Abstract
Some new 7-substituted-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione derivatives were synthesized by a sequence of reactions starting from appropriate aryl hydrocarbons. The final compounds were screened for antihypertensive activities by non-invasive method using Tail Cuff method. All the test compounds showed significant antihypertensive activity; 7-(biphenyl-4-yl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine (4p) exhibited antihypertensive activity more than the reference standard drugs.
Introduction
Hypertension is a disease, which if left untreated affects all important organs of human body. It is known as silent killer as without showing significant symptoms, it may quietly lead to stroke, brain haemorrhage, cardiac disorders, renal failure and vision loss. Hypertension has affected 10–15% of global population and has killed a large number of human race in every region of worldCitation1. The interesting pharmacological activity displayed by pyridazine derivatives has been demonstrated in recent years not only by the growing number of papers and patents describing them but also by the development of several pyridazine-based drugs and pharmacological tools. Nowadays, pyridazine derivatives have received special attention in the search for drugs acting on the cardiovascular systemCitation2 as shown in .
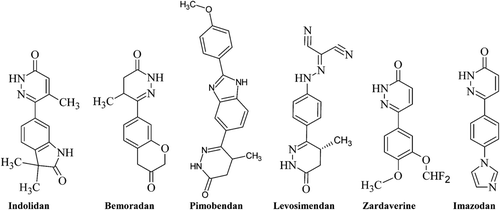
The quite noticeable efforts have been made on obtaining novel antihypertensive agents acting on different mechanisms to control blood pressureCitation3. Pyridazines are important biologically active scaffolds, possessing antihypertensiveCitation4, cardiotonicCitation5, analgesicCitation6, anti-inflammatoryCitation7, antidepressantCitation8, antibacterialCitation9, anticancerCitation10, nephrotopic drugsCitation11, antithrombicCitation12, diureticCitation13 and anti-HIVCitation14 activities. Some pyridazine derivatives, such as bemoradanCitation15, indolidanCitation16, levosimendanCitation17, pimobendanCitation18, zardaverineCitation19 and imazodanCitation20, have already appeared in the clinical market.
Nitrogen containing heterocycles play an important role not only for life science industry but also in many other industrial fields related to special and fine chemistry. Among them s-triazines represent a widely used lead structure with multitude of interesting applications in numerous fieldsCitation21. In the present work, some new 7-substituted-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione derivatives were synthesized and evaluated for antihypertensive activity by non-invasive Tail Cuff method. This work is based on the intensive literature survey on biologically active pyridazine in relation to antihypertensive activities; it has been observed that various pyridazine derivatives possess antihypertensive activity due to their vasorelaxant activity considering the pyridazine nucleus residue as the pharmacophoric group for the activity.
Experimental protocols
Chemistry
Melting points were determined by open tube capillary method and are uncorrected. Purity of the compounds was checked by thin layer chromatography (TLC) method. The FT-IR spectra were recorded on Bio-rad FTS-135 spectrophotometer using KBr pellets; υmax values are given in cm−1. The 1H and 13C NMR spectra were recorded on Bruker Spectrospin DPX 300 MHz using CDCl3 as solvent and tetramethylsilane (TMS) as an internal standard. Chemical shifts are given in δ (ppm) scale and coupling constants (J values) are expressed in Hz. The FAB Mass spectra were obtained on JEOL-JMS-DX 303 system, equipped with direct inlet probe system. Solvent system for TLC was toluene: ethyl acetate: formic acid (5: 4: 1, v/v/v). Elemental analysis was carried out on CHNS Elementar (Vario EL III) using sulphanilic acid as a standard and tungsten (VI) oxide as a combusting agent and analyses for C, H, N were within ± 0.4% of the theoretical values.
General procedure for the synthesis of substituted β-aroyl propionic acids (1a–1g)
The substituted β-aroyl propionic acids (1a–1g) were synthesized from respective aromatic hydrocarbon and characterized on the basis of spectral data as per reported procedureCitation22.
General procedure for the synthesis of 6-substituted-phenyl-4,5-dihydropyridazin-3(2H)-one (2a–2g)
The appropriate substituted β-aroyl propionic acids were reacted with hydrazine hydrate to get corresponding pyridazinone and characterized on the basis of spectral data as per earlier reported procedureCitation23.
General procedure for the synthesis of 2-(hydroxymethyl)-6-(substituted-phenyl)-4,5-dihydropyridazin-3(2H)-one derivatives (3a–3g).
To a solution of 6-(substituted-phenyl)-4,5-dihydropyridazine-3(2H)-one (0.001 mole) derivatives 2 in methanol (30 mL) formaldehyde (37–41%) (2.5 mL) was added and the contents refluxed for 6 h. After completion of the reaction, methanol was distilled off and the residue poured into crushed ice to separate out the 2-(hydroxymethyl)-6-(substituted-phenyl)-4,5-dihydropyridazin-3(2H)-one derivatives 3. The solid, which separated out, was filtered and crystallized from methanol.
General procedure for the synthesis of 7-substituted-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione derivatives (4a–4u)
A mixture of 2-(hydroxymethyl)-6-(substituted-phenyl)-4,5-dihydropyridazin-3(2H)-one derivatives 3 (0.001 mole) and guanidine hydrochloride/urea/thiourea (0.001 mole) was heated in an oil bath at 130°C for 3 h, cooled and triturated with ethanol. The whole content was refluxed on water bath for 8 h. After completion of the reaction, ethanol was distilled off and the residue poured into crushed ice to separate out the title compound (4a–4g). The solid, which separated out, was filtered and crystallized from ethanol.
7-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine (4a). Yield: 30%; m.p. 240°C–242°C; Rf 0.45; IR (KBr) υmax (cm−1): 3218 (NH), 3014 (CH), 1425 (C=N); 1H-NMR (δ) CDCl3: 2.17 (s, 1H, NH), 2.63 (t, 2H, CH2), 2.96 (t, 2H, CH2), 5.90 (s, 2H, CH2), 7.38-7.52 (m, 3H, Ar-H), 7.58–7.73 (m, 2H, Ar-H), 8.50 (s, 1H, = NH); 13C-NMR (δ) CDCl3: 23.6, 26.6, 67.7, 87.8, 128.2, 128.8, 131, 136.4, 146.5, 157.2, 158.9 (C=NH); Mass (m/z): 227/228 (M+/M++1); Anal. Calc. for C12H13N5 (mol. wt. 227.26): C: 63.42, H: 5.77, N: 30.82. Found: C: 63.38, H: 5.75, N: 30.78.
7-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-one (4b). Yield: 82%; m.p. 236°C–238°C; Rf 0.49; IR (KBr) υmax (cm−1): 3256 (NH), 2986 (CH), 1665 (C=O), 1475 (C=N); 1H-NMR (δ) CDCl3: 2.62 (t, 2H, CH2), 2.92 (t, 2H, CH2), 5.93 (s, 2H, CH2), 7.46–7.86 (m, 5H, Ar-H), 8.2 (s, 1H, NHCO); 13C-NMR (δ) CDCl3: 23.2, 25.8, 63.8, 102.2, 128.2, 128.8, 131, 136.4, 146.5, 164.1, 165.7 (C=O); Mass (m/z): 228/229 (M+/M++1); Anal. Calc. for C12H12N4O (mol. wt. 228.24): C: 63.15, H: 5.30, N: 24.55. Found: C: 63.18, H: 5.25, N: 24.68.
7-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-thione (4c). Yield: 72%; m.p. 252°C–254°C; Rf 0.42; IR (KBr) υmax (cm−1): 3278 (NH), 2964 (CH), 1232 (C=S), 1458 (C=N), 770; 1H-NMR (δ) CDCl3: 2.61 (t, 2H, CH2), 2.88 (t, 2H, CH2), 5.94 (s, 2H, CH2), 7.48–7.86 (m, 5H, Ar-H), 8.22 (s, 1H, NHCS); 13C-NMR (δ) CDCl3: 24.2, 26.5, 72.4, 106, 128.1, 128.8, 131.2, 136.4, 146.6, 165.3, 191.7 (C=S); Mass (m/z): 244/245 (M+/M++1); Anal. Calc. for C12H12N4S (mol. wt. 244.31): C: 58.99, H: 4.95, N: 22.93. Found: C: 58.88, H: 5.05, N: 22.88.
7-(4-methylphenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine (4d). Yield: 58%; m.p. 232°C–234°C; Rf 0.58; IR (KBr) υmax (cm−1): 3324 (NH), 2922 (CH), 1517 (C=N); 1H-NMR (δ) CDCl3: 2.19 (s, 1H, NH), 2.38 (s, 3H, CH3), 2.60 (t, 2H, CH2), 2.98 (t, 2H, CH2), 4.90 (m, 2H, CH2), 7.23 (d, 2H, J = 8.1, H-3′, H-5′), 7.26 (d, 2H, J = 8.1, H-2′, H-6′), 8.45 (s, 1H, = NH); 13C-NMR (δ) CDCl3: 21.6, 23.5, 26.1, 65.2, 104.3, 127.8, 130.1, 132.8, 146.4, 158.3, 159.2 (C=NH); Mass (m/z): 241/242 (M+/M++1); Anal. Calc. for C13H15N5 (mol. wt. 241.29): C: 64.71, H: 6.27, N: 29.02. Found: C: 64.68, H: 6.25, N: 28.96.
7-(4-methylphenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-one (4e). Yield: 88%; m.p. 226°C–228°C; Rf 0.60; IR (KBr) υmax (cm−1): 3320 (NH), 2944 (CH), 1672 (C=O), 1524 (C=N); 1H-NMR (δ) CDCl3: 2.18 (s, 1H, NH), 2.37 (s, 3H, CH3), 2.61 (t, 2H, CH2), 2.97 (t, 2H, CH2), 4.93 (m, 2H, CH2), 7.22 (d, 2H, J = 8.1, H-3′, H-5′), 7.26 (d, 2H, J = 8.1, H-2′, H-6′), 8.38 (s, 1H, NHCO); 13C-NMR (δ) CDCl3: 21.3, 23.2, 25.8, 63.8, 102.2, 127, 129.1, 133.4, 140.7, 146.5, 164.1, 165.8 (C=O); Mass (m/z): 242/243 (M+/M++1); Anal. Calc. for C13H14N4O (mol. wt. 242.27): C: 64.45, H: 5.82, N: 23.13. Found: C: 64.48, H: 6.02, N: 22.96.
7-(4-methylphenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-thione (4f). Yield: 77%; m.p. 238°C–240°C; Rf 0.62; IR (KBr) υmax (cm−1): 3326 (NH), 2968 (CH), 1224 (C=S), 1546 (C=N); 1H-NMR (δ) CDCl3: 2.16 (s, 1H, NH), 2.38 (s, 3H, CH3), 2.60 (t, 2H, CH2), 2.98 (t, 2H, CH2), 4.92 (m, 2H, CH2), 7.23 (d, 2H, J = 8.1, H-3′, H-5′), 7.26 (d, 2H, J = 8.1, H-2′, H-6′), 8.45 (s, 1H, NHCS); 13C-NMR (δ) CDCl3: 21.3, 24.2, 26.5, 72.4, 106, 127, 129, 129.1, 133.4, 146.5, 165.3, 191.8 (C=S); Mass (m/z): 258/259 (M+/M++1); Anal. Calc. for C13H14N4S (mol. wt. 258.34): C: 60.44, H: 5.46, N: 21.69. Found: C: 60.40, H: 5.38, N: 21.72.
7-(4-methoxyphenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine (4g). Yield: 45%; m.p. 252°C–254°C, Rf 0.55; IR (KBr) υmax (cm−1): 3350 (NH), 3016 (CH), 1608 (C=N); 1H-NMR (δ) CDCl3: 2.20 (s, 1H, NH), 2.61 (t, 2H, CH2), 2.91 (t, 2H, CH2), 3.78 (s, 3H, CH3O), 4.94 (m, 2H, CH2), 7.32 (dd, 2H, J = 8.8, H-3′, H-5′), 7.74 (dd, 2H, J = 8.8, H-2′, H-6′), 8.45 (s, 1H, = NH); 13C-NMR (δ) CDCl3: 23.5, 26.8, 56.3, 65.7, 101.8, 112.6, 128.8, 144.9, 157.1, 159.8 (C=NH); Mass (m/z): 257/258 (M+/M++1); Anal. Calc. for C13H15N5O (mol. wt. 257.29): C: 60.09, H: 5.88, N: 27.22. Found: C: 60.02, H: 5.78, N: 27.12.
7-(4-methoxyphenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-one (4h). Yield: 88%; m.p. 246°C–248°C, Rf 0.58; IR (KBr) υmax (cm−1): 3342 (NH), 2986 (CH), 1664 (C=O), 1586 (C=N); 1H-NMR (δ) CDCl3: 2.22 (s, 1H, NH), 2.63 (t, 2H, CH2), 2.91 (t, 2H, CH2), 3.81 (s, 3H, CH3O), 4.92 (m, 2H, CH2), 7.32 (dd, 2H, J = 8.8, H-3′, H-5′), 7.74 (dd, 2H, J = 8.8, H-2′, H-6′), 8.58 (s, 1H, NHCO); 13C-NMR (δ) CDCl3: 23.2, 25.8, 55.8, 63.8, 102.2, 114.4, 128.7, 146.5, 164.1, 165.2 (C=O); Mass (m/z): 258/259 (M+/M++1); Anal. Calc. for C13H14N4O2 (mol. wt. 258.34): C: 60.45, H: 5.46, N: 21.69. Found: C: 60.38, H: 5.42, N: 21.58.
7-(4-methoxyphenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-thione (4i). Yield: 86%; m.p. 238°C–240°C, Rf 0.52; IR (KBr) υmax (cm−1): 3336 (NH), 2992(CH), 1228 (C=S), 1578 (C=N); 1H-NMR (δ) CDCl3: 2.19 (s, 1H, NH), 2.62 (t, 2H, CH2), 2.91 (t, 2H, CH2), 3.80 (s, 3H, CH3O), 4.94 (m, 2H, CH2), 7.32 (dd, 2H, J = 8.8, H-3′, H-5′), 7.74 (dd, 2H, J = 8.8, H-2′, H-6′), 8.45 (s, 1H, NHCS); 13C-NMR (δ) CDCl3: 24.2, 26.5, 55.8, 72.4, 106, 114.4, 128.7, 146.5, 162.9, 165.3, 191.6 (C=S); Mass (m/z): 274/275 (M+/M++1); Anal. Calc. for C13H14N4OS (mol. wt. 274.34): C: 56.91, H: 5.14, N: 20.42. Found: C: 56.88, H: 5.08, N: 20.36.
7-(4-ethylphenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine (4j). Yield: 64%; m.p. 226°C–228°C; Rf 0.62 IR (KBr) υmax (cm−1): 3308 (NH), 2965 (CH), 1598 (C=N), 1069, 762; 1H-NMR (δ) CDCl3: 0.93 (t, 3H, CH3), 2.17 (s, 1H, NH), 2.52 (q, 2H, CH2), 2.59 (t, 2H, C-CH2), 2.63 (t, 2H, CH2), 2.92 (t, 2H, CH2), 3.96 (m, 2H, CH2), 7.33 (dd, 2H, J = 8.8, H-3′, H-5′), 7.74 (dd, 2H, J = 8.8, H-2′, H-6′), 8.48 (s, 1H, = NH); 13C-NMR (δ) CDCl3: 14.3, 22.8, 26.2, 28.7, 67.1, 99.2, 126.7, 128.6, 131.5, 146.6, 148.2, 157.6, 158.4 (C=NH); Mass (m/z): 255/256 (M+/M++1); Anal. Calc. for C14H17N5 (mol. wt. 255.31): C: 65.86, H: 6.71, N: 27.43. Found: C: 65.82, H: 6.66, N: 27.42.
7-(4-ethylphenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-one (4k). Yield: 92%; m.p. 234°C–236°C; Rf 0.64 IR (KBr) υmax (cm−1): 3212 (NH), 2974 (CH), 1672 (C=O), 1542 (C=N); 1H-NMR (δ) CDCl3: 0.95 (t, 3H, CH3), 2.16 (s, 1H, NH), 2.52 (q, 2H, CH2), 2.60 (t, 2H, C-CH2), 2.64 (t, 2H, CH2), 2.91 (t, 2H, CH2), 3.93 (m, 2H, CH2), 7.32 (dd, 2H, J = 8.8, H-3′, H-5′), 7.74 (dd, 2H, J = 8.8, H-2′, H-6′), 8.54 (s, 1H, NHCO); 13C-NMR (δ) CDCl3: 14.5, 23.2, 25.8, 28.2, 63.8, 107.2, 127, 127.8, 133.6, 146.5, 148.2, 164.1, 166 (C=O); Mass (m/z): 256/257 (M+/M++1); Anal. Calc. for C14H16N4O (mol. wt. 256.30): C: 65.61, H: 6.29, N: 21.86. Found: C: 65.46, H: 6.18, N: 21.76.
7-[4-(2-methylpropyl)phenyl]-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine (4m). Yield: 60%; m.p. 204°C–206°C; Rf 0.56; IR (KBr) υmax (cm−1): 3412 (NH), 3021 (CH), 1516 (C=N); 1H-NMR (δ) CDCl3: 0.91 (d, 6H, 2xCH3), 1.73 (m, H, C-CH2), 2.18 (s, 1H, NH), 2.53 (t, 2H, CH2), 2.90 (t, 2H, CH2), 4.95 (m, 2H, CH2), 7.33 (dd, 2H, J = 8.2, H-3′, H-5′), 7.66 (dd, 2H, J = 8.8, H-2′, H-6′), 8.51 (s, 1H, = NH); 13C-NMR (δ) CDCl3: 22.6, 23.6, 26.4, 29, 45.1, 66.3, 101.8, 126.1, 128.8, 131, 143.5, 146.8, 156.6, 159.2 (C=NH); Mass (m/z): 283/284 (M+/M++1); Anal. Calc. for C16H21N5 (mol. wt. 283.37): C: 67.82, H: 7.47, N: 24.71. Found: C: 67.80, H: 7.38, N: 24.62.
7-[4-(2-methylpropyl)phenyl]-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-one (4n). Yield: 84%; m.p. 212°C–214°C; Rf 0.58; IR (KBr) υmax (cm−1): 3216 (NH), 3019 (CH), 1674 (C=O), 1509 (C=N); 1H-NMR (δ) CDCl3: 0.90 (d, 6H, 2xCH3), 1.75 (m, H, C-CH2), 2.16 (s, 1H, NH), 2.52 (t, 2H, CH2), 2.92 (t, 2H, CH2), 4.94 (m, 2H, CH2), 7.34 (dd, 2H, J = 8.2, H-3′, H-5′), 7.68 (dd, 2H, J = 8.8, H-2′, H-6′), 8.52 (s, 1H, NHCO); 13C-NMR (δ) CDCl3: 22.8, 23.2, 25.8, 29, 44.5, 63.8, 102.2, 126.2, 128.6, 133.2, 143.7, 146.5, 164.1, 165.2 (C=O); Mass (m/z): 284/285 (M+/M++1); Anal. Calc. for C16H20N4O (mol. wt. 284.35): C: 67.58, H: 7.09, N: 19.70. Found: C: 67.54, H: 7.04, N: 19.68.
7-(biphenyl-4-yl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine (4p). Yield: 50%; m.p. 228°C–230°C; Rf 0.48; IR (KBr) υmax (cm−1): 3204 (NH), 3076 (CH), 1518 (C=N); 1H-NMR (δ) CDCl3: 2.16 (s, 1H, NH), 2.48 (t, 2H, CH2), 2.96 (t, 2H, CH2), 4.94 (m, 2H, CH2), 7.50 (m, 5H, H-3′, H-5′), 7.60-7.80 (dd, 4H, J = 8.8, H-2′, H-6′, H-3′, H-5′), 8.56 (s, 1H, = NH); 13C-NMR (δ) CDCl3: 22.9, 26.3, 63.4, 105.1, 127.9, 128.3, 128.8, 129.5, 133.7, 140.2, 144.6, 145.8, 157.6, 159.3 (C=NH); Mass (m/z): 303/304 (M+/M++1); Anal. Calc. for C18H17N5 (mol. wt. 303.36): C: 71.27, H: 5.65, N: 23.09. Found: C: 71.22, H: 5.61, N: 23.04.
7-(biphenyl-4-yl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-one (4q). Yield: 86%; m.p. 240°C–242°C; Rf 0.50; IR (KBr) υmax (cm−1): 3208 (NH), 3066 (CH), 1685 (C=O), 1488 (C=N); 1H-NMR (δ) CDCl3: 2.15 (s, 1H, NH), 2.46 (t, 2H, CH2), 2.98 (t, 2H, CH2), 4.93 (m, 2H, CH2), 7.53 (m, 5H, H-3′, H-5′), 7.62-7.80 (dd, 4H, J = 8.8, H-2′, H-6′, H-3′, H-5′), 8.58 (s, 1H, NHCO); 13C-NMR (δ) CDCl3: 23.2, 25.8, 63.8, 102.2, 127.6, 127.9, 128, 129.2, 129.7, 135.3, 140.8, 143.1, 146.5, 164.1, 165.4 (C=O); Mass (m/z): 304/305 (M+/M++1); Anal. Calc. for C18H16N4O (mol. wt. 304.34): C: 71.04, H: 5.30, N: 18.41. Found: C: 70.96, H: 5.26, N: 18.36.
7-(4-chlorophenyl)-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine (4s). Yield: 40%; m.p. 188°C–190°C; Rf 0.55; IR (KBr) υmax (cm−1): 3218 (NH), 3014 (CH), 1425 (C=N); 1H-NMR (δ) CDCl3: 2.52 (t, 2H, CH2), 2.91 (brs, 1H, NH), 2.95 (t, 2H, CH2), 3.88 (s, 1H, CH2), 7.32 (d, 2H, J = 8.6, H-3′, H-5′), 7.62 (d, 2H, J = 8.6, H-2′, H-6′), 8.49 (s, 1H, = NH); 13C-NMR (δ) CDCl3: 22.5, 25.6, 63.4, 102.8, 127.6, 128.3, 135.1, 136.7, 147.4, 156.6, 158.9(C=NH); Mass (m/z): 261/263 (M+/M++2); Anal. Calc. for C12H12ClN5 (mol. wt. 261.71): C: 55.07, H: 4.62, N: 26.76. Found: C: 55.02, H: 4.56, N: 26.74.
Pharmacology: Antihypertensive activity
Procurement, identification and housing of animals
Albino rats (body weight 200–250 g) were supplied by Central Animal House facility, Hamdard University and kept under standard laboratory conditions in 12 h light/dark cycle at 25°C ± 2°C. Animals were provided with pellet diet (Lipton, Calcutta, India) and water ad libitum. They were marked for easy identification. All the experimental protocols were carried out with the permission from Institutional Animal Ethics Committee (IAEC), Form number 563 and the guidelines provided by the Committee for the Purpose of Control and Supervision of Experiments in Animal (CPCSEA). Registration number and date of registration is 173/CPCSEA, 28 January 2000.
Conditioning/training of animals
The non-invasive blood pressure system was designed to reduce the animal′s stress and enhance blood flow to the tail. Every rat in each group was given training in the restrainer for a period of 15 min every day at least 10–15 days prior to the day of measurement of the haemodynamic parameters. This exercise was done to avoid the fluctuation in blood pressure due to aggressive behaviour of animal while keeping into the restrainer for measuring the activity. The room temperature was maintained at or above 26°C.
Induction of hypertension in normotensive rats
After recording the initial blood pressure of rats, the animals were divided into groups of five animals each. One group was taken as control. Hypertension was induced in the remaining groups by subcutaneous injection of methyl prednisolone acetate (20 mg/kg/week) for 2 weeksCitation24.
Animal preparation
The animals were placed in the holder 10–15 min prior to obtaining pressure measurements. Acclimated animals provided faster blood pressure measurements then non-acclimated animals. A nervous, stressed animal may have diminished circulation in the tail.
Measurement of mean blood pressure of rats
Mean arterial blood pressure (MABP) was measured in conscious rats using CODA Non-Invasive Blood Pressure Recorder by Tail-Cuff method (Kent Scientific Corporation, USA). The restrainer carrying the rat was placed in the blood pressure instrument with tail protruding out. The tail was gently placed in contact with a transducer membrane, which was connected to the digital blood pressure display panel. The instrument was then turned on and allowed to stabilize until steady pulse rate was observed. Once the ‘pulse level ready′ signal appeared, the blood pressure recording button was pressed and the MABP was recorded. Albino rats (body weight 200–250 g) were used in present study. Rats were assigned to groups of four animals in each. Each compound (20 mg/kg body weight) was injected intraperitoneally after suspending in 1% carboxymethyl cellulose (CMC) solution. The MABP was recorded after 1, 3, 6, 12, 24, 48, 72, 96, 120 h.
Statistical analysis of data
The statistical analysis was performed using GRAPHPAD INSTAT 3 software (Graph Pad Software Inc, San Diego, CA, USA). Data obtained from animal experiments were expressed as arithmetic mean ± SEM. The comparison between various groups was performed by one-way analysis of variance (ANOVA), and the effect in treatment groups were compared with toxic control group by Dunnet multiple comparison test. A p < 0.05 was considered to be significant [*p < 0.05; **p < 0.01]. The percentage reduction in MABP for all the treatment groups was also calculated and compared.
Results and discussion
Chemistry
The synthesis of various 7-substituted-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione derivatives (4a–4u) have been carried out according to the steps shown in the Scheme 1.
Scheme 1. Synthesis protocol for various 7-substituted-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione derivatives.
![Scheme 1. Synthesis protocol for various 7-substituted-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione derivatives.](/cms/asset/029a7c4d-3267-466b-a2b1-49ac189fe7ea/ienz_a_656623_f0002_b.gif)
In the initial step, β-aroyl propionic acids (1a–1g) were synthesized by Friedel–Crafts acylation of appropriate hydrocarbons with succinic anhydride in the presence of anhydrous aluminium chloride. The β-aroyl propionic acid (1) was cyclized on hydrazinolysis to yield 6-(substituted-phenyl)-4,5-dihydropyridazine-3(2H)-one (2a–2g). Pyridazinone (2) was treated with formaldehyde to give the 2-hydroxymethyl derivative, that is, 2-(hydroxymethyl)-6-(substituted-phenyl)-4,5-dihydropyridazin-3(2H)-one (3a–3g) which on cyclocondensation with guanidine hydrochloride/urea/thiourea yielded 7-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione (4a–4u).
The final compounds (4a–4u) were structurally elucidated on the basis of spectral data, explained with the example of compound 4a. In the initial step, benzene was acylated using Friedel–Craft′s reaction to yield β-benzoyl propionic acid 1a. It gave positive effervescence test with sodium bicarbonate solution and showed sharp melting point, 126°C. The IR spectrum showed characteristic band at 3428, 1679 and 1235 for hydroxyl, carbonyl and C-C-O moiety. The 1H-NMR spectrum indicated the two triplets at δ 2.59 and 3.23 for CH2 and CH2CO- moieties, respectively. The aromatic protons were visualized in the form of doublet due to H-2′ and H-6′ protons at δ 7.72. The H-3′ to H-5′ protons appeared as multiplet at δ 7.53 to 7.62. The characteristic peak of singlet at 12.17 showed the presence of COOH group. The β-benzoyl propionic acid 1a was cyclized on hydrazinolysis to yield 6-phenyl-4,5-dihydropyridazine-3(2H)-one 2a. It showed different Rf value and melting point while comparing with intermediate 1a and did not react with sodium bicarbonate solution. The IR spectrum showed characteristic band at 3206 and 1678 cm−1 due to cyclic amide. The band due to C=N appeared at 1619 cm−1. The 1H-NMR spectrum indicated the two triplets at δ 2.45 and 2.93 for C-CH2- and -CH2CO- moiety, respectively. The aromatic protons appeared as multiplets around δ 7.41 due to H-3′ to H-5′ protons and doublet at δ 7.74 for H-2′ and H-6′ protons. The amide proton appeared as singlet δ 10.94. The mass spectrum of compound 2a exhibited molecular ion peak at m/z 174 according to molecular formula C10H10N2O. In third step, the above pyridazinone 2a was treated with formaldehyde to give 2-(hydroxymethyl)-6-phenyl-4,5-dihydropyridazin-3(2H)-one 3a. The IR spectrum showed characteristic band at 3340, 1659 and 1605 for hydroxyl, carbonyl and C=N moiety, respectively. The 1H-NMR spectrum indicated the two triplets at δ 2.63 and δ 3.0 for CH2 and CH2CO- moieties, respectively. The multiplets appeared at δ 3.30 and at δ 5.29 due to presence of −OH group (exchangeable with D2O) and CH2-N-O moiety, respectively. The multiplet at δ 7.42 was due to three protons of aromatic ring attached at 6-position. The other aromatic two protons appeared in downfield at δ 7.76 as multiplet. The mass spectrum of intermediate 3a showed an M++1 peak at m/z 205 according to molecular formula C11H12N2O2. In final step, 2-(hydroxymethyl)-6-phenyl-4,5-dihydropyridazin-3(2H)-one 3a on cyclocondensation with guanidine hydrochloride yielded 7-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine 4a, which showed different Rf value and sharp melting point at 242°C. The IR spectrum showed characteristic band at 3218 cm−1, 3014 cm−1 and 1425 cm−1 for NH, CH stretching and C=N functional group, respectively. The singlet at δ 2.17 was due to NH group. The 1H-NMR showed triplet at δ 2.63 and 2.96 for the cyclic CH2 group at positions 5 and 4, respectively. The singlet at δ 5.90 was due to cyclic CH2 group of triazin-2-imine ring. The aromatic protons appeared as multiplets around δ 7.38 and δ 7.73. The imine proton appeared as singlet δ 8.50. The structure is also supported by elemental analysis data and 13C NMR data. The 13C-NMR showed the peak at δ 157.2 and 158.9 for tertiary carbon (C-6) and imine moiety (C-8). The mass spectrum showed the peak at m/z 228 for M++1 in accordance with molecular formula C12H13N5. The other compounds are also identified in a similar manner.
Pharmacology
The newly synthesized 7-substituted-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione derivatives and standard drugs hydralazineCitation25 and propranolol were screened for antihypertensive activity by non-invasive Tail Cuff method. The pharmacological effects observed contribute to give information about therapeutic interest of pyridazine derivatives in hypertension.
The final compounds (4a–4u) were evaluated for antihypertensive activity by non-invasive method using Tail Cuff method. Compound 4p was found to show highly significant reduction in MABP in comparison to standard drugs (). On the basis of activity reported, it can be concluded that groups like p-C2H5, p-CH2CH(CH3)2, p-C6H5 in phenyl ring at 6-position increases the activity as shown by the compound 4j, 4m and 4p with percentage reduction in MABP 40.88%, 40.98% and 42.04%, respectively.
Table 1. Percent reduction in mean arterial blood pressure (mm Hg) in rats.
All compounds possessing substituted aryl moiety at C-7 of pyridazino[1,6-a]-s-triazin-2-imine/one/thione ring showed significant percentage reduction in comparison to the compounds possessing unsubstituted aryl ring. It indicated that substitution on aromatic ring is essential for antihypertensive activity. The compounds substituted with groups like ethyl, chloro at para position of phenyl ring on pyridazinone moiety did not show appreciable activity. Substitution with isobutyl group at para position of phenyl ring on pyridazinone moiety increases the antihypertensive activity significantly. Substitution by methoxy group at para position of phenyl ring on pyridazinone moiety did not produce significant activity. Maximum activity was also shown by the biphenyl substituent at C-6 position of pyridazine ring.
Conclusion
The 7-substituted-phenyl-3,4,8,9-tetrahydro-2H-pyridazino[1,6-a][1,3,5]triazin-2-imine/one/thione derivatives (4a–4u) can be further modified to exhibit better potency than the standard drugs. Further studies to acquire more information about quantitative structure activity relationships are in progress in the laboratory. The pyridazine derivatives discovered in this study may provide valuable therapeutic intervention for the treatment of hypertension because it is one of the most serious health problems of the modern world with a continuous rise in the number of patients.
Acknowledgements
The authors are also thankful to Jamia Hamdard, New Delhi, India for providing facility for the research work.
Declaration of interest
Ravinesh Mishra expresses sincere thanks to the University Grant Commission (UGC), New Delhi, India, for the award of Research Fellowship in Science for Meritorious Students (RFSMS).
References
- Williams AD, Lemke LT. Foye’s Principles of Medicinal Chemistry, in: David Troy (Ed.), fifth ed. Lippincott Williams & Wilkins, Baltimore, 2002, pp. 824–1195.
- Frank H, Heinisch G. In Pharmacological Active Pyridazines. Part 2. Progress in Medicinal Chemistry; Ellis, GP, Luscombe, DK Eds; Elsevier: Amsterdam, 1992; Vol. 29, p 141–168.
- Bristol JA, Sircar I, Moss WH, Evans DB, Weishmaan E. Cardiotonic agents. 1. 4,5-Dihydro-6-[4-(1H-imidazol-1-yl)phenyl]-3(2H)-pyridazinones: novel positive inotropic agents for the treatment of congestive heart failure. J. Med. Chem. 1984;27:1099–1101.
- Demirayak S, Karaburun AC, Beis R. Some pyrrole substituted aryl pyridazinone and phthalazinone derivatives and their antihypertensive activities. Eur. J. Med. Chem. 2004;39:1089–1095.
- Smolyar NN, Yutilov YM, Gresko SV. Synthesis of 4-amino-6-(hetaryl)pyridazin-3-ones as analogs of pyridazine-based cardiotonic agents. Pharm. Chem. J. 2009;43:87–88.
- Unsal-Tan O, Ozden K, Rauk A, Balkan A. Synthesis and cyclooxygenase inhibitory activities of some N-acylhydrazone derivatives of isoxazolo[4,5-d]pyridazin-4(5H)-ones. Eur. J. Med. Chem. 2010;45(6):2345–2352.
- Mirzoeva S, Sawkar A, Zasadzki M, Guo L, Velentza AV, Dunlap V, Bourguignon JJ. Discovery of a 3-amino-6-phenyl-pyridazine derivative as a new synthetic antineuroinflammatory compound. J. Med. Chem. 2002;45:563–566.
- Sotelo E, Coelho A, Ravina E., Pyridazines. Part 34: Retro-ene-assisted palladium-catalyzed synthesis of 4,5-disubstituted-3(2H)-pyridazinones. Tetrahedron Lett. 2003;44:4459–4462.
- Foks H, Wisterowicz K, Miszke A, Brozewicz K, Wisnlewska K, Szponar MD. Synthesis, fungicidal and antibacterial activity of new pyridazine derivatives. Heterocycles. 2009;78:961–975.
- Kaizerman J, Lucas B, McMinn DL, Zamboni R. Annelated pyridazines for the treatment of tumors driven by inappropriate hedgehog signaling. PCT Int. Appl. 2009, WO 2009035568.
- Ishida A, Honma K, Yato M, Nishiyama S, Okumura F. The syntheses of several heterocyclic skeletons of pyridazine. Eur. Pat. Appl. 1995, EP 661273.
- Monge A, Parrado P, Font M, Alvarez EF. Selective thromboxane synthetase inhibitors and antihypertensive agents. New derivatives of 4-hydrazino-5H-pyridazino[4,5-b]indole, 4-hydrazinotriazino[4,5-a]indole, and related compounds. J. Med. Chem. 1987;30(6):1029–1035.
- Akahane A, Katayama H, Mitsunaga T, Kato T, Kinoshita T, Kita Y, Kusunoki T, Terai T, Yoshida K, Shiokawa Y. Discovery of 6-oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-1(6H)-pyridazinebutanoic acid (FK 838): A novel non-xanthine adenosine A1 receptor antagonist with potent diuretic activity. J. Med. Chem. 1999;42(5):779–783.
- Livermone DGH, Bethell RC, Cammack N, Hancock AP, Hann MM, Green DVS, Lamont RB, Noble SA, Orr DC. Synthesis and anti-HIV-1 activity of a series of imidazo[1,5-b]pyridazines. J. Med. Chem. 1993;36:3784–3794.
- Combs DW, Rampulla MS, Bell SC, Klaubert DH, Tobia AJ, Falotico R, Haertlein B, Weiss CL, Moore JB. 6-Benzoxazinylpyridazin-3-ones: potent, long-acting positive inotrope and peripheral vasodilator agents. J. Med. Chem. 1990;33(1):380–386.
- Robertson DW, Jones ND, Krushinski JH, Pollock GD, Swartzendruber JK, Hayes JS. Molecular structure of the dihydropyridazinone cardiotonic 1,3-dihydro-3,3-dimethyl-5-(1,4,5,6-tetrahydro-6-oxo-3-pyridazinyl)-2H-indol-2-one, a potent inhibitor of cyclic AMP phosphodiesterase. J. Med. Chem. 1987;30(4):623–627.
- Archan S, Toller W. Levosimendan: current status and future prospects. Curr. Opin. Anesthesiol 2008;21(1):78–84.
- Thota S, Bansal R. Synthesis of new pyridazinone derivatives as platelet aggregation inhibitors. Med. Chem. Res. 2010;19:808-816. DOI: 10.1007/s00044-009-9232-6.
- Schade FU, Schudt C. The specific type III and IV phosphodiesterase inhibitor zardaverine suppresses formation of tumor necrosis factor by macrophages. Eur. J. Pharmacol. 1993;230(1):9–14.
- Sotelo E, Fraiz N, Yanez M, Terrades V, Laguna R, Cano E, Ravina E., Pyridazines. Part XXIX: synthesis and platelet aggregation inhibition activity of 5-substituted-6-phenyl-3(2H)-pyridazinones. Novel aspects of their biological actions. Bioorg. Med. Chem. 2002;10:2873–2882.
- Gajare AS, Shingare MS. Synthesis of 2,4-Diarylamino-6-(3′,5′,6′-trichloropyridin-2′-yl)oxy Triazine and Its Herbicidal Activity. Ind. J. Chem. 1998;37B:510–513.
- Khan MSY, Siddiqui AA. Pyridazinone derivatives: A potent anti-inflammatory agents. Ind. J. Chem. 2000;39B:614–620.
- Siddiqui AA, Ahamad SR, Mira MS, Hussain SA, Raish M, Kaur R. Synthesis and in-vitro antifungal activity of 6-substituted-phenyl-2- {[(4′-substituted phenyl-5′-thioxo)-1,2,4-triazol-3-yl]-methyl}-2,3,4,5-tetrahydropyridazin-3-one derivatives. Acta. Pol. Pharm. 2008;65 (2):223–228.
- Krakoff LR, Selvadurai R, Sytter E. Effect of methylprednisolone upon arterial pressure and the renin angiotensin system in the rat. Am. J. Physiol. 1975;228:613–617.
- Borchard RE, Barnes CD, Eltherington LG. Drug Dosage in Laboratory Animals: A Handbook, third ed. The Telford Press Inc., New Jersey, 1991.