mol) and appropriate phenacyl bromide (0.001 mol) was refluxed in ethanol (15 mL) for 3 h. After cooling, the resulting solid was collected by filtration, dried, and recrystallized from ethanol.
N′-(4-phenylthiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4a)
1H NMR (400 MHz, DMSO-d6): δ 4.77 (2H, s), 7.10–7.45 (6H, m), 7.68 (2H, m), 7.84 (2H, m), 9.64 (1H, brs), 10.75 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.22 (CH2), 103.4 (CH), 111.3 (CH), 121.2 (C), 122.8 (CH), 123.3 (CH), 125.6 (2CH), 126.5 (CH), 127.5 (CH), 128.5 (2CH), 134.5 (C), 137.0 (C), 150.4 (C), 166.3 (C), 169.1 (C), 171.5 (C).
For C18H14N4O2S2, calculated: C, 56.53; H, 3.69; N, 14.65; found: C, 56.52; H, 3.71; N, 14.61.
MS (ES): [M + 1]+: 383
N′-(4-p-tolylthiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4b)
1H NMR (400 MHz, DMSO-d6): δ 2.32 (3H, s), 4.77 (2H, s), 7.10–7.40 (5H, m), 7.68 (2H, m), 7.72 (2H, m), 9.60 (1H, brs), 10.73 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 20.77 (CH3), 43.21 (CH2), 102.5 (CH), 111.4 (CH), 121.2 (C), 122.9 (CH), 123.3 (CH), 125.5 (2CH), 126.5 (CH), 129.1 (2CH), 131.9 (C), 136.8 (C), 137.0 (C), 150.5 (C), 166.3 (C), 169.1 (C), 171.5 (C).
For C19H16N4O2S2, calculated: C, 57.56; H, 4.07; N, 14.13; found: C, 57.55; H, 4.05; N, 14.15.
MS (ES): [M + 1]+: 397
N′-(4-(4-chlorophenyl)thiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4c)
1H NMR (400 MHz, DMSO-d6): δ 4.78 (2H, s), 7.15–7.40 (3H, m), 7.46 (2H, m), 7.68 (2H, m), 7.85 (2H, m), 9.68 (1H, brs), 10.76 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.22 (CH2), 104.2 (CH), 111.3 (CH), 121.2 (C), 122.9 (CH), 123.3 (CH), 126.5 (CH), 127.2 (2CH), 128.6 (2CH), 131.9 (C), 133.4 (C), 137.0 (C), 149.2 (C), 166.3 (C), 169.1 (C), 171.7 (C).
For C18H13ClN4O2S2, calculated: C, 51.86; H, 3.14; N, 13.44; found: C, 51.85; H, 3.15; N, 13.40.
MS (ES): [M + 1]+: 418
N′-(4-(4-bromophenyl)thiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4d)
1H NMR (400 MHz, DMSO-d6): δ 4.77 (2H, s), 7.15–7.30 (3H, m), 7.36 (2H, m), 7.55–7.70 (2H, m), 7.78 (2H, m), 9.66 (1H, brs), 10.74 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.22 (CH2), 104.3 (CH), 111.3 (CH), 120.5 (C), 121.2 (C), 122.9 (CH), 123.3 (CH), 126.5 (CH), 127.6 (2CH), 131.5 (2CH), 133.7 (C), 137.0 (C), 149.3 (C), 166.3 (C), 169.2 (C), 171.7 (C).
For C18H13BrN4O2S2, calculated: C, 46.86; H, 2.84; N, 12.14; found: C, 46.85; H, 2.80; N, 12.15.
MS (ES): [M + 1]+: 462
N′-(4-(4-nitrophenyl)thiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4e)
1H NMR (400 MHz, DMSO-d6): δ 4.78 (2H, s), 7.15–7.40 (3H, m), 7.66 (2H, s), 8.10 (2H, m), 8.27 (2H, m), 9.80 (1H, brs), 10.81 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.22 (CH2), 108.3 (CH), 111.3 (CH), 121.2 (C), 122.9 (CH), 123.3 (CH), 124.0 (2CH), 126.4 (2CH), 126.5 (CH), 137.0 (C), 140.5 (C), 146.2 (C), 148.4 (C), 166.4 (C), 169.2 (C), 172.0 (C).
For C18H13N5O4S2, calculated: C, 50.58; H, 3.07; N, 16.38; found: C, 50.55; H, 3.10; N, 16.35.
MS (ES): [M + 1]+: 428
N′-(4-(3-chlorophenyl)thiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4f)
1H NMR (400 MHz, DMSO-d6): δ 4.77 (2H, s), 7.0–8.0 (9H, m), 9.69 (1H, s), 10.75 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.21 (CH2), 105.0 (CH), 111.3 (CH), 121.2 (C), 122.9 (CH), 123.3 (CH), 124.1 (CH), 125.2 (CH), 126.5 (CH), 127.2 (CH), 130.5 (CH), 133.4 (C), 136.5 (C), 137.0 (C), 148.8 (C), 166.3 (C), 169.2 (C), 171.6 (C).
For C18H13ClN4O2S2, calculated: C, 51.86; H, 3.14; N, 13.44; found: C, 51.85; H, 3.16; N, 13.42.
MS (ES): [M + 1]+: 418
N′-(4-(3-nitrophenyl)thiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4g)
1H NMR (400 MHz, DMSO-d6): δ 4.78 (2H, s), 7.05–7.40 (3H, m), 7.61 (1H, s), 7.70 (2H, m), 8.14 (1H, dd, J = 8.1, 2.3 Hz), 8.27 (1H, d, J = 7.9 Hz), 8.64 (1H, t, J = 2.0 Hz), 9.78 (1H, brs), 10.80 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.21 (CH2), 106.2 (CH), 111.3 (CH), 119.9 (CH), 121.2 (C), 122.1 (CH), 122.9 (CH), 123.3 (CH), 126.5 (CH), 130.2 (CH), 131.6 (CH), 136.0 (C), 137.0 (C), 148.1 (C), 148.3 (C), 166.4 (C), 169.1 (C), 172.1 (C).
For C18H13N5O4S2, calculated: C, 50.58; H, 3.07; N, 16.38; found: C, 50.59; H, 3.09; N, 16.34.
MS (ES): [M + 1]+: 428
N′-(4-(3,4-dichlorophenyl)thiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4h)
1H NMR (400 MHz, DMSO-d6): δ 4.77 (2H, s), 7.0–7.4 (3H, m), 7.49 (1H, s), 7.62–7.67 (2H, m), 7.80 (1H, dd, J = 8.5, 2.0 Hz), 8.06 (1H, d, J = 2.0 Hz), 9.70 (1H, brs), 10.75 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.23 (CH2), 105.7 (CH), 111.3 (CH), 121.2 (C), 122.9 (CH), 123.3 (CH), 125.6 (CH), 126.5 (CH), 127.1 (CH), 129.7 (C), 130.8 (CH), 131.4 (C), 135.0 (C), 137.0 (C), 147.8 (C), 166.3 (C), 169.1 (C), 171.7 (C).
For C18H12Cl2N4O2S2, calculated: C, 47.90; H, 2.68; N, 12.41; found: C, 47.92; H, 2.70; N, 12.38.
MS (ES): [M + 1]+: 452
N′-(4-(2,4-dichlorophenyl)thiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4i)
1H NMR (400 MHz, DMSO-d6): δ 4.77 (2H, s), 7.05–7.35 (3H, m), 7.37 (1H, s), 7.48 (1H, dd, J = 8.5, 2.2 Hz), 7.63–7.70 (2H, m), 7.88 (1H, d, J = 8.5 Hz), 9.68 (1H, brs), 10.75 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.22 (CH2), 109.0 (CH), 111.3 (CH), 121.2 (C), 122.8 (CH), 123.3 (CH), 126.5 (CH), 127.4 (CH), 129.7 (CH), 131.4 (C), 131.9 (C), 132.3 (CH), 132.4 (C), 137.0 (C), 145.7 (C), 166.3 (C), 169.1 (C), 170.7 (C).
For C18H12Cl2N4O2S2, calculated: C, 47.90; H, 2.68; N, 12.41; found: C, 47.89; H, 2.71; N, 12.39.
MS (ES): [M + 1]+: 452
N′-(4-(2,5-dichlorophenyl)thiazol-2-yl)-2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetohydrazide (4j)
1H NMR (400 MHz, DMSO-d6): δ 4.77 (2H, s), 7.15–7.32 (3H, m), 7.40 (1H, dd, J = 8.6, 2.7 Hz), 7.47 (1H, s), 7.55 (1H, d, J = 8.6 Hz), 7.66 (1H, dd, J = 7.8, 1.2 Hz), 7.92 (1H, d, J = 2.7 Hz), 9.71 (1H, brs), 10.76 (1H, s).
13C NMR (100 MHz, DMSO-d6): δ 43.21 (CH2), 109.8 (CH), 111.3 (CH), 121.2 (C), 122.8 (CH), 123.3 (CH), 126.4 (CH), 128.5 (CH), 129.1 (C), 130.3 (CH), 131.8 (C), 132.1 (CH), 134.3 (C), 137.0 (C), 145.3 (C), 166.3 (C), 169.1 (C), 170.7 (C).
For C18H12Cl2N4O2S2, calculated: C, 47.90; H, 2.68; N, 12.41; found: C, 47.88; H, 2.70; N, 12.38.
MS (ES): [M + 1]+: 452
AChE and BuChE inhibitory activity
AChE and BuChE inhibitory activity was determined by Ellman’s method with minor modifications (Electric eel AChE enzyme was used instead of bovine AChE enzyme and buffer was added 2.4 mL instead of 3 mL)Citation19. Compounds 4a–j were dissolved in DMSO and tested at final concentration range 5–80 µg/mL. Twenty micro litre of enzyme (AChE or BuChE, 1 U/mL), 10 µL sample added to 2.4 mL buffer, the mixture was incubated at 37°C for 15 min. After 15 min incubation, 50 µL of 0.01 M 5,5′-dithio-bis(2-nitrobenzoic acid) and 20 µL of 75 mM acetylthiocholine iodide or 25 mM butyrylthiocholine iodide were added, and the final mixture was incubated at room temperature for 30 min. Blank was prepared using 10 µL of DMSO instead of the test sample with all other procedures similar to those used in the case of the sample mixture. Absorbances were measured at 412 nm and 37°C using polystyrol cuvets with spectrophotometer (Shimadzu, UV-1700). Experiment was done in triplicate. Data are expressed as mean ± standard deviation (SD).
The inhibition (percent) of AChE or BuChE was calculated using the following equation:
Toxicity
The level of cellular MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma) reduction was quantified as previously described in the literature with small modifications as represented below in detailCitation20,Citation21.
Cell culture and drug treatment
NIH/3T3 cells were obtained from the American Type Culture Collection (ATCC, USA). The cells were incubated in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (Life Technologies, UK), 100 IU/mL penicillin (Gibco, Paisley, Scotland) and 100 mg/mL streptomycin (Gibco) at 37°C in a humidified atmosphere of 95% air and 5% CO2. Exponentially growing cells were plated at 2 × 104 cells/mL into 96-well microtiter tissue culture plates (Nunc, Denmark) and incubated for 24 h before the addition of the drugs (the optimum cell number for cytotoxicity assays was determined in preliminary experiments). Stock solutions of compounds were prepared in DMSO (Sigma-Aldrich, Poole, UK) and further dilutions were made with fresh culture medium (the concentration of DMSO in the final culture medium was <0.1% which had no effect on the cell viability).
MTT assay for cytotoxicity of the compounds
The MTT assay is widely used as a measure of cytotoxicity. After 24 h of preincubation, the tested compounds were added to give final concentration in the range 0.5–500 µg/mL and the cells were incubated for 24 h. At the end of this period, MTT was dissolved in phosphate buffered saline at 5 mg/mL and filtered to sterilize. At the time indicated above, stock MTT solution (20 μL/200 μL medium) was added to all wells of assay, and the plates were incubated for 4 h at 37°C. After the medium was removed, the formazan crystals formed by MTT metabolism were solubilized by addition of 200 μL DMSO (instead of acid-isopropanol) to each well and after a few minutes at room temperature to ensure that all crystals were dissolved, absorbance was read at 540 nm with a microtitre plate spectrophotometer (Bio-Tek plate reader). Each concentration was repeated in three wells and IC50 values were defined as the drug concentrations that reduced absorbance to 50% of control values.
Results and discussion
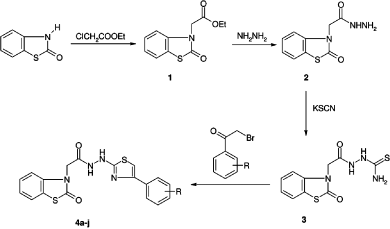
Initially, ethyl 2-(2-oxobenzo[d]thiazol-3(2H)-yl)acetate (1) was synthesized via the reaction of benzo[d]thiazol-2(3H)-one with ethyl chloroacetate in the presence of potassium carbonate. Then, this ester (1) was converted to the corresponding hydrazide derivative (2).
1-[2-(2-Oxobenzo[d]thiazol-3(2H)-yl)acetyl]thiosemicarbazide (3) was obtained by the reaction of the hydrazide derivative (2) with potassium thiocyanate and conc. HCl. The ring closure of compound 3 with phenacyl bromides gave the target compounds (4a–j). These reactions are summarized in and some properties of the compounds are given in . The structures of these compounds (4a–j) were confirmed by 1H NMR, 13C NMR and mass spectral data and elemental analyses.
Table 1. Some properties of the synthesized compounds (4a–j).
The anticholinesterase effects of the compounds (4a–j) on AChE and BuChE were determined by a modification of Ellman’s spectrophotometric method ().
Table 2. The anticholinesterase activities of the compounds (4a–j) as IC50 values (µg/mL).
Among these compounds (4a–j), compound 4e can be identified as the most promising anticholinesterase agent due to its inhibitory effect on AChE with an IC50 value of 25.5 ± 2.12 µg/mL when compared with eserine (IC50 = 0.025 ± 0.01 µg/mL). In our previous study, we also reported that galantamine exhibited its inhibitory effect on AChE with an IC50 value of 0.28 ± 0.04 µg/mL22. Compound 4e also showed the highest AChE inhibitory effect when compared with galantamine. Although compounds 4e and 4g possess nitro substituent on phenyl ring, they show different levels of anticholinesterase activity. The former bearing p-nitro group exhibits the inhibitory effect on AChE with an IC50 value of 25.5 ± 2.12 µg/mL, whereas the latter bearing m-nitro group exhibits the inhibitory effect on AChE with an IC50 value of 68 ± 2.12 µg/mL. Compounds 4e and 4g also exhibit different levels of cytotoxicity with IC50 values of 71.67 ± 7.63 and 4.93 ± 0.11 µg/mL, respectively. These observations indicate that the position of nitro substituent on phenyl ring has a crucial influence on anticholinesterase activity and cytotoxicity. It is apparent that there is a positive correlation between anticholinesterase activity and p-nitro group. Compound 4i carrying 2,4-dichloro group on phenyl ring exhibits the inhibitory effect on AChE with an IC50 value of 38.50 ± 2.12 µg/mL, whilst compound 4c bearing p-chloro group on phenyl ring exhibits the inhibitory effect on AChE with an IC50 value of 58.42 ± 3.14 µg/mL. Compounds 4d, 4f, 4h, 4j exhibit weak inhibition on AChE (IC50 > 80 µg/mL), whereas compounds 4a and 4b are inactive.
Compounds 4a, 4b, 4c, 4e, 4f, 4g, 4h, 4i showed weak inhibitory effects on BuChE (IC50 > 80 µg/mL), whilst compounds 4d and 4j were inactive.
The compounds were also evaluated for their cytotoxic properties using MTT assay (). The biological study indicated that compound 4g possessed the highest cytotoxicity, whereas compound 4j exhibited the lowest cytotoxicity against mouse fibroblast (NIH/3T3) cell line among the title compounds.
Table 3. In vitro cytotoxicity of the compounds (4a–j).
Conclusion
In the present paper, we synthesized a series of thiazole derivatives and evaluated their anticholinesterase effects and cytotoxicity.
The biological results indicate that functional groups on the phenyl ring have a considerable influence on anticholinesterase activity and toxicity. In particular, compound 4e is the most promising AChEI due to its inhibitory effect on AChE with an IC50 value of 25.5 ± 2.12 µg/mL. In addition, the cytotoxic dose (IC50 = 71.67 ± 7.63 µg/mL) of compound 4e is higher than its effective dose.
Declaration of interest
The authors report no conflicts of interest.
References
- Lemke TL, Williams DA. (2008). Foye’s Principles of Medicinal Chemistry. Baltimore and Philadelphia: Lippincott Williams & Wilkins USA, 99–391.
- Silverman RB. (2004). The organic chemistry of drug design and drug action. Burlington: Elsevier Academic Press USA, 229–535.
- Shen ZX. Brain cholinesterases: III. Future perspectives of AD research and clinical practice. Med Hypotheses 2004;63:298–307.
- Wilkinson DG, Francis PT, Schwam E, Payne-Parrish J. Cholinesterase inhibitors used in the treatment of Alzheimer’s disease: the relationship between pharmacological effects and clinical efficacy. Drugs Aging 2004;21:453–478.
- Grutzendler J, Morris JC. Cholinesterase inhibitors for Alzheimer’s disease. Drugs 2001;61:41–52.
- Giacobini E. Cholinesterases: new roles in brain function and in Alzheimer’s disease. Neurochem Res 2003;28:515–522.
- Johannsen P. Long-term cholinesterase inhibitor treatment of Alzheimer’s disease. CNS Drugs 2004;18:757–768.
- Martinez A, Castro A. Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer’s disease. Expert Opin Investig Drugs 2006;15:1–12.
- Pepeu G, Giovannini MG. Cholinesterase inhibitors and beyond. Curr Alzheimer Res 2009;6:86–96.
- Siddiqui N, Arshad MF, Ahsan W, Alam MS. Thiazoles: a valuable insight into the recent advances and biological activities. Int J Pharm Sci Drug Res 2009;1:136–143.
- Mustafa SM, Naira VA, Chittoorb JP, Krishnapillaic S. Synthesis of 1,2,4-Triazoles and thiazoles from thiosemicarbazide and its derivatives. Mini-Rev Org Chem 2004;1:375–385.
- Andreani A, Burnelli S, Granaiola M, Guardigli M, Leoni A, Locatelli A et al. Chemiluminescent high-throughput microassay applied to imidazo[2,1-b]thiazole derivatives as potential acetylcholinesterase and butyrylcholinesterase inhibitors. Eur J Med Chem 2008;43:657–661.
- Sengupta AK, Garg M. New 5-arylamino-2[N-(2′-mercaptoacetylamino-4′-arylthiazolo)]thiadiazoles(III): as AChE inhibitors. J Indian Chem Soc 1980;57:1241–1243.
- Shaw FH, Bentley GA. The pharmacology of some new anti-cholinesterases. Aust J Exp Biol Med Sci 1953;31:573–576.
- Matsunaga Y, Tanaka T, Yoshinaga K, Ueki S, Hori Y, Eta R et al. Acotiamide hydrochloride (Z-338), a new selective acetylcholinesterase inhibitor, enhances gastric motility without prolonging QT interval in dogs: comparison with cisapride, itopride, and mosapride. J Pharmacol Exp Ther 2011;336:791–800.
- Alspach JD, Ingraham LL. Inhibition of acetylcholinesterase by thiamine. A structure-function study. J Med Chem 1977;20:161–164.
- Tomassoli I, Ismaili L, Pudlo M, de Los Rí, os C, Soriano E, Colmena I et al. Synthesis, biological assessment and molecular modeling of new dihydroquinoline-3-carboxamides and dihydroquinoline-3-carbohydrazide derivatives as cholinesterase inhibitors, and Ca channel antagonists. Eur J Med Chem 2011;46:1–10.
- Cakir B, Yildirim E, Ercanli T, Erol K, Sahin MF. Synthesis and anticonvulsant activity of some (2/4-substituted)benzaldehyde (2-oxobenzothiazolin-3-yl)acetohydrazones. Farmaco 1999;54:842–845.
- Ellman GL, Courtney KD, Andres V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 1961;7:88–95.
- Mossmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 1983;65:55–63.
- Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod 2000;26:288–291.
- Altintop MD, Kaplancikli ZA, Ozdemir A, Turan-Zitouni G, Temel HE, Akalın G. Synthesis and anticholinesterase activity and cytotoxicity of novel amide derivatives. Arch Pharm 2011. DOI: 10.1002/ardp.201100124.