Abstract
Phosphates and tensin homologue deleted on chromosome 10 (PTEN) is a tumour suppressor gene which dephosphorilates phosphoinositol 3,4,5 triphosphates. Therefore PTEN can regulate PI3K/AKT pathway in cells. Because of promoter methylation or gene deletion, PTEN expression is commonly decreased or lost in non-small cell lung cancer (NSCLC) cell lines. Therefore, we hypothesized that PTEN could regulate the activity of superoxide dismutase (CuZnSOD), glutathione peroxidase (GPx) and catalase. We first recreated PTENwt, G129R and G129E expressions in lung cell lines, in which endogenous PTEN expression was not detected. Then, we showed that PTEN could suppress AKT activity by its lipid phosphatase domain. We then examined the effect of recreated PTEN expressions in NSCLC cells. While PTENwt expression caused enhanced activity of SOD, GPx and catalase in transfected cells lines, neither G129R nor G129E expression effected enzyme activities. These results suggest that PTEN can up-regulate SOD, GPx and catalase activity by inhibition of PI3K/AKT pathway in NSCLC cell lines.
Introduction
Lung cancer continues to be one of the major causes of cancer-related deaths in the world, and smoking is the primary cause of lung cancerCitation1. Lung is a unique tissue for oxidant stress among most other organs because it is directly exposed to higher oxygen tensions. Because of their direct exposure to ambient air, lung cells experience enhanced oxidant stress by environmental irritants and pollutants including oxidants such as cigarette smoke, environmental carcinogens which generate free radicalsCitation2. Therefore, antioxidant enzyme activity and expression is very important for lung tissue.
The PTEN (phosphates and tensin homologue deleted on chromosome 10) tumour suppressor gene is frequently deleted or mutated in a wide variety of human cancers, including glioblastomaCitation3, melanomaCitation4, prostate cancerCitation5, breast cancerCitation6, lung cancerCitation7, and endometrial cancerCitation8. PTEN is also essential for embryonic developmentCitation9,Citation10. PTEN encodes a protein that has sequence homology with phosphatases which dephosphorylate both tyrosine and serine/threonin phosphates on proteinsCitation11–13. PTEN is also capable of dephosphorylating inositol phospholipidsCitation14. In particular, PTEN dephosphorylates phosphatidylinositol-3,4,5-triphosphate (PIP3) and phosphatidylinositol-4.5-biphosphate (PIP2). Therefore, PTEN can suppress AKT activity and inhibit the PI3K/AKT/NFκB pathway.
Nuclear factor kappa B (NF-κB) is a transcription factor regulating expression of multiple genesCitation15, and has been shown to govern various cellular functions, including inflammatory and stress-induced responses and survivalCitation16. NF-κB activation is regulated by negative feedback mediated by IκB, an inhibitor protein that binds to NF-κB, but can undergo ubiquitination and proteasomal degradationCitation17, thus freeing NF-κB to translocate to the nucleus and initiate transcriptionCitation18. NF-κB is a redox-sensitive transcription factorCitation19, that has been proposed to be the sensor for oxidative stressCitation20. It has also been reported that NFκB activity down-regulate Catalase and SOD activityCitation21. Therefore, we designed this study to investigate the suppressive effect of PTEN tumour suppressor gene on intracellular oxidative stress in NSCLC cell lines.
Materials and methods
Cell culture
PC3, PC9 and PC14 cells were cultured in RPMI1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA) and incubated at 37°C in humidified incubator with 5% CO2.
Expression plasmids and transfections
PTENwt, lipidfosfatase dead PTEN G129E and kinase dead PTEN G129R were cloned in both, pcDNA3 and pcDNA6/TK expression vectors as described in our previous workCitation22. By using FUGEN (Roche, Indianapolis, IN), PC14 cells were first stably transfected with regulatory plasmid pcDNA6/TK, and clones were selected by blasticidin (10 µg/ml). Selected clones were then transfected with pcDNA4/TO-PTENwt, pcDNA4/TO-PTENG129R, and pcDNA4/TO-PTENG129E expression vectors. Double transfectants were selected in medium containing blasticidin (10 µg/ml) and zeocin (50 µg/ml), then stable transfected cells were treated 2 µg/ml tetracycline for 24 h to induce PTEN expression on these cells. PC9 and PC3 cells were transiently transfected with pcDNA-PTENwt, pcDNA-PTENG129R and pcDNA-PTENG129E, the expressions verified by western blotting.
Lysate preparation and western blot
Cells were grown to 80% confluency, washed with phosphate buffered saline, and incubated in serum free medium containing tetracyclin (2 µg/ml) for 24 h. Cell lysates were prepared in ice-cold RIPA buffer (10 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1% sodiumdeoxycholate, 0.1% SDS). Cellular debri was removed by centrifugation at 12,000g for 5 min at 4°C. One hundred micrograms of proteins were subjected to SDS-PAGE using 2–15% or 7.5% poly-acrylamide gels (Pierce, Rockford, IL) at pH 7.0, proteins were immunoblotted onto Hybond-PVDF membrane (Amersham-Pharmacia Biotech, Buckinghamshire, UK), and labeled with antibodies. AKT, phospho-AKT (Ser473), antibodies were obtained from Cell Signaling Technology (Beverly, MA). PTEN and horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). After primary and secondary antibody labeling blots were treated with Super Signal West Pico chemiluminescent substrate (Pierce), exposed to Hyperfilm ECL (Amersham-PharmaciaBiotech) and developed.
Measurement of superoxide dismutase activity
Cell pellets were sonicated (Bandelin Sonopuls, Bandelin Electronic GmbH&Co., Berlin, Germany) in cold 20 mM HEPES buffer, pH 7.2, containing 1 mM EDTA (Sigma, Sigma-Aldrich Chemie, Steinheim, Germany). Cell homogenates were centrifuged at 1,500g for 5 min at 4°C and supernatants were kept at −80°C until assayed. Superoxide activity was measured using a SOD activity assay kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. The SOD activity assay kit utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD activity was defined as the amount of enzyme needed to exhibit 50% dismutation of superoxide radical. The SOD assay measured both the cytosolic and mitochondrial activity of the enzyme.
Measurement of catalase activity
We measured catalase activity as described beforeCitation23. Briefly, cell pellets were sonicated in cold 50 mM potassium phosphate, pH 7.0, containing 1 mM EDTA (Sigma, Sigma-Aldrich Chemie, Steinheim, Germany). Cell homogenates were centrifuged at 10,000g for 15 min at 4°C and supernatants were kept at −80°C until assayed. Catalase activity was measured using a CAT activity assay kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. The CAT activity assay kit utilizes the peroxidatic function of CAT for determination of enzyme activity. The method is based on the reaction of the enzyme with methanol in the presence of H2O2. The formaldehyde produced, is measured spectrophotometrically with purpald (4-amino-3-hydrazino-5-marcapto-1,2,4-traizole) as the chromogen. The formaldehyde produced by each sample was calculated from a standard curve obtained via the supplied standard within the kit. One unit of enzyme activity was defined as the amount of enzyme that caused the formation of 1 μmol formaldehyde per minute at 25°C.
Measurement of glutathione peroxidase activity
We measured glutathione peroxidase activity as described beforeCitation23. Briefly, cell pellets were sonicated in cold 50 mM Tris-HCl (SERVA, Heidelberg, Germany) buffer at pH 7.5, containing 1 mM EDTA (Sigma, Sigma-Aldrich Chemie, Steinheim, Germany). Cell homogenates were centrifuged at 10,000g for 15 min at 4°C and supernatants were kept at −80°C until assayed. Glutathione peroxidase was measured using a GPx activity assay kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer’s instructions. The GPx activity assay kit measures enzyme activity indirectly by a coupled reaction with glutathione reductase (GR). The oxidation of NADPH to NADP is accompanied by as decrease in absorbance at 340 nm. Absorbance kinetics was assessed spectrophotometrically at 340 nm by using the NADPH extinction coefficient of 0.00622 μM/cm. One unit of enzyme activity was defined as the amount of enzyme that caused the oxidation of 1 μmol NADPH to NADP per minute at 25°C.
Results
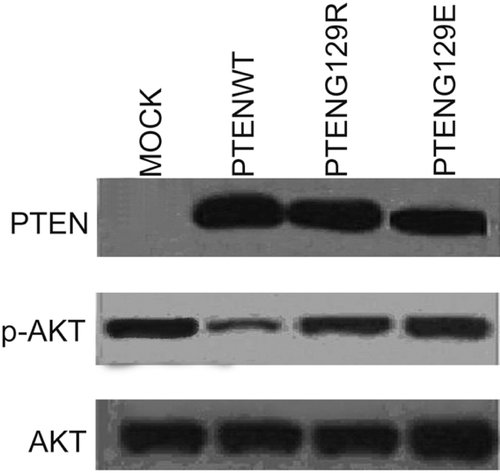
To show the possible effects of recreated PTEN expression on antioxidant enzymes catalase, GPx and SOD activity, we use NSCLC PC14, PC9 and PC3 cells which have no endogenous PTEN expressionCitation22,Citation24. First, we transfected PC14 cells with pcDNA6-PTENwt, pcDNA6-PTENG129R and pcDNA6-PTENG129E and selected stably transfected cells as described in the Materials and methods section. Stably transfected PTENwt, PTENG129R and PTENG129E expressing cells were lysed and PTEN expression was detected by western blot. In , it is clearly shown that PTENwt, G129R and G129E expressions were recreated in PC14 cells. PTEN wt expression leads to decreased p-AKT level. Lipid phosphatase dead (G129E) and kinase dead (G129R) PTEN expressions had no effect on p-AKT level, implicating that PTEN can suppress AKT activity by its lipid phosphatase ability.
Figure 1. Western blot analysis of stably transfected PC14 cells. Figure represent the stable expression of Mock, PTENwt, PTENG129E and PTENG129R and the effect of these expressions on AKT activity in NSCLC PC14 cell lines.
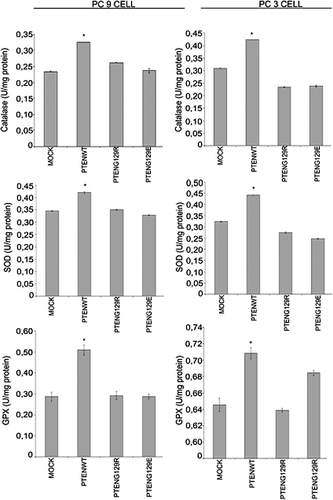
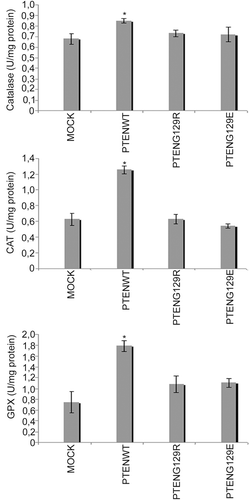
It has previously been reported that SOD and catalase activity increase when NFκB activity is suppressedCitation21. Because PTEN suppresses PI3K/AKT/NFκB pathway and inhibits NFκB activity, we hypothesized that PTEN could also change the activity of antioxidant enzymes by suppression of the PI3K/AKT/NFκB pathway. Therefore, we first examined recreated PTEN and its mutant effects on GPx, SOD and catalase activity in stable PTEN expressing PC14 cells. Interestingly, our results indicate that, only PTENwt expression can induce the activity of GPx, SOD and catalase. Neither PTENG129R nor PTENG129E had an effect on these enzyme activities ().
Figure 2. The effects of stable expression of Mock, PTENwt, PTENG129E and PTENG129R on catalase, GPx and SOD enzymes activity in NSCLC PC 14 cells.
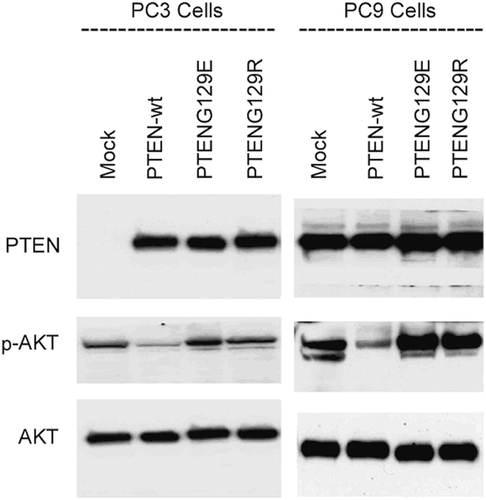
To understand whether the observed effects were cell specific or not, we transiently transfected PC9 and PC3 cells with mock, PTENwt, PTENG129R and PTENG129E. These NSCLC cell lines, PC9 and PC3, have no endogenous PTEN expressionCitation22,Citation24. We first transiently transfected PC9 and PC3 cells with mock, pcDNA3-PTENwt, pcDNA3-PTENG129R and pcDNA3-PTENG129E. After 24 hours of transfection, cells were lysed for detection of PTEN expression and the effects of this expression on GPx, catalase, and SOD enzyme activities were analyzed. shows that stably transfected PC9 and PC3 cells start the expression of PTENwt, PTENG129R and PTENG129E. Only PTENwt expression reduced AKT activity. Lipid phosphatase dead PTENG129R and kinase dead PTENG129E could not change the activity of AKT, inferring that PTEN reduced AKT activity by its lipid phosphatase domain. We examined the effects of PTENwt, G129R and G129E expression on GPx, catalase and SOD enzyme activities in these transfected cells. In , it is clearly shown that PTENwt can induce the activity of GPx, catalase and SOD. Lipid phosphatase dead PTENG129R and kinase dead PTENG129E could not change the activity of antioxidant enzymes, inferring that PTEN can induce antioxidant enzyme activity by suppression of PI3K/AKT pathway.
Discussion
In this study, we report that PTEN up-regulates catalase, GPx and SOD activity with inhibition of PI3K/AKT pathway by its lipid dephosphorylase activity. Previously published data show that catalase enzyme activity can down-regulate PI3K/AKT signaling in mesangial cells. Venkatesan et al.Citation25 and Choi et al.Citation21 showed that when NFκB activity was suppressed with gemsitabin, SOD and catalase activity increase in macrophages. With reference to these data, we hypothesized that PTEN could possibly regulate antioxidant enzyme activity by inhibition of PI3K/AKT/NFκB pathway. We first transfected NSCLC cell line PC14 with mock, PTENwt, lipid phosphatase activity dead PTEN, G129E, and catalytically inactive PTEN, G129R. PTEN wt and 2 mutants of PTEN, G129E and G129R, were created and cloned before by our groupCitation22. As shown in , PC14 cells have no detectable endogenous PTEN expression, and after transfection with PTENwt, G129E and G129R, expressions are recreated in these cells. clearly indicates that PTEN suppresses AKT with its lipid phosphatase activity inferring that PTEN inhibits PI3K/AKT pathway in PC14 cells with its lipid phosphatase activity. We then examined the effect of PTENwt, G129E and G129R expression on GPx, catalase and SOD enzymes activity in PC14 cells. Interestingly, only PTENwt expression induced the activity of GPx, catalase and SOD enzymes. G129E and G129R did not have an effect on antioxidant enzyme activities. Obtained data implicates that PTEN up-regulates antioxidant enzyme activity via inhibition of PI3K/AKT pathway by its lipid dephosphatase activity in PC14 cells. These results are in good agreement with previous reportsCitation25,Citation26. Venkatesan et al.Citation25 reported that PI3K/AKT pathway activation down-regulate catalase activity and Choi et al.Citation21 also showed that gemsitabin inhibition of NFκB activity leads to an increase in SOD and catalase activity. Moreover, Leung et al.Citation26 reported that antioxidant enzyme activity was involved in apoptosis in human lung squamous carcinoma CH27 cells. These results are supported by our data. Knowing that PI3K/AKT/NFκB pathway works as a survival pathway in cells and that PTEN works as a tumour suppressor gene, it is so meaningful that PTEN mediated inhibition of PI3K/AKT/NFκB pathway leads to up-regulation of antioxidant enzymes activity.
To understand whether the observed effects were cell specific or not, we transiently transfected PC9 and PC3 cells with mock, PTENwt, PTENG129R and PTENG129E. These NSCLC cell lines, PC9 and PC3, have no endogenous PTEN expressionCitation22,Citation24. In it is clearly shown that PTENwt, G129E and G129R expressions are successfully recreated in PC9 and PC3 cells. Then we examined these expressions effects on GPx, SOD and catalase enzymes activity. As seen in , PTEN up-regulate catalase, SOD and GPx activity with its lipid phosphatase activity by inhibiting PI3K/AKT/NFκB pathway.
Conclusion
We clearly indicate in this study that tumour suppressor PTEN can enhance the activity of SOD, catalase and GPx enzymes activity through the inhibition of PI3K/AKT pathway.
Acknowledgments
This study was supported by a grant from TUBİTAK (no:108S187).
Declaration of interest
The authors declared no conflict of interest.
References
- Svensk AM, Soini Y, Pääkkö P, Hiravikoski P, Kinnula VL. Differential expression of superoxide dismutases in lung cancer. Am J Clin Pathol 2004;122:395–404.
- Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 2003;167:1600–1619.
- Wang SI, Puc J, Li J, Bruce JN, Cairns P, Sidransky D et al. Somatic mutations of PTEN in glioblastoma multiforme. Cancer Res 1997;57:4183–4186.
- Guldberg P, thor Straten P, Birck A, Ahrenkiel V, Kirkin AF, Zeuthen J. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res 1997;57:3660–3663.
- Cairns P, Okami K, Halachmi S, Halachmi N, Esteller M, Herman JG et al. Frequent inactivation of PTEN/MMAC1 in primary prostate cancer. Cancer Res 1997;57:4997–5000.
- Rhei E, Kang L, Bogomolniy F, Federici MG, Borgen PI, Boyd J. Mutation analysis of the putative tumor suppressor gene PTEN/MMAC1 in primary breast carcinomas. Cancer Res 1997;57:3657–3659.
- Kohno T, Takahashi M, Manda R, Yokota J. Inactivation of the PTEN/MMAC1/TEP1 gene in human lung cancers. Genes Chromosomes Cancer 1998;22:152–156.
- Tashiro H, Blazes MS, Wu R, Cho KR, Bose S, Wang SI et al. Mutations in PTEN are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res 1997;57:3935–3940.
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet 1998;19:348–355.
- Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol 1998;8:1169–1178.
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1997;275:1943–1947.
- Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH et al. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet 1997;15:356–362.
- Li DM, Sun H. TEP1, encoded by a candidate tumor suppressor locus, is a novel protein tyrosine phosphatase regulated by transforming growth factor beta. Cancer Res 1997;57:2124–2129.
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 1998;273:13375–13378.
- Wang T, Zhang X, Li JJ. The role of NF-κB in the regulation of cell stress responses. Int Immunopharmacol 2002;2:1509–1520.
- Baichwal VR, Baeuerle PA. Activate NF-κB or die? Curr Biol 1997;7:R94–R96.
- Henkel T, Machleidt T, Alkalay I, Krönke M, Ben-Neriah Y, Baeuerle PA. Rapid proteolysis of IκB-α is necessary for activation of transcription factor NF-κB. Nature 1993;365:182–185.
- Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IκB-NF-κB signaling module: temporal control and selective gene activation. Science 2002;298:1241–1245.
- D’Angio CT, Finkelstein JN. Oxygen regulation of gene expression: a study in opposites. Mol Genet Metab 2000;71:371–380.
- Li N, Karin M. Is NF-κB the sensor of oxidative stress? FASEB J 1999;13:1137–1143.
- Choi C, Cho H, Park J, Cho C, Song Y. Suppressive effects of genistein on oxidative stress and NFκB activation in RAW 264.7 macrophages. Biosci Biotechnol Biochem 2003;67:1916–1922.
- Akca H, Demiray A, Tokgun O, Yokota J. Invasiveness and anchorage independent growth ability augmented by PTEN inactivation through the PI3K/AKT/NFkB pathway in lung cancer cells. Lung Cancer 2011;73:302–309.
- Akinci O, Mihci E, Tacoy S, Kardelen F, Keser I, Aslan M. Neutrophil oxidative metabolism in Down syndrome patients with congenital heart defects. Environ Mol Mutagen 2010;51:57–63.
- Akca H, Tani M, Hishida T, Matsumoto S, Yokota J. Activation of the AKT and STAT3 pathways and prolonged survival by a mutant EGFR in human lung cancer cells. Lung Cancer 2006;54:25–33.
- Venkatesan B, Mahimainathan L, Das F, Ghosh-Choudhury N, Ghosh Choudhury G. Downregulation of catalase by reactive oxygen species via PI 3 kinase/Akt signaling in mesangial cells. J Cell Physiol 2007;211:457–467.
- Leung HW, Kuo CL, Yang WH, Lin CH, Lee HZ. Antioxidant enzymes activity involvement in luteolin-induced human lung squamous carcinoma CH27 cell apoptosis. Eur J Pharmacol 2006;534:12–18.