Abstract
A series of novel 1,4-substituted semicarbazides 5a–g with a primaquine moiety bridged by a carbonyl group at position 1 and a cycloalkyl, aryl, benzyloxy or hydroxy substituent at position 4 were prepared and biologically evaluated. The synthetic pathways applied for preparation of the title compounds involved benzotriazole as synthetic auxiliary. Primaquine semicarbazides 5a–g and their synthetic precursors benzotriazolecarbonyl semicarbazides 4 were evaluated for cytostatic, antiviral and antioxidative activities. All compounds of the series 5 showed high selectivity towards MCF-7 cells (breast carcinoma) with IC50 values in the low micromolar range and the most active was benzyl derivative 5c (IC50 1 ± 0.2 µM). The benzhydryl derivative 5e showed significant cytostatic activities towards all the tested cell lines (IC50 4–18 µM). The same compound was the strongest lipoxygenase inhibitor as well (51%). The highest antioxidant activity was demonstrated for the hydroxy derivative 5g and benzotriazolecarbonyl semicarbazides 4b,c (61.2–68.5%). No antiviral activity was observed against a wide variety of DNA and RNA viruses.
| Abbreviations: | ||
| AAPH, | = | 2,2′-azobis(2-amidinopropane) hydrochloride; |
| Btc, | = | 1-benzotriazolecarbonyl; |
| BtH, | = | benzotriazole; |
| DPPH, | = | 1,l-diphenyl-2-picrylhydrazyl; |
| LOX, | = | lipoxygenase; |
| LP, | = | lipid peroxidation; |
| NDGA, | = | nordihydroguaiaretic acid; |
| PQ, | = | primaquine; |
| TEA, | = | triethylamine |
Introduction
Hydroxyurea, hydroxysemicarbazide, semicarbazide, semicarbazone and thiosemicarbazone functionalities have been identified as essential pharmacophores for antitumoural, antiviral, antibacterial, antimalarial, anticonvulsant, herbicidal and fungicidal activitiesCitation1–6. Several reports show potent activity of Schiff bases of hydroxysemicarbazides against L1210 murine leukaemia cellsCitation7,Citation8. The 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) is a metal chelator that potently inhibits ribonucleotide reductaseCitation9,Citation10. Semicarbazide-based derivatives of peptides (azapeptides) are known as inhibitors of human dipeptidyl peptidase I, the enzyme that plays an important role in the immune systemCitation11, while goserelin, an azapeptide analogue of the luteinising-hormone releasing hormone, is used for the treatment of prostate cancer. Hydroxyurea is a useful drug in therapy of leukaemia and other malignant tumour diseases and its derivative zileuton is an orally active inhibitor of 5-lipoxygenase which is used for the maintenance treatment of asthmaCitation12. Hydroxyurea is an inhibitor of ribonucleotide reductase, the enzyme described as a target for the chemotherapy of both malignant diseases and malariaCitation13,Citation14. Mahajan et al.described 7-chloroquinolyl thioureas as potential antimalarial and anticancer agentsCitation15. Several research groups found dual antimalarial and anticancer activity of both quinoline and trioxane derivatives as well16–18. Based on these findings, we have synthesized a series of compounds containing the antimalarial drug primaquine (PQ) substituted by an N-4-cycloalkyl, aryl, benzyloxy or hydroxy semicarbazide functionality. The newly designed compounds are closely related to biurets, hydroxyureas, hydroxamic acids or azapeptides. Here their synthesis, full chemical characterization, cytostatic, antiviral and antioxidative evaluations are reported. This paper is a continuation of our previous papers dealing with PQ ureas and NSAID semicarbazide derivatives as potential antitumor agentsCitation19–22.
Material and methods
Chemistry
General experimental details
Melting points were determined on a Stuart Melting Point Apparatus SMP3 and were uncorrected. IR spectra were recorded on a FTIR Perkin Elmer Paragon 500 spectrometer. 1H and 13C NMR spectra were recorded on a Varian Gemini 300 spectrometer, operating at 300 and 75.5 MHz for the 1H and 13C nuclei, respectively. Samples were measured in DMSO-d6 solutions at 20°C in 5 mm NMR tubes. Chemical shifts (δ) in ppm were referred to TMS. Elemental analyses were performed on CHNS LECO analyzer and mass spectra were taken on a HPLC-MS/MS (HPLC, Agilent Technologies 1200 Series; MS, Agilent Technologies 6410 Triple Quad). Solvent systems cyclohexane/ethyl acetate (EtOAc)/methanol (3:1:0.5), cyclohexane/EtOAc (1:1), petrol ether/EtOAc/methanol (3:1:0.75), dichloromethane/methanol (9.5:0.5) and (8.5:1.5) and pre-coated Merck silica gel 60 F254 plates were used for thin-layer chromatography (TLC). Spots were visualized by short-wave UV light and iodine vapour. Column chromatography was performed on silica gel (0.063−0.200 mm), with the same eluents used in TLC. Benzotriazole (BtH), triphosgene, cyclopentylamine, cyclohexylamine, benzylamine, 2-phenylethanamine, benzhydrylamine, O-benzylhydroxylamine hydrochloride, triethylamine (TEA), hydrazine hydrate and 10% Pd/C were purchased from Aldrich. The PQ base was prepared from primaquine diphosphate (Aldrich) prior to use. The PQ solution was protected from light during the whole procedure. The 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azobis(2-amidinopropane) hydrochloride (AAPH), Trolox and nordihydroguaiaretic acid (NDGA) were purchased from Aldrich Chemical Co. Soybean lipoxygenase (LOX) and linoleic acid sodium salt were obtained from Sigma Chemical Co.
1-Benzotriazole carboxylic acid chloride (1)
Compound 1 was prepared from BtH and triphosgene, according to the previously published procedureCitation23,Citation24.
1-(N-Cycloalkyl/arylcarbamoyl)benzotriazoles (2a–f)
The title compounds were synthesized from chloride 1 and corresponding amine, following our previously published procedureCitation22,Citation25. The following derivatives were prepared: 1-(N-cyclopentylcarbamoyl)benzotriazole (2a), 1-(N-cyclohexylcarbamoyl)benzotriazole (2b), 1-(N-benzylcarbamoyl)benzotriazole (2c), 1-(N-[2-phenylethyl]carbamoyl)benzotriazole (2d), 1-(N-benzhydrylcarbamoyl)benzotriazole (2e) and 1-(N-benzyloxycarbamoyl)benzotriazole (2f).
General procedure for preparation of 4-cycloalkyl or aryl semicarbazides (3a–f)
The title compounds were synthesized from 1-(N-alkyl/arylcarbamoyl)benzotriazoles (2a–f) and hydrazine hydrate, according to our slightly modified procedure (molar ration of reactants 1:3, dioxane, 18 h, r.t.)Citation22,Citation25. The following derivatives were prepared: 4-cyclopentylsemicarbazide (3a), 4-cyclohexylsemicarbazide (3b), 4-benzylsemicarbazide (3c), 4-(2-phenylethyl)semicarbazide (3d), 4-benzhydrylsemicarbazide (3e) and 4-benzyloxysemicarbazide (3f). These compounds are commercially available or published elsewhere.
General procedure for preparation of 1-(1-benzotriazolecarbonyl)-4-cycloalkyl/aryl semicarbazides (4a–f)
To a solution of BtcCl (1) (0.908 g, 5.0 mmol) in anhydrous toluene (15 mL), a solution of semicarbazide 3a–f (5.0 mmol) and TEA (0.697 mL, 5 mmol) in anhydrous dioxane (20 mL) was added drop-wise (ice bath). The reaction mixture was stirred 1 h at room temperature or under an ice-bath and used in the next reaction step without further work-up (4a and 4b) or evaporated under reduced pressure (4c–f). The residue was dissolved in 30 mL EtOAc/water mixture (1:1). The organic layer was extracted with water (3 × 40 mL), dried over anhydrous sodium sulphate and evaporated under reduced pressure. Compound 4e precipitated from EtOAc/water mixture and the crude product was filtered off.
1-(1-Benzotriazolecarbonyl)-4-cyclopentylsemicarbazide (4a)
From the reaction of 0.716 g (5.0 mmol) 3a, 0.908 g (5.0 mmol) 1 and 0.697 mL (5 mmol) TEA under an ice bath for 1 h the crude product 4a was obtained and used in further reaction step without purification. Its structure was confirmed indirectly.
1-(1-Benzotriazolecarbonyl)-4-cyclohexylsemicarbazide (4b)
From the reaction of 0.786 g (5.0 mmol) 3b, 0.908 g (5.0 mmol) 1 and 0.697 mL (5 mmol) TEA under an ice bath for 1 h the crude product 4b was obtained and used in further reaction step without purification. Its structure was confirmed indirectly.
1-(1-Benzotriazolecarbonyl)-4-benzylsemicarbazide (4c)
From the reaction of 0.826 g (5.0 mmol) 3c, 0.908 g (5.0 mmol) 1 and 0.697 mL (5 mmol) TEA at room temperature for 1 h and purification of the product from ether/petrol ether, 0.884 g (57%) of 4c was obtained; mp 146°C−147°C; IR (KBr, ν/cm−1) 3310, 1753, 1659, 1576, 1495, 1450, 1380, 1290, 1254, 1029, 747, 698, 615; 1H NMR (300 MHz, DMSO) δ 10.92 (1s, 1H, 1′), 8.40 (1s, 1H, 2′), 8.23, 8.20 (2d, 2H, 3, 6, J = 8.27 Hz), 7.75, 7.58 (2t, 2H, 4, 5, J = 7.40 Hz), 7.35–7.21 (m, 6H, arom., 4′), 4.28 (d, 2H, 5′, J = 6.09 Hz); 13C NMR (75.5 MHz, DMSO) δ 158.41 (3′), 150.15 (1), 145.65, 132.05 (2, 7), 140.93 (6′), 130.58, 126.99, 126.18 (4, 5, 9′), 128.56, 127.42 (7′, 8′, 10′, 11′), 120.29 (6), 113.98 (3), 43.10 (5′); elemental analysis: calcd for C15H14N6O2: C, 58.06; H, 4.55; N, 27.08. Found: C, 58.40; H, 4.65; N, 27.36.
1-(1-Benzotriazolecarbonyl)-4-(2-phenylethyl)semicarbazide (4d)
From the reaction of 0.896 g (5.0 mmol) 3d, 0.908 g (5.0 mmol) 1 and 0.697 mL (5 mmol) TEA at room temperature for 1 h and purification of the product from ether/petrol ether, 0.778 g (48%) of 4d was obtained; mp 135°C−136°C; IR (KBr, ν/cm−1) 3299, 1766, 1745, 1650, 1488, 1449, 1380, 1288, 1236, 1030, 750, 700; 1H NMR (300 MHz, DMSO) δ 10.83 (1s, 1H, 1′), 8.30 (1s, 1H, 2′), 8.23, 8.19 (2d, 2H, 3, 6, J = 8.39 Hz), 7.75, 7.58 (2t, 2H, 4, 5, J = 7.59 Hz), 7.30–7.16 (m, arom., 5H), 6.81 (s, 1H, 4′), 3.27 (q, 2H, 5′, J = 6.75 Hz), 2.73 (t, 2H, 6′, J = 7.59 Hz); 13C NMR (75.5 MHz, DMSO) δ 158.20 (3′), 150.12 (1), 145.65, 132.02 (2, 7), 140.05 (7′), 130.60, 126.47, 126.20 (4, 5, 10′), 129.12, 128.78 (8′, 9′, 11′, 12′), 120.31 (6), 113.96 (3), 41.51, 36.42 (5′, 6′); elemental analysis: calcd for C16H16N6O2: C, 59.25; H, 4.97; N, 25.91. Found: C, 59.60; H, 5.03; N, 25.66.
1-(1-Benzotriazolecarbonyl)-4-benzhydrylsemicarbazide (4e)
From the reaction of 1.206 g (5.0 mmol) 3e, 0.908 g (5.0 mmol) 1 and 0.697 mL (5 mmol) TEA at room temperature for 1 h and purification of the product from ether/petrol ether 1.468 g (76%) of 4e was obtained; mp 164°C−166°C; IR (KBr, ν/cm−1) 3322, 1771, 1663, 1570, 1495, 1446, 1376, 1367, 1286, 1231, 1018, 793, 750, 703, 630; 1H NMR (300 MHz, DMSO) δ 10.88 (1s, 1H, 1′), 8.30 (1s, 1H, 2′), 8.23, 8.20 (2d, 2H, 3, 6, J = 8.43 Hz), 7.75, 7.57 (2t, 2H, 4, 5, J = 7.66 Hz), 7.66 (d, 1H, 4′), 7.37–7.25 (m, arom., 10H), 6.05 (d, 1H, 5′, J = 8.43 Hz); 13C NMR (75.5 MHz, DMSO) δ 157.56 (3′), 150.19 (1), 145.66, 132.00 (2, 7), 143.41 (6′, 12′), 130.64, 126.22 (4, 5), 128.74, 127.80 (7′, 8′, 10′, 11′, 13′, 14′, 16′, 17′), 127.33 (9′, 15′), 120.32 (6), 113.92 (3), 57.22 (5′); elemental analysis: calcd for C21H18N6O2: C, 65.27; H, 4.70; N, 21.75. Found: C, 65.40; H, 4.99; N, 21.55.
1-(1-Benzotriazolecarbonyl)-4-benzyloxysemicarbazide (4f)
From the reaction of 0.906 g (5.0 mmol) 3f, 0.908 g (5.0 mmol) 1 and 0.697 mL (5 mmol) TEA at room temperature for 1 h and purification of the product from ether/petrol ether, 1.191 g (73%) of 4f was obtained; mp 90°C−93°C (decomp.) mpCitation22 90°C−93°C.
General procedure for preparation of primaquine semicarbazide derivatives (5a–f)
Method A: To a solution of 4a–f (5.0 mmol) in toluene (15 mL), a solution of PQ (1.297 g, 5.0 mmol) and TEA (2.09 mL, 15.0 mmol) in dioxane (20 mL) was added drop-wise. The reaction mixture was stirred on ice bath for 2 (5a) or 8 h (5b) and evaporated under reduced pressure. The obtained residue was triturated and stirred with a dioxane/water and EtOAc/water mixtures and finally with water.
Method B: Solution of compounds 4a–f (4 mmol), PQ (1.037 g, 4.0 mmol) and TEA (1.12 mL, 8.0 mmol) in dioxane (30 mL) was stirred at room temperature for 3 h and evaporated under reduced pressure.
Method C: Solution of compounds 4a–f (4 mmol), PQ (1.037 g, 4.0 mmol) and TEA (1.67 mL, 12.0 mmol) in dioxane (30 mL) was stirred at room temperature for 3 h and evaporated under reduced pressure. The obtained residue was dissolved in EtOAc (30 mL) and extracted with 0.1% sodium hydroxide solution (3 × 30 mL), washed with water, dried over anhydrous sodium sulphate and evaporated under reduced pressure.
N1-cyclopentyl-N2-[4-(6-methoxyquinolin-8-ylamino)pentyl]hydrazine-1,2-dicarboxamide (5a)
Method A: From the reaction of 1.442 g (5.0 mmol) 4a, 1.297 g (5.0 mmol) PQ and 2.09 mL (15 mmol) TEA on ice bath for 2 h and purification of the product by column chromatography (eluent dichloromethane/MeOH 9.5:0.5 and 9:1) and trituration with ether, 0.279 g (13%) of 5a was obtained; mp 190°C−194°C; IR (KBr, ν/cm−1) 3306, 3223, 3088, 2939, 2868, 1661, 1618, 1551, 1520, 1455, 1422, 1388, 1326, 1218, 1199, 1158, 1051, 1031, 971, 937, 899, 820, 790, 681, 625; MS/MS m/z 429.5 (M + H)+; 1H NMR (300 MHz, DMSO) δ 8.54 (d, 1H, 11, J = 2.76 Hz), 8.08 (d, 1H, 13, J = 7.81 Hz), 7.46–7.42 (m, 3H, 1′, 2′, 12), 6.48 (s, 1H, 17), 6.36 (t, 1H, 2, J = 4.84 Hz), 6.27 (s, 1H, 15), 6.11, 6.08 (2d, 2H, 8, 4′, J = 8.96 Hz, 8.04 Hz), 3.90–3.83 (m, 1H, 5′), 3.83 (s, 3H, 18), 3.63–3.61 (m, 1H, 6), 3.04 (q, 2H, 3), 1.76–1.20 (m, 15H, 4, 5, 7, 6′–9′); 13C NMR (75.5 MHz, DMSO) δ 159.47 (1), 159.18 (16), 158.64 (3′), 145.11 (9), 144.70 (11), 135.26 (13), 134.99 (10), 130.05 (14), 122.56 (12), 96.54 (17), 92.05 (15), 55.45 (18), 51.38 (5′), 47.52 (6), 39.54 (3), 33.84 (5), 33.07 (6′, 9′), 27.15 (4), 23.67 (7′, 8′), 20.67 (7); Elemental analysis: calcd for C22H32N6O3: C, 61.66; H, 7.53; N, 19.61. Found: C, 61.80; H, 7.64; N, 19.75.
N1-cyclohexyl-N2-[4-(6-methoxyquinolin-8-ylamino)pentyl]hydrazine-1,2-dicarboxamide (5b)
Method A: From the reaction of 1.512 g (5.0 mmol) 4b, 1.297 g (5.0 mmol) PQ and 2.09 mL (15 mmol) TEA on ice bath for 8 h and re-crystallization from methanol/water, 1.394 g (63%) of 5b was obtained; mp 163°C−168°C; IR (KBr, ν/cm−1) 3312, 3228, 3091, 2932, 2855, 1664, 1618, 1548, 1521, 1456, 1423, 1389, 1336, 1285, 1239, 1220, 1201, 1158, 1133, 1052, 1031, 822, 791, 680, 625, 532; 1H NMR (300 MHz, DMSO) δ 8.53 (d, 1H, 11, J = 4.04 Hz), 8.07 (d, 1H, 13, J = 8.07 Hz), 7.47–7.40 (2s, q, 3H, 1′, 2′, 12), 6.47 (s, 1H, 17), 6.35 (t, 1H, 2, J = 4.84 Hz), 6.26 (s, 1H, 15), 6.11, 5.96 (2d, 2H, 8, 4′, J = 8.07 Hz), 3.82 (s, 3H, 18), 3.65–3.57 (m, 1H, 6), 3.40 (m, 1H, 5′), 3.03 (q, 2H, 3), 1.72–1.04 (m, 17H, 4, 5, 7, 6′-10′); 13C NMR (75.5 MHz, DMSO) δ 159.47 (1), 159.15 (16), 158.27 (3′), 145.10 (9), 144.69 (11), 135.27 (13), 134.98 (10), 130.05 (14), 122.56 (12), 96.55 (17), 92.06 (15), 55.45 (18), 48.41 (5′), 47.52 (6), 39.53 (3), 33.84 (5), 33.44 (6′, 10′), 27.14 (4), 25.64 (8′), 25.08 (7′, 9′), 20.66 (7); MS/MS m/z 443.3 (M + H)+; elemental analysis: calcd for C23H34N6O3: C, 62.42; H, 7.74; N, 18.99. Found: C, 62.21; H, 7.55; N, 18.60.
N1-benzyl-N2-[4-(6-methoxyquinolin-8-ylamino)pentyl]hydrazine-1,2-dicarboxamide (5c)
Method B: From the reaction of 1.241 g (4.0 mmol) 4c, 1.037 g (4.0 mmol) PQ and 1.12 mL (8 mmol) TEA at room temperature for 3 h and purification of the product by trituration with ether/EtOAc/petrol ether and ether/petrol ether, 1.153 g (64%) of 5c was obtained; mp 200°C−203°C; IR (KBr, ν/cm−1) 3306, 3223, 3089, 2933, 1664, 1616, 1572, 1555, 1519, 1455, 1423, 1387, 1322, 1219, 1201, 1158, 1051, 1030, 820, 790, 735, 700, 626; 1H NMR (300 MHz, DMSO) δ 8.54 (d, 1H, 11, J = 3.87 Hz), 8.08 (d, 1H, 13, J = 8.12 Hz), 7.66 (1s, 1H, 1′), 7.61 (1s, 1H, 2′), 7.42 (q, 1H, 12, J = 4.06 Hz), 7.26–7.14 (m, arom., 5H, 7′–11′), 6.90 (t, 1H, 4′, J = 5.42 Hz), 6.47 (s, 1H, 17), 6.42 (t, 1H, 2, J = 4.84 Hz), 6.26 (s, 1H, 15), 6.12 (d, 1H, 8, J = 8.70 Hz), 4.21 (d, 2H, 5′, J = 5.80 Hz), 3.82 (s, 3H, 18), 3.66–3.57 (m, 1H, 6), 3.05 (q, 2H, 3), 1.65–1.46 (m, 4H, 4, 5), 1.20 (d, 3H, 7, J = 6.00 Hz); 13C NMR (75.5 MHz, DMSO) δ 159.48 (1), 159.25 (16), 159.08 (3′), 145.12 (9), 144.71 (11), 141.09 (6′), 135.27 (13), 135.00 (10), 130.06 (14), 128.48, 127.40 (7′, 8′,10′, 11′), 126.85 (9′), 122.58 (12), 96.54 (17), 92.05 (15), 55.46 (18), 47.54 (6), 43.00 (5′), 39.65 (3), 33.85 (5), 27.20 (4), 20.69 (7); MS/MS m/z 451.3 (M + H)+; elemental analysis: calcd for C24H30N6O3: C, 63.98; H, 6.71; N, 18.65. Found: C, 63.63; H, 6.77; N, 18.78.
N1-[4-(6-methoxyquinolin-8-ylamino)pentyl]-N2-phenylethylhydrazine-1,2-dicarboxamide (5d)
Method B: From the reaction of 1.297 g (4 mmol) 4d, 1.037 g (4.0 mmol) PQ and 1.12 mL (8 mmol) TEA at room temperature for 3 h and purification of the product by trituration with ether/EtOAc, 1.264 g (68%) of 5d was obtained; mp 174°C−176°C; IR (KBr, ν/cm−1) 3302, 3226, 3088, 2933, 1662, 1616, 1575, 1554, 1520, 1455, 1423, 1387, 1326, 1219, 1200, 1157, 1131, 1051, 1131, 820, 790, 750, 700, 624; 1H NMR (300 MHz, DMSO) δ 8.50 (d, 1H, 11, J = 3.79 Hz), 8.04 (d, 1H, 13, J = 7.81 Hz), 7.54 (1s, 1H, 1′), 7.47 (1s, 1H, 2′), 7.39 (q, 1H, 12, J = 4.24 Hz), 7.26–7.11 (m, arom., 5H, 8′–12′), 6.44 (s, 1H, 17), 6.33–6.28 (2t, 2H, 2, 4′), 6.24 (s, 1H, 15), 6.09 (d, 1H, 8, J = 8.70 Hz), 3.80 (s, 3H, 18), 3.62–3.57 (m, 1H, 6), 3.20 (q, 2H, 5′, J = 6.47 Hz), 3.02 (q, 2H, 3), 2.66 (t, 2H, 6′, J = 7.14 Hz), 1.63–1.43 (m, 4H, 4, 5), 1.18 (d, 3H, 7, J = 6.03 Hz); 13C NMR (75.5 MHz, DMSO) δ 159.49, 159.10, 159.02 (1, 16, 3′), 145.14 (9), 144.71 (11), 140.11 (7′), 135.26 (13), 135.02 (10), 130.06 (14), 129.09, 128.73 (8′, 9′,11′, 12′), 126.40 (10′), 122.55 (12), 96.57 (17), 92.11 (15), 55.46 (18), 47.56 (6), 41.28 (5′), 39.64 (3), 36.48 (6′), 33.89 (5), 27.15 (4), 20.70 (7); MS/MS m/z 465.3 (M + H)+; elemental analysis: calcd for C25H32N6O3: C, 64.63; H, 6.94; N, 18.09. Found: C, 64.88; H, 7.11; N, 18.48.
N1-benzhydryl-N2-[4-(6-methoxyquinolin-8-ylamino)pentyl]hydrazine-1,2-dicarboxamide (5e)
Method C: From the reaction of 1.546 g (4 mmol) 4e, 1.037 g (4.0 mmol) PQ and 1.67 mL (12.0 mmol) TEA at room temperature for 3 h and purification of the product by column chromatography (eluent dichloromethane/MeOH 9.5:0.5) and re-crystallized from ether/petrol ether, 1.369 g (65%) of 5e was obtained; mp 90°C (decomp.); IR (KBr, ν/cm−1) 3311, 2960, 2933, 2869, 1662, 1616, 1575, 1555, 1519, 1454, 1423, 1387, 1220, 1202, 1158, 1052, 1030, 822, 791, 747, 700, 625; 1H NMR (300 MHz, DMSO) δ 8.51 (d, 1H, 11, J = 3.13 Hz), 8.05 (d, 1H, 13, J = 8.13 Hz), 7.61 (s, 1H, 1′), 7.54 (s, 1H, 2′), 7.39 (q, 1H, 12, J = 4.17 Hz, 3.75 Hz), 7.29–7.17 (m, arom., 10H, 7′–11′, 13′–17′), 6.98 (d, 1H, 4′, J = 8.34 Hz), 6.45 (s, 1H, 17), 6.35 (t, 1H, 2), 6.24 (s, 1H, 15), 6.09 (d, 1H, 8, J = 8.55 Hz), 5.91 (d, 1H, 5′, J = 8.34 Hz), 3.80 (s, 3H, 18), 3.63–3.55 (m, 1H, 6), 3.02 (q, 2H, 3), 1.62–1.41 (m, 4H, 4, 5), 1.17 (d, 3H, 7, J = 5.84 Hz); 13C NMR (75.5 MHz, DMSO) δ 159.51 (1), 159.12 (16), 158.29 (3′), 145.14 (9), 144.71 (11), 143.57 (6′, 12′), 135.26 (13), 135.03 (10), 130.07 (14), 128.74, 127.60 (7′, 8′,10′, 11′, 13′, 14′, 16′, 17′), 127.26 (9′, 15′), 122.56 (12), 96.57 (17), 92.13 (15), 57.21(5′), 55.47 (18), 47.56 (6), 39.68 (3), 33.89 (5), 27.19 (4), 20.69 (7); MS/MS m/z 527.3 (M + H)+; elemental analysis: calcd for C30H34N6O3: C, 68.42; H, 6.51; N, 15.96. Found: C, 68.80; H, 6.44; N, 16.13.
N1-benzyloxy-N2-[4-(6-methoxyquinolin-8-ylamino)pentyl]hydrazine-1,2-dicarboxamide (5f)
Method C: From the reaction of 1.305 g (4 mmol) 4f, 1.037 g (4.0 mmol) PQ and 1.67 mL (12.0 mmol) TEA at room temperature for 3 h and purification of the product by column chromatography (eluent dichloromethane/MeOH 9.5:0.5) and trituration with ether, 0.802 g (43%) of 5f was obtained; mp 102°C−104°C; IR (KBr, ν/cm−1) 3300, 3216, 3089, 2934, 1670, 1617, 1573, 1520, 1455, 1423, 1387, 1328, 1219, 1201, 1157, 1051, 1030, 820, 790, 738, 697, 625; 1H NMR (300 MHz, DMSO) δ 9.48, 8.42, 7.50 (3s, 3H, 1′, 2′, 4′), 8.55 (dd, 1H, 11, J = 3.92 Hz), 8.08 (d, 1H, 13, J = 8.19 Hz), 7.45–7.31 (m, arom., 6H, 7′–11′, 12), 6.48 (d, 1H, 17, J = 2.26 Hz), 6.28 (d, 1H, 15, J = 2.14 Hz), 6.18 (t, 1H, 2, J = 5.70 Hz), 6.13 (d, 1H, 8, J = 8.78 Hz), 4.77 (s, 2H, 5′), 3.83 (s, 3H, 18), 3.68–3.59 (m, 1H, 6), 3.04 (q, 2H, 3), 1.66–1.43 (m, 4H, 4, 5), 1.22 (d, 3H, 7, J = 6.17 Hz); 13C NMR (75.5 MHz, DMSO) δ 160.16 (1), 159.47 (16), 158.98 (3′), 145.11 (9), 144.72 (11), 136.95 (6′), 135.27 (13), 135.00 (10), 130.05 (14), 129.23, 128.59 (7′, 8′,10′, 11′), 128.42 (9′), 122.58 (12), 96.58 (17), 92.08 (15), 77.85 (5′), 55.46 (18), 47.51 (6), 39.71 (3), 33.87 (5), 27.24 (4), 20.70 (7); MS/MS m/z 467.3 (M + H)+; elemental analysis: calcd for C24H30N6O4: C, 61.79; H, 6.48; N, 18.01. Found: C, 61.83; H, 6.40; N, 17.86.
N1-hydroxy-N2-[4-(6-methoxyquinolin-8-ylamino)pentyl]hydrazine-1,2-dicarboxamide (5g)
A suspension of 5e (0.467 g, 1.0 mmol) in methanol (15 mL) was hydrogenated for 1.5 h in the presence of 10% Pd/C (100 mg). The catalyst was filtered off and the solvent was evaporated under reduced pressure. The crude product was re-crystallized from ether/petrol ether. Yield: 0.226 g (60%). mp 112°C; IR (KBr, ν/cm−1) 3262, 2929, 2857, 1654, 1616, 1576, 1558, 1520, 1456, 1423, 1388, 1221, 1202, 1159, 1052, 1031, 822, 791, 625; 1H NMR (300 MHz, DMSO) δ 8.70, 8.55, 8.21, 7.42 (4s, 4H, 1′, 2′, 4′, OH), 8.54 (d, 1H, 11), 8.08 (dd, 1H, 13, J = 8.36 Hz), 7.43–7.41 (q, 1H, 12), 6.48 (d, 1H, 17, J = 2.33 Hz), 6.27 (d, 1H, 15, J = 2.33 Hz), 6.17 (t, 1H, 2, J = 5.82 Hz), 6.14–6.11 (m, 1H, 8), 3.83 (s, 3H, 18), 3.66–3.58 (m, 1H, 6), 3.04 (q, 2H, 3), 1.64–1.46 (m, 4H, 4, 5), 1.21 (d, 3H, 7, J = 6.21 Hz); 13C NMR (75.5 MHz, DMSO) δ 160.6 (1), 158.92 (16), 158.51 (3′), 144.52 (9), 144.15 (11), 134.76 (13), 134.38 (10), 129.50 (14), 122.02 (12), 96.05 (17), 91.54 (15), 54.91 (18), 46.96 (6), 39.08 (3), 33.30 (5), 26.67 (4), 20.12 (7); MS/MS m/z 377.3 (M + H)+; elemental analysis: calcd for C17H24N6O4: C, 54.24; H, 6.43; N, 22.33. Found: C, 54.41; H, 6.55; N, 21.98.
Biological evaluation
Cytostatic activity assays
The HCT 116 (colon carcinoma), MCF-7 (breast carcinoma), H 460 (lung carcinoma) cells were cultured as monolayers and maintained in Dulbecco’s modified Eagle medium (DMEM), supplemented with 10% foetal bovine serum (FBS), 2 mmol L−1 L-glutamine, 100 U mL−1 penicillin and 100 μg mL−1 streptomycin in a humidified atmosphere with 5% CO2 at 37°C. The growth inhibition activity was assessed, as described previously, according to the slightly modified procedure of the National Cancer Institute, Developmental Therapeutics ProgramCitation26,Citation27. Briefly, the cells were inoculated onto standard 96-well microtiter plates on day 0. Test agents were then added in five consecutive 10-fold dilutions (10−8 to 10−4 mol L−1) and incubated for further 72 h. Working dilutions were freshly prepared on the day of testing. The solvent (DMSO) was also tested for eventual inhibitory activity by adjusting its concentration to be the same as in working concentrations (maximal concentration of DMSO was 0.25%). After 72 h of incubation, the cell growth rate was evaluated by performing the MTT assay which detects dehydrogenase activity in viable cells. The absorbance was measured on a microplate reader at 570 nm. Each test point was performed in quadruplicate in at least two individual experiments. The results are expressed as IC50, which is the concentration necessary for 50% of inhibition. The IC50 values for each compound are calculated from dose–response curves using linear regression analysis by fitting the test concentrations that give percentage of growth (PG) values above and below the reference value (i.e. 50%). Each result is a mean value from three separate experiments.
The cytostatic activity of the test compounds against L1210, HeLa and CEM cells was determined with cells grown in suspension (L1210, CEM) or monolayers (HeLa) in 200 µL-wells of 96-well microtiter plates (initial cell number: 5–7.5 × 104 cells/well). After 48 (L1210) or 72 (CEM, HeLa) h, the tumour cell number was determined by the use of a Coulter counter.
Antiviral activity assays
Antiviral activity against vesicular stomatitis virus, Coxsackie virus B4, respiratory syncytial virus, herpes simplex virus type 1 (KOS) and type 2 (G), thymidine kinase-deficient herpes simplex virus type 1 (TK− KOS ACVr), vaccinia virus, parainfluenza-3 virus, reovirus-1, Sindbis virus, Punta Toro virus, influenza virus A (H1N1; H3N2) and B, feline corona virus (FIPV) and feline herpes virus, HIV-1 and HIV-2 was determined essentially, as described previouslyCitation28. After a 2 h incubation period, residual virus was removed and the infected cells were further incubated with the medium containing different concentrations of the test compounds. After incubation for 3 days at 37°C, virus-induced cytopathogenicity was monitored microscopically. Antiviral activity was expressed as the concentration required to reduce virus-induced cytopathogenicity by 50% (EC50).
Antioxidant activity assays
Determination of the reducing activity of the stable radical DPPH
To an ethanolic solution of DPPH (0.05 mM) in absolute ethanol an equal volume of the compounds (final concentration 100 μM) dissolved in DMSO was added. The mixture was shaken vigorously and allowed to stand for 20 or 60 min. Absorbance at 517 nm was determined spectrophotometrically, and the percentage of activity was calculated. Each experiment was performed at least in triplicate, and the standard deviation of absorbance was less than 10% of the mean ().
Table 1. Interaction with DPPH, in vitro inhibition of LP, soybean LOX and theoretically calculated Clog p values.
Inhibition of linoleic acid lipid peroxidation
The water soluble azo compound AAPH is used as a free radical initiator for in vitro studies of free radical production. Production of conjugated diene hydroperoxide by oxidation of linoleic acid sodium salt in an aqueous solution is monitored at 234 nm. This assay can be used to follow oxidative changes and to understand the contribution of each tested compound. An amount of 10 μL of the 16 mM linoleic acid sodium salt solution was added to the UV cuvette containing 0.93 mL of 0.05 M phosphate buffer, pH 7.4, prethermostated at 37°C. The oxidation reaction was initiated at 37°C under air by the addition of 50 μL of 40 mM AAPH solution. Oxidation was carried out in the presence of aliquots (10 μL) in the assay without antioxidant, and lipid oxidation was measured in the presence of the same level of DMSO. The rate of oxidation at 37°C was monitored by recording the increase in absorption at 234 nm caused by conjugated diene hydroperoxides. All tests were undertaken on three replicates, and the results were averaged.
Soybean LOX inhibition study in vitro
The tested compounds dissolved in DMSO were incubated at room temperature with sodium linoleate (0.1 mL) and 0.2 mL of the enzyme solution (1 part of enzyme/9 parts of saline × 10−4, w/v in saline). The conversion of sodium linoleate to 13-hydroperoxylinoleic acid at 234 nm was recorded and compared with the appropriate standard inhibitor.
Results and discussion
Chemistry
A series of novel 1,4-substituted semicarbazides 5a–g with PQ moiety bridged by carbonyl group at position 1 and cycloalkyl, aryl, benzyloxy or hydroxy substituent at position 4 were prepared and biologically evaluated. The title compounds contain a region of six or seven electronegative atoms bound to primary amino group of PQ. Therefore, they are closely related to biurets, hydroxyureas, hydroxamic acids or azapeptides.
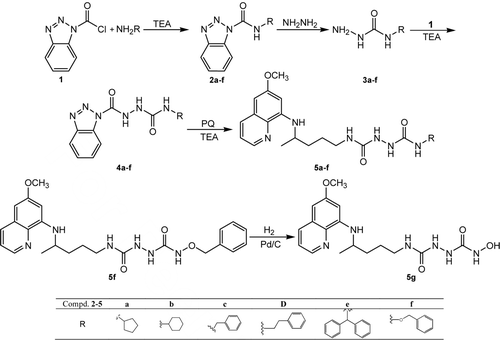
Preparation of compounds 5a–f involved BtH as synthetic auxiliary. The BtH moiety in 1-(N-cycloalkyl/arylcarbamoyl)benzotriazoles (1-benzotriazole carboxylic acid amides) 2a–f and 1-(1-benzotriazolecarbonyl)-4-cycloalkyl/aryl semicarbazides 4a–f activated the molecules for nucleophilic substitution with hydrazine hydrate or PQ, allowing the preparation of semicarbazides 3a–f and PQ-semicarbazide derivatives 5a–f, respectively. The BtH was introduced in the intermediate products 2 and 4 by means of 1-benzotriazole carboxylic acid chloride (BtcCl, 1), which was first described by our research group and now is commercially available23,24. The TEA was used as a catalyst and/or HCl acceptor in several synthetic steps. The synthetic pathway is shown in : the starting chloride 1 in reaction with corresponding amine (cyclopentylamine, cyclohexylamine, benzylamine, 2-phenylethanamine, benzhydrylamine, O-benzylhydroxylamine) gave 1-carbamoylbenzotriazoles 2a–f. In the next reaction step, the BtH was replaced by hydrazine hydrate to give 4-substituted semicarbazides 3a–f. Benzotriazolecarbonyl semicarbazides 4a–f were prepared by the reaction of products 3 with chloride 1. In the final reaction step, benzotriazole ring of 4a–f was substituted with PQ affording PQ-semicarbazide derivatives 5a–f. Compound 5g with a free hydroxyl group at position 4 of semicarbazide backbone was prepared by hydrogenolysis of benzyloxysemicarbazide 5f.
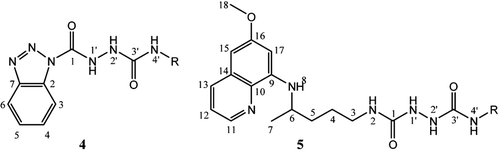
Structures of the synthesized compounds are supported by infrared, mass, 1H and 13C NMR spectra and confirmed by elemental analysis. The chemical shifts are consistent with the proposed structures. The presence of the PQ residue was confirmed in 1H (13C) spectra of 5a–g: hydrogen atoms next to pyridine nitrogen occurred between 8.50 and 8.55 (144.15 and 144.72), OCH3 group appeared as a singlet between 3.80 and 3.83 (54.91 and 55.47), C-6 appeared as a multiplet between 3.54 and 3.68 (46.96 and 47.56), C-7 occurred as a doublet between 1.17 and 1.22 (20.12 and 20.70), while NH group at position 8 appeared as a doublet between 5.96 and 6.13 ppm. Semicarbazide NH groups 1′ and 2′ of compounds 5 showed as two close singlets at 7.47–7.61 ppm, while those of compounds 4 were shifted to 10.83−10.88 and 8.30−8.40, respectively. The NH groups at positions 2 and 4′ were approximately at 6.90 and 6.35 ppm. Signals of two carbonyl C-atoms in the PQ semicarbazide spectra were very close and appeared between 158.27 and 160.6 ppm, while C-1 carbonyl in benzotriazolecarbonyl semicarbazides 4 was shifted to the lower parts per million values (150.12−150.19). Atom enumeration of compounds 4 and 5 is shown in . The IR spectra of 5a–f showed absorption bands at 3410−3262 (NH groups), 1674−1650, 1576−1548 and 1521−1519 cm−1 (semicarbazide carbonyls), while carbonyl group bound to BtH in compound 4 was absorbed at 1771−1738 cm−1.
Biological evaluation
Cytostatic activity
The PQ-semicarbazide derivatives 5a–g and their synthetic precursors benzotriazolecarbonyl semicarbazides 4 were evaluated for their cytostatic activities against a variety of tumour cell lines, which are derived from several cancer types. The following cell lines were used: human HCT 116, MCF-7, H 460, CEM, HeLa and murine L1210. All compounds of the series 5 showed high selectivity towards MCF-7 cells (breast carcinoma) with IC50 values in the low micromolar range (). Benzyl derivative 5c was the most active compound (IC50 1 ± 0.2 µM), while benzhydryl derivative 5e showed significant cytostatic activities towards all the tested cell lines (IC50 4–17 µM) and the highest activity against MCF-7 cells. These data are in agreement with our previous results obtained for NSAID semicarbazide derivativesCitation22, where the presence of a bulky lipophilic group, preferably a benzhydryl group, at the N-4 position of the semicarbazide backbone was strictly required, while the NSAID terminal end of these molecules was less important to display anti-proliferative potency. On the other hand, analogue compounds of the series 4 with the same semicarbazide moiety, containing a BtH ring instead of PQ or NSAID residues were practically inactive, which indicates the importance of the PQ/NSAID part of the molecule.
Table 2. Inhibitory effects of PQ semicarbazide derivatives 5a-g and their synthetic precursors 4 on the proliferation of malignant tumour cell lines.
Most interesting, however, is the high sensitivity of MCF-7 tumour cells to almost all the compounds. This phenomenon was already noted in the authors’ previous work with urea derivatives of PQ Citation20. Some other authors reported selectivity of quinidine, PQ, N-dipeptidylprimaquines and imidazolin-4-one PQ derivatives towards MCF-7 cell line as wellCitation16,Citation29. Selective growth inhibitory activity towards breast cancer cells could be explained by selective induction of cytochrome P450 (CYP-1) enzymes specifically in the MCF-7 line, known as CYP-1 inducible cell lineCitation30. These enzymes catalyse the initial step in either detoxification or bio-activation of environmental toxins and xenobiotics. The transcriptional regulation of the CYP1A1 gene by polycyclic aromatic hydrocarbons (PAH) and halogenated aromatic hydrocarbons (HAH) is mediated via ligand-dependent activation of the aryl hydrocarbon receptor (AhR), which translocates to the nucleus upon activation, dimerizes to the aryl hydrocarbon receptor nuclear translocator (arnt) protein and binds to the xenobiotic response element (XRE) in the regulatory region of the CYP1A1 geneCitation31. Probably, the observed selectivity towards breast cancer cells could be related to the PQ moiety, although PQ does not conform to the structural features of AhR ligands (planar hydrophobic aromatic compounds) and, accordingly, does not bind with high specificity to the AhR. It has been shown that several non-planar compounds with little aromaticity, such as benzimidazole compounds (e.g. some antiparasitic drugs) also induce the CYP1A1 mRNA expression, but the mechanism of its induction has not been well understoodCitation32. Moreover, it was demonstrated that PQ is a potent inducer of CYP1A1 in the rat hepatoma H4IIE cellsCitation31. It uses the AhR for the transcriptional activation of the CYP1A1 gene and causes an inhibition of CYP1A1 enzyme degradation. A similar concept of sensitivity of MCF-7 cells towards benzothiazoles mediated by AhR has been shown concerning the discovery of a class of 2-(4-aminophenyl)benzothiazoles, potential antitumour agents that showed remarkably similar, highly selective profiles of antitumour activityCitation33. The intriguing specificity of PQ semicarbazides towards MCF-7 cells should be further studied in more detail and fostered towards the discovery of novel AhR-dependent toxicity/antitumour activity.
Antiviral activity
Compounds 4b, 4c and 5a–g were evaluated against a broad variety of viral infections, including herpes simplex virus type 1 (HSV-1) and HSV-2, vaccinia virus and vesicular stomatitis virus in HEL cell cultures, parainfluenza-3 virus, reovirus-1, Sindbis virus, Coxsackie virus B4 and Punta Toro virus in Vero cell cultures, vesicular stomatitis virus, Coxsackie B4 virus and respiratory syncytial virus in HeLa cell cultures, feline coronavirus (FIPV) and feline herpes virus in CRFK cell cultures, influenza A (H1N1 and H3N2) and B in MDCK cell cultures and human immunodeficiency virus type 1 (HIV-1/IIIB) and HIV-2(ROD) in CEM cell cultures. No specific antiviral effects were noted for any of the compounds evaluated against any of the tested viruses (data not shown).
Antioxidant activity
In the present investigation, antioxidant activity of the PQ derivatives 4b, 4c and 5a–g were studied and compared with the well-known antioxidant agents, such as NDGA and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox). Two different antioxidant assays were used: (1) interaction with the stable free DPPH radical and (2) interaction with the water-soluble azo compound AAPH, used as a source of peroxyl radicalsCitation34. The DPPH interaction of the tested compounds was examined at 100 μM concentration after 20 and 60 min. The results are shown in . Derivative 5g with hydroxy group at position 4 was the only PQ semicarbazide that showed significant interaction (68.5%), followed by 1-(1-benzotriazolecarbonyl)-4-aryl semicarbazides 4b and 4c (61.2% and 66.6%, respectively). A slight increase (69.2−77.4%) in antioxidant activity was observed after 60 min. Low lipophilicity values (1.03−2.25) are correlated with higher DPPH interaction. The antioxidant ability of the other compounds of the series 5 was very low (2.4−12.6%).
In our studies, AAPH was used as a free radical initiator to follow oxidative changes of linoleic acid to conjugated diene hydroperoxide. Under our experimental conditions, no inhibition of lipid peroxidation (LP) by the tested compounds was observed.
The LOXs play a significant role in membrane LP by forming hydroperoxides in the lipid bilayer from the biotransformation of arachidonic acid catalyzed by LOX. The LOX inhibitors have attracted attention initially as potential agents for the treatment of inflammatory and allergic diseases, certain types of cancer and cardiovascular diseases. In this context, it was decided to evaluate the newly prepared derivatives for their ability to inhibit soybean LOXCitation36. It has been shown that inhibition of plant LOX activity by NSAIDs is qualitatively similar to their inhibition of the rat mast cell LOX and may be used as a simple qualitative screen for such activity. Compound 5e, followed by 5d, was found to be the most potent among the PQ semicarbazides 5, while the tested compound 4 showed no activity. The fact that the most active compounds have high Clog p values (5.05 and 4.08, respectively) support the findings that lipophilicity is an important physicochemical property for LOX inhibitors.
Conclusions
The PQ semicarbazides 5, described here, showed significant selective cytostatic activity against the proliferation of MCF-7 cells with IC50 values between 1 and 16 µM (five compounds in the range 1−4 µM). The most active compound was the benzyl derivative 5c. Benzhydryl derivative 5e showed cytostatic activities towards all the tested cell lines (IC50 4–18 µM). The same compound was the strongest LOX inhibitor as well. It is worth to note that in the series of NSAID semicarbazide derivatives previously described by our groupCitation22, the most active compounds were also the benzhydryl derivatives. On the other hand, the highest antioxidant activity was found for the hydroxy derivative 5g and the benzotriazolecarbonyl semicarbazides 4b, 4c. The prepared PQ semicarbazide derivatives represent useful lead compounds in development of specific agents against breast cancer. They have potential as antimalarial agents as well, but their antimalarial evaluation still remains to be performed.
Acknowledgement
We thank Dr. C. Hansch, Dr. A. Leo, and Biobyte Corp., Claremont, California, for free access to the C-QSAR program and Mrs. Leentje Persoons, Frieda De Meyer, Leen Ingels, Lizette van Berckelaer for excellent technical assistance.
Declaration of interest
The authors report no conflicts of interest. This study is supported by the Ministry of Science, Education and Sports of the Republic of Croatia (Projects No. 006-0000000-3216 and 098-0982464-2514) and the K.U. Leuven (GOA 10/014).
References
- Beraldo H, Gambino D. The wide pharmacological versatility of semicarbazones, thiosemicarba-zones and their metal complexes. Mini Rev Med Chem 2004;4:31–39.
- Asghar SF, Yasin KA, Aziz S. Synthesis and cyclisation of 1,4-disubstituted semicarbazides. Nat Prod Res 2010;24:315–325.
- Zhang R, Durkin JP, Windsor WT. Azapeptides as inhibitors of the hepatitis C virus NS3 serine protease. Bioorg Med Chem Lett 2002;12:1005–1008.
- Bailey MD, Halmos T, Goudreau N, Lescop E, Llinàs-Brunet M. Novel azapeptide inhibitors of hepatitis C virus serine protease. J Med Chem 2004;47:3788–3799.
- Ferrari MB, Capacchi S, Reffo G, Pelosi G, Tarasconi P, Albertini R et al. Synthesis, structural characterization and biological activity of p-fluorobenzaldehyde thiosemicarbazones and of a nickel complex. J Inorg Biochem 2000;81:89–97.
- Klayman DL, Lin AJ, Hoch JM, Scovill JP, Lambros C, Dobek AS. 2-Acetylpyridine thiosemicarbazones XI: 2-(alpha-Hydroxyacetyl)pyridine thiosemicarbazones as antimalarial and antibacterial agents. J Pharm Sci 1984;73:1763–1767.
- Ren S, Wang R, Komatsu K, Bonaz-Krause P, Zyrianov Y, McKenna CE et al. Synthesis, biological evaluation, and quantitative structure-activity relationship analysis of new Schiff bases of hydroxysemicarbazide as potential antitumor agents. J Med Chem 2002;45:410–419.
- Shao J, Zhou B, Zhu L, Bilio AJ, Su L, Yuan YC et al. Determination of the potency and subunit-selectivity of ribonucleotide reductase inhibitors with a recombinant-holoenzyme-based in vitro assay. Biochem Pharmacol 2005;69:627–634.
- Finch RA, Liu M, Grill SP, Rose WC, Loomis R, Vasquez KM et al. Triapine (3-aminopyridine-2-carboxaldehyde- thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem Pharmacol 2000;59:983–991.
- Kolesar J, Brundage RC, Pomplun M, Alberti D, Holen K, Traynor A et al. Population pharmacokinetics of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (Triapine®) in cancer patients. Cancer Chemother Pharmacol 2011;67:393–400.
- Bondebjerg J, Fuglsang H, Valeur KR, Kaznelson DW, Hansen JA, Pedersen RO et al. Novel semicarbazide-derived inhibitors of human dipeptidyl peptidase I (hDPPI). Bioorg Med Chem 2005;13:4408–4424.
- Lemke TL, Williams DA, Roche VF, Zito SW. Foye’s Principles of Medicinal Chemistry, 6th Ed.,Philadelphia: Lippincott Williams & Wilkins, 2008.
- Holland KP, Elford HL, Bracchi V, Annis CG, Schuster SM, Chakrabarti D. Antimalarial activities of polyhydroxyphenyl and hydroxamic acid derivatives. Antimicrob Agents Chemother 1998;42:2456–2458.
- Klayman DL, Acton N, Scovill JP. 2-Acetylpyridine thiosemicarbazones. 12. Derivatives of 3-acetylisoquinoline as potential antimalarial agents. Arzneimittelforschung 1986;36:10–13.
- Mahajan A, Yeh S, Nell M, van Rensburg CE, Chibale K. Synthesis of new 7-chloroquinolinyl thioureas and their biological investigation as potential antimalarial and anticancer agents. Bioorg Med Chem Lett 2007;17:5683–5685.
- Fernandes I, Vale N, de Freitas V, Moreira R, Mateus N, Gomes P. Anti-tumoral activity of imidazoquines, a new class of antimalarials derived from primaquine. Bioorg Med Chem Lett 2009;19:6914–6917.
- Jones M, Mercer AE, Stocks PA, La Pensée LJ, Cosstick R, Park BK et al. Antitumour and antimalarial activity of artemisinin-acridine hybrids. Bioorg Med Chem Lett 2009;19:2033–2037.
- Rosenthal AS, Chen X, Liu JO, West DC, Hergenrother PJ, Shapiro TA et al. Malaria-infected mice are cured by a single oral dose of new dimeric trioxane sulfones which are also selectively and powerfully cytotoxic to cancer cells. J Med Chem 2009;52:1198–1203.
- Dzimbeg G, Zorc B, Kralj M, Ester K, Pavelic K, Andrei G et al. The novel primaquine derivatives of N-alkyl, cycloalkyl or aryl urea: synthesis, cytostatic and antiviral activity evaluations. Eur J Med Chem 2008;43:1180–1187.
- Simunovic M, Perkovic I, Zorc B, Ester K, Kralj M, Hadjipavlou-Litina D et al. Urea and carbamate derivatives of primaquine: synthesis, cytostatic and antioxidant activities. Bioorg Med Chem 2009;17:5605–5613.
- Rajic Z, Koncic MZ, Miloloža K, Perkovic I, Butula I, Bucar F et al. Primaquine-NSAID twin drugs: Synthesis, radical scavenging, antioxidant and Fe2+ chelating activity. Acta Pharm 2010;60:325–337.
- Perković I, Butula I, Kralj M, Martin-Kleiner I, Balzarini J, Hadjipavlou-Litina D, Katsori A-M, Zorc B. Novel NSAID 1-acyl-4-cycloalkyl/arylsemicarbazides and 1-acyl-5-benzyloxy/hydroxy)carbamoylcarbazides as potential anticancer agents and antioxidants. Eur J Med Chem (In press).
- Butula I, Proštenik M, Vela V. Reactions with 1-benzotriazole carboxylic acid chloride. I. Synthesis of the 2,6-bis(hydroxymethyl)pyridine dicarbamates. Croat Chem Acta 1977;49:837–42.
- Kalčić I, Zovko M, Jadrijević-Mladar Takač M, Zorc B, Butula I. Synthesis and reactions of some azolecarboxylic acid derivatives. Croat Chem Acta 2003;76:217–28.
- Butula I, Vela V, Ivezić B. Reaktionen mit 1-Benzotriazol carbonsaurechlorid.IV.Synthese von substituierten Harnstoffen, Semicarbaziden und Carbaziden. Croat Chem Acta 1978;51:339–46.
- Rajic Z, Hadjipavlou-Litina D, Pontiki E, Kralj M, Suman L, Zorc B. The novel ketoprofen amides–synthesis and biological evaluation as antioxidants, lipoxygenase inhibitors and cytostatic agents. Chem Biol Drug Des 2010;75:641–652.
- Boyd MR, Kenneth DP. Some practical considerations and applications of the National Cancer Intitute in vitro anticancer drug discovery screen. Drug Dev Res 1995;34:91–109.
- Tzioumaki N, Manta S, Tsoukala E, Vande Voorde J, Liekens S, Komiotis D et al. Synthesis and biological evaluation of unsaturated keto and exomethylene D-arabinopyranonucleoside analogs: novel 5-fluorouracil analogs that target thymidylate synthase. Eur J Med Chem 2011;46:993–1005.
- Zhou Q, Melkoumian ZK, Lucktong A, Moniwa M, Davie JR, Strobl JS. Rapid induction of histone hyperacetylation and cellular differentiation in human breast tumor cell lines following degradation of histone deacetylase-1. J Biol Chem 2000;275:35256–35263.
- Iwanari M, Nakajima M, Kizu R, Hayakawa K, Yokoi T. Induction of CYP1A1, CYP1A2, and CYP1B1 mRNAs by nitropolycyclic aromatic hydrocarbons in various human tissue-derived cells: chemical-, cytochrome P450 isoform-, and cell-specific differences. Arch Toxicol 2002;76:287–298.
- Werlinder V, Backlund M, Zhukov A, Ingelman-Sundberg M. Transcriptional and post-translational regulation of CYP1A1 by primaquine. J Pharmacol Exp Ther 2001;297:206–214.
- Backlund M, Weidolf L, Ingelman-Sundberg M. Structural and mechanistic aspects of transcriptional induction of cytochrome P450 1A1 by benzimidazole derivatives in rat hepatoma H4IIE cells. Eur J Biochem 1999;261:66–71.
- Loaiza-Pérez AI, Trapani V, Hose C, Singh SS, Trepel JB, Stevens MF et al. Aryl hydrocarbon receptor mediates sensitivity of MCF-7 breast cancer cells to antitumor agent 2-(4-amino-3-methylphenyl) benzothiazole. Mol Pharmacol 2002;61:13–19.
- Pontiki E, Hadjipavlou-Litina D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Bioorg Med Chem 2007;15:5819–5827.
- C-QSAR Database: Biobyte Corp., 201 West 4th Street, Suite 204, Claremont, CA 91711.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med 1999;26:1231–37.