Abstract
A series of fourteen 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues were synthesized and evaluated for anticonvulsant activity according to the Antiepileptic Drug Development Programme (ADD) protocol. Some of the synthesized compounds showed significant activity in minimal clonic seizure model (6 Hz psychomotor seizure test). 3-(4-Fluorophenyl)-N-(4-bromophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4c) was found to be the most active compound of the series showing 75% (3/4, 0.25–2.0 h) and 50% (2/4, 4.0 h) protection against minimal clonic seizure at 100 mg/kg without any toxicity. 3-(Pyridin-4-yl)-N-(4-chlorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4f) showed protection in maximal electroshock (MES) seizure and subcutaneous metrazol (scMET) seizure at 300 mg/kg.
Introduction
Epilepsy is a common neurological disorder, affecting 1–2% world’s populationCitation1. In recent years, several new drugs such as oxcarbazepine, lamotrigine, topiramate, gabapentine and vigabatrin have been added as therapeutic agents for the treatment of epilepsy. However, there is a significant group of patients (up to 30%) who are resistant to the available antiepileptic drugs (AEDs). Also most of the AEDs have dose related toxicity and idiosyncratic side effectsCitation2,Citation3. Hence, there is an urgent need to develop new AEDs that lead to substantial benefit to the patient population in the form of increase seizure control, increase tolerability, and better safety and pharmacokinetic properties. CPP 115 and vigabatrin were found to be effective in 6 Hz minimal clonic seizure test in mice at both 32 mA and 44 mA stimuli (3 s).Citation4
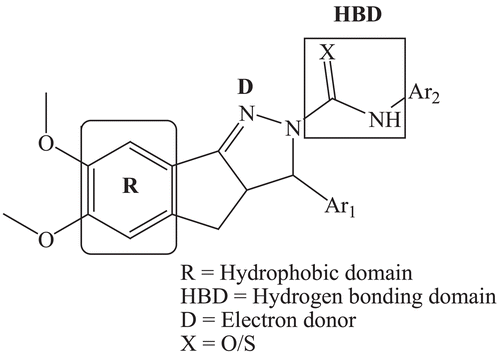
Pyrazoline is an important class of heterocyclic compounds, and were found to have various biological activities including antiamoebic, anticonvulsant, anticancer, antimicrobial, anti-inflammatory, antiviral, antiarrhythmic, antidepressant, antidiabetic, antitubercular and moreCitation5–13. Hence, it is worth to synthesize such compounds. In the present investigation, we have focussed on the anticonvulsant screening of 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues. Earlier, we have reported the antitubercular activity of pyrazoline analoguesCitation14–16. The proposed pharmacophore model contains three binding site for interaction with a macromolecular complex in vivo ().
Results and discussion
Chemistry
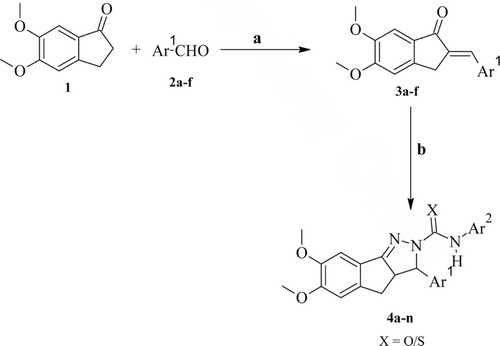
The 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues (4a–n) described in this study are shown in and the reaction sequence for the synthesis is summarized in . In the initial step, 5,6-dimethoxy-2,3-dihydro-1H-inden-1-one (0.1 mol) and appropriate aromatic aldehydes (0.1 mol) in diluted methanolic sodium hydroxide solution were stirred at room temperature giving the 2-substituted-5,6-dimethoxy-2,3-dihydro-1H-indene-1-one derivatives (3a–f). In the subsequent step, 2-substituted-5,6-dimethoxy-2,3-dihydro-1H-indene-1-one derivatives were treated with appropriate semicarbazides furnishing the titled compounds (4a–n). The substituted phenyl semicarbazides were synthesized as per the reported methodCitation17. The yields of the titled compounds were ranged from 66% to 84% after recrystallization with absolute ethanol. The reactions were monitored by thin layer chromatography (TLC) using benzene-acetone (9:1) and the purity of the compounds was checked by elemental analyses and mass spectroscopy. Both the analytical and spectral data (IR, 1H NMR and MS) of all the synthesized compounds were in full accordance with the proposed structures. In general, the IR spectra of the compounds afforded pyrazoline C=N stretching at 1560–1573 cm−1, C-H deformation at 1362–1464 cm−1, C2-N1 stretching at 1135–1180 cm−1, and carbamoyl group N-H stretching at 3330–3341 cm−1 and C=O stretching at 1645–1681 cm−1 bands. In the Nuclear Magnetic Resonance spectra (1H NMR), the signals of the respective protons of the synthesized titled compounds were verified on the basis of their chemical shift, multiplicities and coupling constants in Dimethyl sulfoxide (DMSO) -d6. The spectra showed multiplet at δ 3.03–3.35 ppm corresponding to CH; a doublet at δ 3.33–3.45 ppm corresponding to CH2 group; a singlet at δ 3.79–3.90 ppm corresponding to OCH3 group; a doublet at 4.1–5.2 ppm corresponding to CH; a broad singlet at δ 4.42–4.48 corresponding to CONH2, a broad singlet at δ 4.92–4.96 corresponding to CSNH2, multiplet at δ 6.98–8.39 ppm corresponding to aromatic protons; broad singlet at δ 8.89–10.05 ppm corresponding to CONH (D2O exchangeable). The elemental analysis results were within ±0.4% of the theoretical values.
Table 1. Physical constant of 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide.
Anticonvulsant screening
All the compounds were evaluated for their anticonvulsant activity according to the Antiepileptic Drug Development Programme (ADD) protocol reported elsewhereCitation18–24. Initially, the compounds were administered i.p. at doses of 30, 100 and 300 mg/kg in mice and activity was established using the maximal electroshock (MES) induced seizure and subcutaneous metrazole (scMET) induced seizure to identify their anticonvulsant activity at two intervals (0.5 and 4.0 h). Neurotoxicity or toxicity was observed by minimal motor impairment which was measured in mice by rotorod test. Ten compounds were screened in 6 Hz psychomotor seizure test to identify their anticonvulsant activity at five different time points viz. 0.25 h, 0.5 h, 1.0 h, 2.0 h and 4.0 h after i.p. administration in mice. The results are shown in . The MES, scMET and neurotoxicity screening results of four compounds are given in . Two of the compounds (4f and 4j) were also evaluated in the MES test and toxicity after oral administration to rat at 30 mg/kg dose. The results are shown in . Three compounds (4a, 4g and 4h) were also screened in in vitro hippocampal slice culture neuroprotection assay (NP). Only four of the title compounds (4f, 4g, 4h and 4i) showed protection in MES and scMET screens but were associated with toxicity while some of the compounds showed promising activity in 6 Hz psychomotor seizure test without any toxicity at five different time points, i.e. 0.25 h, 0.5 h, 1.0 h, 2.0 h and 4.0 h. Like the MES test, the minimal clonic seizure (6 Hz) test is used to assess a compound’s efficacy against electrically induced seizures but at a lower frequency (6 Hz) and longer duration of stimulation (3 s) to identify potential AEDs, which are ineffective in MES and scMET screen but still have anticonvulsant activities in vivo. Among the title compounds 4k, 4l, 4e, 4f, 4g and 4c were found to have moderate to good activity in 6 Hz psychomotor seizure test. The compound, 4c was found to be the most promising compound of the series showing 75% (3/4, 0.25–2.0 h) and 50% (2/4, 4.0 h) protection without any toxicity. The compound 4g showed 75% (3/4, 0.25–0.5 h), 100% (4/4, 1.0 h) and 50% (2/4, 2.0 h) protection. The compound 4f showed 100% (4/4, 0.25 h), 75% (3/4, 1.0 h) and 50% (2/4, 0.5 h) protection. The compound 4e showed 100% (4/4, 0.25 h), 75% (3/4, 0.5 h) and 50% (2/4, 1.0 h) protection. The compounds 4k and 4l were nearly equipotent. The compound 4h showed 25% (1/4 0.25–0.5 h) and 50% (2/4, 1.0 h) protection. The compounds, 4a, 4d and 4i showed absence of activity in 50% mice population. The compound, 4j showed 50% (2/4, 0.5–2.0 h) and 25% (0.25 and 4.0 h) protection while compound 4f showed 50% (2/4, 1.0 h) and 25% (1/4, 2.0 h) in MES screen after oral administration in rat at dose 30 mg/kg without any toxicity. The in vitro hippocampal slice culture neuroprotection assay (Test 76) of compounds 4a, 4g and 4h showed no neuroprotection against either kainic acid (KA) or N-methyl-D-aspartate (NMDA) induced cytotoxicity. We have observed that, the compound with 3-aryl substitution with 4-fluorophenyl, 3,4-dimethoxyphenyl, 4-pyridinyl, 4-methoxyphenyl and phenyl group showed good to moderate activity.
Table 2. 6 Hz psychomotor seizure test and neurotoxicity of 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues.
Table 3. Anticonvulsant activity of 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide analogues.
Table 4. Evaluation of compound, 4f and 4j in the MES test and toxicity after oral administration to rat.
Experimental
All chemicals were supplied by E. Merck (Darmstadt, Germany) and S. D. Fine Chemicals (Mumbai, India). Melting points were determined by open tube capillary method and are uncorrected. The completion of the reactions was monitored by TLC plates (silica gel G) using eluants benzene-acetone (9:1), the spots were identified by iodine vapours or UV light. IR spectra were obtained on a Schimadzu 8201 PC, FT-IR spectrometer (KBr pellets). 1H NMR spectra were recorded on a Bruker AC 300 MHz spectrometer using tetramethylsilane (TMS) as internal standard in DMSO. Mass spectra were recorded on a Bruker Esquire LCMS using ESI and elemental analyses were performed on Perkin-Elmer 2400 Elemental Analyzer.
General procedure
Synthesis of 2-substituted-5,6-dimethoxy-2,3-dihydro-1H-indene-1-one (3a–f)
5,6-dimethoxy-2,3-dihydro-1H-inden-1-one (0.001 mol) with appropriate aldehyde (0.001 mol) in diluted methanolic sodium hydroxide solution was stirred under room temperature for 4 h. The resulting solution was allowed to stand overnight and then the reaction mixture was poured into cold water and neutralized with dilute HCl. The solid was filtered, dried and recrystallized with ethanol furnished the 2-substituted-5,6-dimethoxy-2,3-dihydro-1H-indene-1-one (3a–f).
Synthesis of 3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide analogues (4a–n)
2-substituted-5,6-dimethoxy-2,3-dihydro-1H-indene-1-one (3a–g) (0.01 mol) and substituted phenyl semicarbazide (0.01 mol) were refluxed in 20 mL glacial acetic acid for 12 h. The excess of solvent was removed under reduced pressure and then the reaction mixture was poured into the crushed ice. The solid mass was filtered dried and recrystallized with ethanol furnished the 3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide analogues.
3-(Pyridin-4-yl)-N-(4-fluorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4a) IR: (KBr) cm−1: 3331 (NH), 1680 (C=O), 1563 (C=N), 1142 (C-N), 788 (C-F). 1H NMR (300MHz, DMSO-d6): δ 3.29–3.33 (1H, m, CH), 3.42–3.45 (2H, d, J = 9.0 Hz, CH2), 3.80 (3H, s, OCH3), 3.82 (3H, s, OCH3), 5.2 (1H, d, J = 6.1 Hz, CH), 6.96–8.23 (10H, m, Ar), 8.91 (1H, s, CONH); m/z = 432 (M+), 433 (M+1)+.
3-(2-Chlorophenyl)-N-(4-bromophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4b) IR: (KBr) cm−1: 3335 (NH), 1685 (C=O), 1567 (C=N), 1151 (C-N), 767 (C-Cl). 1H NMR (300MHz, DMSO-d6): δ 3.03–3.05 (1H, m, CH), 3.33–3.35 (2H, d, J = 6.0 Hz, CH2), 3.84 (3H, s, OCH3), 3.90 (3H, s, OCH3), 4.04 (1H, d, J = 6.4 Hz, CH), 7.23–7.81 (10H, m, Ar), 10.06 (1H, s, CONH); m/z = 526 (M+), 527 (M+1)+.
3-(4-Fluorophenyl)-N-(4-bromophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4c) IR: (KBr) cm−1: 3341 (NH), 1645 (C=O), 1573 (C=N), 1381 (CH), 1180 (C-N), 786 (C-F). 1H NMR (300MHz, DMSO-d6): δ 3.23–3.31 (1H, m, CH), 3.39–3.43 (2H, d, J = 12.0 Hz, CH2), 3.81 (6H, s, OCH3), 5.2 (1H, d, J = 6.1 Hz, CH), 7.02–8.39 (10H, m, Ar), 8.96 (1H, s, CONH); m/z = 510 (M+), 511 (M+1)+.
3-(3,4-Dimethoxyphenyl)-N-(4-bromophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4d) IR: (KBr) cm−1: 3339 (NH), 1678 (C=O), 1556 (C=N), 1141 (C-N). 1H NMR (300MHz, DMSO-d6): δ 3.04–3.07 (1H, m, CH), 3.35–3.36 (2H, d, J = 3.0 Hz, CH2), 3.83 (6H, s, OCH3), 3.91 (6H, s, OCH3), 4.02 (1H, d, J = 6.4 Hz, CH), 7.06–7.56 (9H, m, Ar), 10.06 (1H, s, CONH); m/z = 552 (M+), 553 (M+1)+.
3-(4-Methoxyphenyl)-N-(4-bromophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4e) IR: (KBr) cm−1: 3334 (NH), 1681 (C=O), 1561 (C=N), 1151 (C-N). 1H NMR (300MHz, DMSO-d6): δ 3.03–3.05 (1H, m, CH), 3.33–3.34 (2H, d, J = 6.2 Hz, CH2), 3.83 (6H, s, OCH3), 3.90 (3H, s, OCH3), 3.96 (1H, d, J = 6.4 Hz, CH), 5.2 (1H, d, J = 6.1 Hz, CH), 7.05–7.73 (10H, m, Ar), 10.05 (1H, s, CONH); m/z = 522 (M+), 523 (M+1)+.
3-(Pyridin-4-yl)-N-(4-chlorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4f) IR: (KBr) cm−1: 3334 (NH), 1680 (C=O), 1563 (C=N), 1144 (C-N), 767 (C-Cl). 1H NMR (300MHz, DMSO-d6): δ 3.29–3.35 (1H, m, CH), 3.41–3.43 (2H, d, J = 6.0 Hz, CH2), 3.81 (3H, s, OCH3), 3.83 (3H, s, OCH3), 5.1 (1H, d, J = 6.4 Hz, CH), 6.98–8.31 (10H, m, Ar), 8.89 (1H, s, CONH); m/z = 448 (M+), 449 (M+1)+.
3-(3,4-Dimethoxyphenyl)-N-(4-chlorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4g) IR: (KBr) cm−1: 3336 (NH), 1685 (C=O), 1565 (C=N), 1174 (C-N). 1H NMR (300 MHz, DMSO-d6): δ 3.29–3.33 (1H, t, CH), 3.40–3.44 (2H, d, J = 6.7 Hz, CH2), 3.80 (6H, s, OCH3), 3.83 (6H, s, OCH3), 5.2 (1H, d, J = 6.4 Hz, CH), 6.78–8.19 (9H, m, Ar), 9.39 (1H, s, CONH); m/z = 437 (M+), 438 (M+1)+.
3-Phenyl-N-(4-chlorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4h) IR: (KBr) cm−1: 3331 (NH), 1681 (C=O), 1565 (C=N), 1144 (C-N), 766 (C-Cl). 1H NMR (300MHz, DMSO-d6): δ 3.27–3.31 (1H, m, CH), 3.41–3.43 (2H, d, J = 6.0 Hz, CH2), 3.81 (3H, s, OCH3), 3.83 (3H, s, OCH3), 5.1 (1H, d, J = 6.3 Hz, CH), 6.98–8.33 (11H, m, Ar), 9.49 (1H, s, CONH); m/z = m/z = 447 (M+), 448 (M+1)+.
3-(Pyridin-4-yl)-N-(3-chloro-4-fluorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4i) IR: (KBr) cm−1: 3331 (NH), 1678 (C=O), 1560 (C=N), 1144 (C-N), 789 (C-F), 789 (C-F), 766 (C-Cl). 1H NMR (300MHz, DMSO-d6): δ 3.18–3.21 (1H, m, CH), 3.33–3.35 (2H, d, J = 6.0 Hz, CH2), 3.81 (3H, s, OCH3), 3.83 (3H, s, OCH3), 4.02 (1H, d, J = 6.7 Hz, CH), 7.22–7.99 (9H, m, Ar), 10.03 (1H, s, CONH); m/z = 467 (M+), 468 (M+1)+.
3-(2-Chlorophenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4j) IR: (KBr) cm−1: 3337 (NH), 1685 (C=O), 1561 (C=N), 1151 (C-N). 1H NMR (300MHz, DMSO-d6): δ 3.22–3.24 (1H, m, CH), 3.33–3.35 (2H, d, J = 6.0 Hz, CH2), 3.80 (3H, s, OCH3), 3.82 (3H, s, OCH3), 4.9 (1H, d, J = 6.3 Hz, CH), 5.46 (2H, s, CONH2), 7.08–7.68 (6H, m, Ar); m/z = 372 (M+), 373 (M+1)+.
3-(3,4-Dimethoxyphenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4k) IR: (KBr) cm−1: 3330 (NH), 1678 (C=O), 1565 (C=N), 1135 (C-N). 1H NMR (300MHz, DMSO-d6): δ 3.22–3.25 (1H, m, CH), 3.33–3.35 (2H, d, J = 6.0 Hz, CH2), 3.79 (6H, s, OCH3), 3.83 (6H, s, OCH3), 5.2 (1H, d, J = 6.3 Hz, CH), 5.44 (2H, s, CONH2), 7.09–7.62 (5H, m, Ar); m/z = 497 (M+).
3-Phenyl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide (4l) IR: (KBr) cm−1: 3333 (NH), 1675 (C=O), 1564 (C=N), 1135 (C-N). 1H NMR (300MHz, DMSO-d6): δ 3.22–3.24 (1H, m, CH), 3.34–3.36 (2H, d, J = 6.0 Hz, CH2), 3.80 (3H, s, OCH3), 3.82 (3H, s, OCH3), 5.1 (1H, d, J = 6.2 Hz, CH), 5.48 (2H, s, CONH2), 7.08–7.72 (7H, m, Ar); m/z = 337 (M+).
3-(3,4-Dimethoxyphenyl)-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carbothioamide (4m) IR: (KBr) cm−1: 3334 (NH), 1563 (C=N), 1271 (C=S), 1135 (C-N). 1H NMR (300MHz, DMSO-d6): δ 3.23–3.24 (1H, m, CH), 3.36–3.38 (2H, d, J = 6.0 Hz, CH2), 3.81 (6H, s, OCH3), 3.83 (6H, s, OCH3), 5.2 (1H, d, J = 6.2 Hz, CH), 5.89 (2H, s, CSNH2), 7.12–7.76 (5H, m, Ar); m/z = 413 (M+).
3-Phenyl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carbothioamide (4n) IR: (KBr) cm−1: 3336 (NH), 1562 (C=N), 1270 (C=S), 1135 (C-N). 1H NMR (300MHz, DMSO-d6): δ 3.21–3.23 (1H, m, CH), 3.34–3.36 (2H, d, J = 6.0 Hz, CH2), 3.79 (3H, s, OCH3), 3.81 (3H, s, OCH3), 5.1 (1H, d, J = 6.1 Hz, CH), 5.90 (2H, s, CONH2), 7.18–7.82 (7H, m, Ar); m/z = 453 (M+).
Biology
MES seizure
The anticonvulsant evaluations were undertaken by the National Institute of Health, using their reported procedures. For all tests based on MES convulsions, 60Hz of alternating current (50 mA in mice and 150 mA in rat) was delivered for 0.2 s by corneal electrodes which have been primed with an electrolyte solution containing anaesthetic agent (0.5% tetracaine HCl). The mice were tested at various (0.5 and 4 h) intervals following doses of 30, 100 and 300 mg/kg of test compound given by i.p. injection of a volume of 0.01 mL/g. An animal is considered “protected” from MES-induced seizures upon abolition of the hind limb tonic extensor component of the seizure.
Subcutaneous metrazole-induced seizure (scMET)
Subcutaneous injection of the convulsant metrazol produces clonic seizures in laboratory animals. The scMET test detects the ability of a test compound to raise the seizure threshold of an animal and thus protect it from exhibiting a clonic seizure. Animals were pretreated with various doses of the test compound (in a similar manner to the MES test, although a dose of 50 mg/kg (p.o.) is the standard for scMET). An animal is considered “protected” from scMET induced seizures upon absence of episode of clonic spasms, approximately 3–5 s, of the fore and/or hindlimbs, jaws or vibrissae.
6 Hz psychomotor seizure test
Some clinically useful AEDs are ineffective in the standard MES and scMET tests but still have anticonvulsant activities in vivo. In order to identify potential AEDs with this profile, compounds were tested in the 6 Hz or “psychomotor” test (minimal clonic seizure). The title compounds were tested in the minimal clonic seizure test at dose of 100 mg/kg to four mice. Like the MES test, the minimal clonic seizure (6 Hz) test is used to assess a compound’s efficacy against electrically induced seizures but uses a lower frequency (6 Hz) and longer duration of stimulation (3 s).
Neurotoxicity or minimal motor impairment
Minimal motor impairment was measured in mice by the rotorod test. The mice were trained to stay on an accelerating rotorod that rotate at 10 revolutions per minute. The rod diameter was 3.2 cm. trained animals were given i.p. injection of the test compound in doses of 30, 100 and 300 mg/kg, neurotoxicity was indicated by the inability of the mice to maintain equilibrium on the rod for at least 1 min in each of the three trails.
In vitro hippocampal slice culture NP: primary screen experiment
The “primary screen experiment” is a qualitative assessment of the ability of a compound to prevent excitotoxic cell death. Organotypic hippocampal slice cultures are treated with NMDA or KA to induce neuronal cell death. Propidium iodide, a membrane-impermeant compound, is included in all wells of the culture plate. Dying cells have compromised cell membranes, thus propidium iodide may diffuse into the cell, intercalate with DNA and fluoresce. Thus, the intensity of the propidium iodide fluorescence is proportional to the amount of cell death in the individual slices. Hippocampal slice cultures are treated with the excitotoxin alone or where indicated above, with the excitotoxin and either one or two investigational compounds at the concentrations indicated. If neuroprotection occurs as a consequence of the added compound, slice cultures will have a visibly reduced fluorescent intensity when compared to the slice cultures that have been treated with the excitotoxin alone.
Declaration of interest
The authors declare no conflict of interest.
Acknowledgments
Authors are thankful to the management people of Alwar Pharmacy College, Alwar, Rajasthan, India for providing research facilities. The authors also wish to express their thanks to all the staffs of Anticonvulsant Drug Development Programme, NINDS, National Institute of Health, USA for anticonvulsant activity.
References
- White HS. Preclinical development of antiepileptic drugs: past, present, and future directions. Epilepsia 2003;44 Suppl 7:2–8.
- Dimmock JR, Pandeya SN, Quail JW, Pugazhenthi U, Allen TM, Kao GY, Balzarini J, DeClereq E. Nicotinic acid hydrazone: a novel anticonvulsant pharmacophore. Eur J Med Chem 1995;30:303–314.
- Duncan JS. The promise of new antiepileptic drugs. Br J Clin Pharmacol 2002;53:123–131.
- Antiepileptic Drug and Device Trials XI Conference. (2011). CPP-115 for refractory complex partial seizures and infantile spasm. Aventura, FL, April 27–29.
- Budakoti A, Bhat AR, Athar F, Azam A. Syntheses and evaluation of 3-(3-bromo phenyl)-5-phenyl-1-(thiazolo [4,5-b] quinoxaline-2-yl)-2-pyrazoline derivatives. Eur J Med Chem 2008;43:1749–1757.
- Parmar SS, Pandey BR, Dwivedi C, Harbison RD. Anticonvulsant activity and monoamine oxidase inhibitory properties of 1,3,5-trisubstituted pyrazolines. J Pharm Sci 1974;63:1152–1155.
- Dawane BS, Konda SG, Mandawad GG, Shaikh BM. Poly(ethylene glycol) (PEG-400) as an alternative reaction solvent for the synthesis of some new 1-(4-(4′-chlorophenyl)-2-thiazolyl)-3-aryl-5-(2-butyl-4-chloro-1H-imidazol-5yl)-2-pyrazolines and their in vitro antimicrobial evaluation. Eur J Med Chem 2010;45:387–392.
- Sharma PK, Kumar S, Kumar P, Kaushik P, Kaushik D, Dhingra Y et al. Synthesis and biological evaluation of some pyrazolylpyrazolines as anti-inflammatory-antimicrobial agents. Eur J Med Chem 2010;45:2650–2655.
- Bilgin AA, Palaska E, Sunal R. Studies on the synthesis and antidepressant activity of some 1-thiocarbamoyl-3,5-diphenyl-2-pyrazolines. Arzneimittelforschung 1993;43:1041–1044.
- Turan-Zitouni G, Chevallet P, Kiliç FS, Erol K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur J Med Chem 2000;35:635–641.
- Mui MS, Siew BN, Buss AD, Crasta SC, Kah LG, Sue KL. Synthesis of N-1 acidic functionality affording analogues with enhanced antiviral activity against HIV. Bioorg Med Chem Lett 2002;12:679–699.
- Manna F, Chimenti F, Fioravanti R, Bolasco A, Secci D, Chimenti P et al. Synthesis of some pyrazole derivatives and preliminary investigation of their affinity binding to P-glycoprotein. Bioorg Med Chem Lett 2005;15:4632–4635.
- Ali MA, Shaharyar M, Siddiqui AA. Synthesis, structural activity relationship and anti-tubercular activity of novel pyrazoline derivatives. Eur J Med Chem 2007;42:268–275.
- Ahsan MJ, Samy JG, Dutt KR, Agrawal UK, Yadav BS, Vyas S et al. Design, synthesis and antimycobacterial evaluation of novel 3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide analogues. Bioorg Med Chem Lett 2011;21:4451–4453.
- Ahsan MJ, Samy JG, Soni S, Jain N, Kumar L, Sharma LK et al. Discovery of novel antitubercular 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues. Bioorg Med Chem Lett 2011;21:5259–5261.
- Ahsan MJ, Samy JG, Khalilullah H, Bakht MA, Hassan MZ. Synthesis and antimycobacterial evaluation of 3a,4-dihydro-3H-indeno [1,2-c] pyrazole-2-carboxamide analogues. Eur J Med Chem 2011;46:5694–5697.
- Amir M, Ahsan MJ, Ali I. Synthesis of N1-(3-chloro-4-fluorophenyl)-N4-substituted semicarbazones as novel anticonvulsant agents. Ind J Chem 2010;49B:1509–1514.
- Swinyard EA, Woodhead JH, White HS, Franklin MR. (1989). General principles: experimental selection, quantification, and evaluation of anticonvulsants. In: Levy RH,Mattson RH, Melmm B, Penry JK, Dreifuss FE, ed. Antiepileptic Drugs. New York: Raven Press, 85–102.
- White HS, Johnson M, Wolf HH, Kupferberg HJ. The early identification of anticonvulsant activity: role of the maximal electroshock and subcutaneous pentylenetetrazol seizure models. Ital J Neurol Sci 1995;16:73–77.
- White HS, Woodhead JH, Franklin MR. (1995). General principles: experimental selection, quantification, and evaluation of antiepileptic drugs. In: Levy RH,Mattson RH, Meldrum B, ed. Antiepileptic Drugs. New York: Raven Press, 99–110.
- DUNHAM NW, MIYA TS. A note on a simple apparatus for detecting neurological deficit in rats and mice. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1957;46:208–209.
- Barton ME, Klein BD, Wolf HH, White HS. Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res 2001;47:217–227.
- TOMAN JE, EVERETT GM, RICHARDS RK. The search for new drugs against epilepsy. Tex Rep Biol Med 1952;10:96–104.
- Noraberg J, Poulsen FR, Blaabjerg M, Kristensen BW, Bonde C, Montero M et al. Organotypic hippocampal slice cultures for studies of brain damage, neuroprotection and neurorepair. Curr Drug Targets CNS Neurol Disord 2005;4:435–452.
- Pandeya SN, Raja AS, Stables JP. Synthesis of isatin semicarbazones as novel anticonvulsants–role of hydrogen bonding. J Pharm Pharm Sci 2002;5:266–271.