Abstract
A series of novel Mannich bases of chlorokojic acid (2-chloromethyl-5-hydroxy-4H-pyran-4-one) were synthesized and their biological activities were investigated. Anticonvulsant activity results according to phase-I tests of Antiepileptic Drug Development (ADD) Program revealed that compound 13 was the most effective one at 4 h against subcutaneous pentylenetetrazole (scPTZ)-induced seizure test. Antimicrobial activities were evaluated in vitro against bacteria and fungi by using broth microdilution method. The antitubercular activities against Mycobacterium tuberculosis and M. avium were discussed with Resazurin microplate assay (REMA). The antimicrobial activity results indicated that compounds 1 and 12 (MIC: 8–16 µg/mL) showed higher activity against Gram negative bacteria while compound 12 had MIC: 4–16 µg/mL against Gram positive bacteria. Compound 1 was the most active one with MIC values of 8–32 µg/mL against fungi. Mannich bases also exhibit significant antitubercular activity in a MIC range of 4 to 32 µg/mL, especially compound 18 against M. avium.
Introduction
Kojic acid (5-hydroxy-2-hydroxymethyl-4H-pyran-4-one), is a biologically important fungal metabolite, produced by many species of Aspergillus, Acetobacter and Penicillium. Because of providing a promising skeleton for development of both new and more potent derivatives, it has been used by many researchers as a starting material in preparation of new compoundsCitation1–11. Chlorokojic acid (2-chloromethyl-5-hydroxy-4H-pyran-4-one), which is synthesized by chlorination of kojic acid exhibits distinct antibacterial and antifungal activitiesCitation3–5. Moreover, these hydroxypyrone derivatives are known as effective chelating agents forming complexes with metalsCitation5,Citation12,Citation13 and this ability plays a significant role in antimicrobial activityCitation14. Kojic acid and its derivatives also display a variety of biological activities, such as antiepilepticCitation4,Citation6–9, modest anti-inflammatory agentCitation5, food additiveCitation15, antioxidant or antibrowning agentCitation16, skin-lightening product in cosmetics as a result of inhibition of melanin productionCitation17, herbicidalCitation18, anti-speckCitation19, pesticide and insecticideCitation20, antitumor activityCitation21 and anti-diabetic agentCitation22.
Epilepsy, one of the more common neurological disorders, affects a large section of people. Since available marketed antiepileptic drugs possess the risk of tolerance development, dose-related toxicity, and idiosyncratic side effects, the search for a new agent is still a popular investigation area of medicinal chemists worldwideCitation23. In the literature many compounds bearing hydroxypyrone ring such as kojic acid, kojic amine, maltol and etil maltol have been reported as anticonvulsant agentsCitation24–27. The lipid solubility of a drug is an important factor in connection with its transfer into the central nervous system. The increase of anticonvulsant effect in Mannich bases of kojic acid and allomaltol (5-hydroxy-2-methyl-4H-pyran-4-one) is attributed to increase of lipophilicityCitation4,Citation8,Citation9.
Tuberculosis (TB), one of the earliest recorded human diseases and a chronic bacterial infection, continues to be an important global one with mortality worldwide killing more than 2 million people a yearCitation28,Citation29. Mycobacterium tuberculosis, which causes TB, infects approximately one-third of the world’s population and is comparable only to human immunodeficiency virus (HIV) as an infectious cause of deathCitation30. Also, Mycobacterium avium complex (MAC) is the most common cause of human infection due to nontuberculous mycobacteria. MAC is not only an opportunistic pathogen in acquired immunodeficiency syndrome (AIDS) patients but also cause of progressive pulmonary disease even in immunocompetent humansCitation31. Clinical management of patients with M. avium infections is difficult, even with macrolides such as clarithromycin and azithromycin as first-line drugs in multidrug regimensCitation32. The prevalence of multi-drug resistant and extensively drug resistant tuberculosis (MDR- and XDR-TB) strains is reported to be high in the countries where adequate supplies of the drugs are not available. The frightening TB is due to use and misuse of existing antibiotics and poor compliance with the long duration of current chemotherapy. The current trends suggest that TB will be among the 10 leading causes of global disease burden in the year 2020Citation33. Thus, there is an urgent need for development of new antitubercular agents with improved properties such as enhanced activity against MDR and XDR strains, effectiveness against latent TB, shortened duration of therapy, reduced toxicity and rapid mycobactericidal mechanism of actionCitation34.
Although pharmaceutical industries have produced a number of new antibiotics in the last three decades, resistance by microorganisms has increased. Especially, widespread use of antibiotics has led many bacteria to evolve resistance to multiple versions of drugs such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and XDR-TB. Generally, bacteria have the genetic ability to transmit and acquire resistance to drugs, which are utilized as therapeutic agents and the concern is mainly on the number of patients in hospitals who have suppressed immunity, and due to new bacterial strains, which are multi-resistantCitation35.
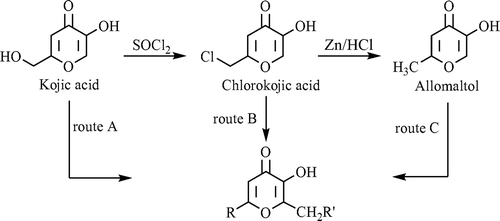
In our laboratory, a large number of Mannich bases of kojic acid (route A), chlorokojic acid (route B), and allomaltol (route C) were prepared () for their various types of biological activitiesCitation4,Citation6–11. Most of these compounds seemed to be promising candidates for anticonvulsant, antimicrobial and antiviral agents. Mannich bases of kojic acid and allomaltol which contain lipophilic aryl portions were synthesized by our research group in order to increase the penetration to the blood-brain barrierCitation4,Citation6–9. These compounds demonstrated significant anticonvulsant activities in vivo against maximal electroshock (MES)- and subcutaneous pentylenetetrazole (scPTZ)-induced seizure tests. Also, in our recent studies Mannich bases of chlorokojic acid (route B) exhibited good antimicrobial and antiviral activitiesCitation10–11.
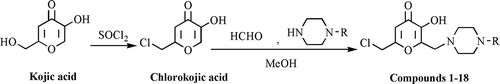
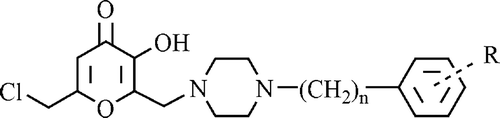
Therefore in present work we planned to synthesize eighteen novel 6-chloromethyl-3-hydroxy-2-substituted 4H-pyran-4-one derivatives including piperazine ring and evaluate for their anticonvulsant and antimicrobial couplet with antitubercular activities ().
Adding a phenyl ring or equivalent hydrocarbon substitute, and a carbonyl or another electronegative group adjacent to the phenyl ring were expected to increase lipophilicity and biological effect as succeeded before.
Materials-methods
Chemistry
All chemicals used for the synthesis of the compounds were supplied by Merck (Darmstadt, Germany) and Aldrich Chemical Co. (Steinheim, Germany). Melting points were determined by a Thomas Hoover Capillary Melting Point Apparatus (Philadelphia, PA, USA) and were uncorrected. IR spectra were recorded on a Perkin Elmer FT-IR-420 System, Spectrum BX spectrometer. 1H-NMR and 13C-NMR spectra were obtained with a Varian Mercury 400 MHz spectrophotometer in deutorochloroform (CDCl3) and dimethylsulphoxyde (DMSO-d6). Tetramethylsilane (TMS) was used as an internal standard (chemical shift in δ, ppm). Mass spectral analysis was carried out with a Micromass ZQ LC-MS with Masslynx Software Version 4.1 by using electrospray ionization (ESI+) method. The elemental analyses were performed with a Elementar Analysensysteme GmbH varioMICRO CHNS at The Scientific & Technological Research Council of Turkey-Ankara Testing and Analyses Laboratory (TUBİTAK-ATAL). For the compounds 9 and 17 elementary analysis were performed on Ankara University, Faculty of Pharmacy, Central Laboratory on CHNS-932 (LECO). The purity of the compounds was assessed by thin layer chromatography (TLC) on Kieselgel 60 F254 (Merck, Darmstadt, Germany) chromatoplates.
Chlorokojic acid was synthesized as described in previous studiesCitation8–11. Yield 76%, m.p.: 166-167 °C.
Preparation of Mannich bases of chlorokojic acid derivatives (1–18)
The secondary amine (substituted piperazine derivatives) and 37% formaline were dissolved in MeOH. Chlorokojic acid was added to the solution and the mixture was stirred vigorously for 15 to 25 min. The resulting precipitate was collected by filtration and washed with cold MeOH. All crude products recrystallized from appropriate solvent.
6-(Chloromethyl)-3-hydroxy-2-[(4-phenylpiperazin-1-yl)methyl]-4H-pyran-4-one (1)
IR ν (cm−1): 1622 (C=O), 1455 (C=C), 1201 (C-O); 757 (C-Cl); 1H-NMR (DMSO-d6, 400 MHz) δ ppm: 2.62 (4H; t; J = 4.8; piperazine-H2′, H6′), 3.13 (4H; t; J = 4.8; piperazine-H3′,H5′), 3.63 (2H; s; -CH2-), 4.67 (2H; s; ClCH2-), 6.56 (1H; s; H5), 6.76 (1H; t; J= 7.2; Ar-H4′’), 6.91 (2H; d; J= 8.0; H2′’ and Ar-H6′’), 7.19 (2H; t; J= 7.8; H3′’ and Ar-H5′’), 9.24 (1H; brs; -OH); 13C-NMR (DEPT) (CDCl3, 400 MHz) δ ppm: 173.60, 112.08, 41.02, 49.13, 52.95, 55.26, 161.69, 150.99, 145.81, 129.17, 116.25, 120.09, 144.16; 13C-NMR (APT) (CDCl3, 400 MHz) δ ppm: −55.26, −52.95, −49.12, −41.22; ESI-MS (m/z): 195 (100%), 335 (M++H), 337 (M++H+2), 357 (M++Na).
6-(Chloromethyl)-3-hydroxy-2-[(4-o-tolylpiperazin-1-yl)methyl]-4H-pyran-4-one (2)
IR ν (cm−1): 1622 (C=O), 1453 (C=C), 1196 (C-O); 761 (C-Cl); Citation1H-NMR (CDCl3, 400 MHz) δ ppm: 2.22 (3H; s; -CH3), 2.65 (4H; brs; piperazine-H2′, H6′), 2.84 (4H; t; J= 4.4; piperazine-H3′, H5′), 3.65 (2H; s; -CH2-), 4.67 (2H; s; ClCH2-), 6.57 (1H; s; H5), 6.9-7.10 (4H; m; Ar-H); 13C-NMR (DMSO-d6, 400 MHz) δ ppm: 17.43, 41.35, 51.19; 52.75; 53.39; 112.37; 118.64; 122.71; 126.40, 130.68, 131.62, 144.02, 147.59, 151.09, 161.13, 173.37; ESI-MS (m/z): 349 (M++H), 351 (M++H+2), 371 (100%, M++Na).
6-(Chloromethyl)-3-hydroxy-2-[(4-p-tolylpiperazin-1-yl)methyl]-4H-pyran-4-one (3)
IR ν (cm−1): 1634 (C=O), 1456 (C=C), 1196 (C-O); 749 (C-Cl); Citation1H-NMR (CDCl3, 400 MHz) δ ppm: 2.19 (3H; s; -CH3), 2.61 (4H; t; J= 4.8; piperazine-H2′, H6′), 3.06 (4H; t; J= 5.0; piperazine-H3′, H5′), 3.62 (2H; s; -CH2-), 4.67 (2H; s; ClCH2-), 6.56 (1H; s; H5), 6.81 (2H; d; J= 8.4; Ar-H2′’, Ar-H6′’) 7.01 (2H; d; J= 8.8; Ar-H3′’, Ar-H5′’), 9.24 (1H; brs; -OH); 13C-NMR (DMSO-d6, 400 MHz) δ ppm: 19.93, 41.33, 48.57, 52.24, 53.32, 112.34, 115.58, 127.53, 129.22, 144.02, 147.46, 148.79, 161.14, 173.37; ESI-MS (m/z): 349 (M++H), 351 (M++H+2), 371 (M++Na, 100%).
6-(Chloromethyl)-2-{[4-(2,3-dimethylphenyl)piperazin-1-yl]methyl}-3-hydroxy-4H-pyran-4-one (4)
IR ν (cm−1): 1621 (C=O), 1454 (C=C), 1197 (C-O); 747 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.13 (3H; s; -CH3), 2.19 (3H; s; -CH3), 2.64 (4H; brs; piperazine-H2′, H6′), 2.79 (4H; t; J= 4.4; piperazine-H3′, H5′), 3.64 (2H; s; -CH2-), 4.68 (2H; s; ClCH2-), 6.56 (1H; s; H5), 6.8–7.0 (3H; m; Ar-H); ESI-MS (m/z): 363 (M++H), 365 (M++H+2), 385 (M++Na, %100).
2-{[4-(4-Acetylphenyl)piperazin-1-yl]methyl}-6-(chloromethyl)-3-hydroxy-4H-pyran-4-one (5)
IR ν (cm−1): 1622 (C=O), 1454 (C=C), 1199 (C-O); 747 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.44 (3H; s; CH3CO-), 2.61 (4H; t; J= 5.0; piperazine-H2′, H6′), 3.33 (4H; t; J= 4.8; piperazine-H3′, H5′), 3.63 (2H; s; -CH2-), 4.66 (2H; s; ClCH2-), 6.56 (1H; s; H5), 6.95 (2H; d; J= 9.6; Ar-H2′’,H6′’) 7.79 (2H; d; J= 9.2; Ar-H3′’, H5′’); ESI-MS (m/z): 377 (M++H), 379 (M++H+2), 399 (M++Na, 100%).
6-(Chloromethyl)-3-hydroxy-2-{[4-(4-nitrophenyl)piperazin-1-yl]methyl}-4H-pyran-4-one (6)
IR ν (cm−1): 1630 (C=O), 1458 (C=C), 1201 (C-O); 753 (C-Cl); Citation1H-NMR (CDCl3, 400 MHz) δ ppm: 2.48 (4H; t; J= 1.8; piperazine-H2′, H6′), 2.57 (4H; t; J= 4.8; piperazine-H3′, H5′), 3.61 (2H; s; -CH2-), 4.65 (2H; s; ClCH2-), 6.54 (1H; s; H5), 7.01 (2H; d; J= 9.6; Ar-H2′’, H6′’), 8.02 (2H; d; J= 9.2; Ar-H3′’, H5′’), 9.34 (1H; brs; -OH); 13C-NMR (DMSO-d6, 400 MHz) δ ppm: 42.09, 46.97, 52.51, 53.87, 113.12, 113.33, 126.35, 137.56, 144.83, 148.03, 155.35, 161.93, 174.15; ESI-MS (m/z): 325 (100%), 380 (M++H), 382 (M++H+2), 402 (M++Na).
6-(Chloromethyl)-2-[(4-cyclohexylpiperazin-1-yl)methyl]-3-hydroxy-4H-pyran-4-one (7)
IR ν (cm−1): 1630 (C=O), 1450 (C=C), 1197 (C-O); 740 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.06–1.71 (5H; m; cyclohexane-H); 1.54–1.71 (5H; m; cyclohexane-H); 2.17 (1H; m; cyclohexane-H); 2.46 (8H; brs; piperazine-H), 3.53 (2H; s; -CH2-), 4.65 (2H; s; ClCH2-), 6.53 (1H; s; H5); ESI-MS (m/z): 341 (M++H, 100%), 343 (M++H+2), 363 (M++Na).
2-[(4-Benzylpiperazin-1-yl)methyl]-6-(chloromethyl)-3-hydroxy-4H-pyran-4-one (8)
IR (cm−1): 1627 (C=O), 1452 (C=C), 1200 (C-O); 748 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.37 (4H; brs; piperazine-H2′, H6′), 2.49 (4H; brs; piperazine-H3′, H5′), 3.44 (2H; s; -CH2-), 3.55 (2H; s; -CH2-Ar), 4.65 (2H; s; ClCH2-), 6.54 (1H; s; H5), 7.2–7.3 (5H; m; Ar-H); ESI-MS (m/z): 349 (M++H, 100%), 351 (M++H+2), 371 (M++Na).
6-(Chloromethyl)-3-hydroxy-2-{[4-(2-methylbenzyl)piperazin-1-yl]methyl}-4H-pyran-4-one (9)
IR ν (cm−1): 1622 (C=O), 1455 (C=C), 1197 (C-O); 746 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.29 (3H; s; -CH3); 2.38 (4H; brs; piperazine-H2′, H6′), 2.47 (4H; t; J= 1.8; piperazine-H3′, H5′), 3.40 (2H; s; -CH2-), 3.55 (2H; s; -CH2-Ar), 4.65 (2H; s; ClCH2-), 6.54 (1H; s; H5), 7.10–7.20 (4H; m; Ar-H); 13C-NMR (DEPT) (CDCl3, 400 MHz) δ ppm: 173.85, 112.74, 41.43, 53.05, 53.39, 56.12, 161.45, 146.01, 137.75, 130.51, 125.72, 136.32, 130.02, 127.35, 144.55, 60.80, 19.46; 13C-NMR (APT) (CDCl3, 400 MHz) δ ppm: −60.80, −56.12, −53.39, −53.05, −41.43; ESI-MS (m/z): 363 (M++H), 365 (M++H+2), 385 (M++Na, 100%).
6-(Chloromethyl)-3-hydroxy-2-{[4-(3-methylbenzyl)piperazin-1-yl]methyl}-4H-pyran-4-one (10)
IR ν (cm−1): 1624 (C=O), 1456 (C=C), 1197 (C-O); 747 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.28 (3H; s; -CH3); 2.36-2.50 (8H; m; piperazine-H), 3.40 (2H; s; -CH2-), 3.56 (2H; s; -CH2-Ar), 4.63 (2H; s; ClCH2-), 6.52 (1H; s; H5), 7.03–7.19 (4H; m; Ar-H); ESI-MS (m/z): 363 (M++H, 100%), 365 (M++H+2), 385 (M++Na).
6-(Chloromethyl)-3-hydroxy-2-({4-[3-(trifluoromethyl)benzyl]piperazin-1-yl}methyl)-4H-pyran-4-one (11)
IR ν (cm−1): 1623 (C=O), 1455 (C=C), 1197 (C-O); 749 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.51 (8H; brs; piperazine-H), 3.56 (2H; s; -CH2-), 3.57 (2H; s; -CH2-Ar), 4.65 (2H; s; ClCH2-), 6.54 (1H; s; H5), 7.53–7.61 (4H; m; Ar-H); ESI-MS (m/z): 417 (M++H, 100%), 419 (M++H+2), 439 (M++Na).
6-(Chloromethyl)-3-hydroxy-2-({4-[4-(trifluoromethyl)benzyl]piperazin-1-yl}methyl)-4H-pyran-4-one (12)
IR ν (cm−1): 1623 (C=O), 1457 (C=C), 1198 (C-O); 749 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.40 (4H; brs; piperazine-H2′, H6′), 2.50 (4H; t; J= 8.8; piperazine-H3′, H5′), 3.55 (2H; s; -CH2-), 3.57 (2H; s; -CH2-Ar), 4.66 (2H; s; ClCH2-), 6.55 (1H; s; H5), 7.51 (2H; d; J= 7.6; Ar-H2′’, H6′’) 7.66 (2H; d; J= 8.0; Ar-H3′’, H5′’); ESI-MS (m/z): 417 (M++H, 100%), 419 (M++H+2), 439 (M++Na).
6-(Chloromethyl)-2-{[4-(2,5-difluorobenzyl)piperazin-1-yl]methyl}-3-hydroxy-4H-pyran-4-one (13)
IR ν (cm−1): 1627 (C=O), 1460 (C=C), 1052 (C-O); 732 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.38 (4H; brs; piperazine-H2′, H6′), 2.47 (4H; t; J= 1.8; piperazine-H3′, H5′), 3.40 (2H; s; -CH2-), 3.55 (2H; s; -CH2-Ar), 4.65 (2H; s; ClCH2-), 6.54 (1H; s; H5), 7.10–7.20 (3H; m; Ar-H); ESI-MS (m/z): 363 (M++H), 365 (M++H+2), 385 (M++Na, 100%).
2-{[4-(4-Chlorobenzyl)piperazin-1-yl]methyl}-6-(chloromethyl)-3-hydroxy-4H-pyran-4-one (14)
IR ν (cm−1): 1621 (C=O), 1453 (C=C), 1196 (C-O); 746 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.37 (4H; brs; piperazine-H2′, H6′), 2.50 (4H; brs; piperazine-H3′, H5′), 3.44 (2H; s; -CH2-), 3.56 (2H; s; -CH2-Ar), 4.65 (2H; s; ClCH2-), 6.54 (1H; s; H5), 7.29 (2H; d; J= 8.8; Ar-H2′’, H6′’); 7.35 (2H; d; J = 8.4; Ar-H3′’, H5′’), 9.18 (1H; brs; -OH); 13C-NMR (DMSO-d6, 400 MHz) δ ppm: 41.31, 52.26, 53.32, 60.87, 112.32, 128.00, 130.42, 131.27, 137.17, 143.93, 147.56, 161.06, 173.33; ESI-MS (m/z): 383 (M++H, 100%), 385 (M++H+2, % 66.07), 405 (M++Na).
6-(Chloromethyl)-2-{[4-(2,6-dichlorobenzyl)piperazin-1-yl]methyl}-3-hydroxy-4H-pyran-4-one (15)
IR ν (cm−1): 1620 (C=O), 1454 (C=C), 1196 (C-O); 766 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.49 (8H; brs; piperazine-H), 3.53 (2H; s; -CH2-), 3.54 (2H; s; -CH2-Ar), 4.64 (2H; s; ClCH2-), 6.53 (1H; s; H5), 7.32 (1H; t; J= 8.0; Ar-H4′’), 7.44 (2H; d; J= 7.6; Ar-H3′’, H5′’); ESI-MS (m/z): 325 (100%), 419 (M++H,), 421 (M++H+2), 441 (M++Na).
6-(Chloromethyl)-2-{[4-(2,4-dichlorobenzyl)piperazin-1-yl]methyl}-3-hydroxy-4H-pyran-4-one (16)
IR ν (cm−1): 1626 (C=O), 1454 (C=C), 1199 (C-O); 738 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 2.50 (8H; brs; piperazine-H), 3.53 (2H; s; -CH2-), 3.57 (2H; s; -CH2-Ar), 4.65 (2H; s; ClCH2-), 6.54 (1H; s; H5), 7.38-7.55 (3H; m; Ar-H), 9.20-9.40 (1H; brs; -OH); 13C-NMR (DMSO-d6, 400 MHz) δ ppm: 42.09, 52.10, 54.08, 58.52, 113.11, 127.87, 129.31, 132.69, 134.74, 135.51, 144.74, 148.30, 161.85, 174.12; ESI-MS (m/z): 363 (M++H), 365 (M++H+2), 385 (M++Na, 100%).
6-(Chloromethyl)-2-{[4-(cyclohexylmethyl)piperazin-1-yl]methyl}-3-hydroxy-4H-pyran-4-one (17)
IR ν (cm−1): 1622 (C=O), 1456 (C=C), 1200 (C-O); 742 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 0.78–1.70 (11H; m; cyclohexane-H), 2.04 (2H; d; J= 7.6; -CH2-N=), 2.31 (4H; brs; piperazine-H2′, H6′), 2.46 (4H; brs; piperazine-H3′, H5′), 3.54 (2H; s; -CH2-), 4.65 (2H; s; ClCH2-), 6.53 (1H; s; H5); ESI-MS (m/z): 355 (M++H, 100%), 357 (M++H+2), 377 (M++Na).
6-(Chloromethyl)-2-{[4-(cyclohexanecarbonyl)piperazin-1-yl]methyl}-3-hydroxy-4H-pyran-4-one (18)
IR ν (cm−1): 1638 (C=O), 1447 (C=C), 1216 (C-O); 738 (C-Cl); 1H-NMR (CDCl3, 400 MHz) δ ppm: 1.28–1.70 (8H; m; cyclohexane-H), 2.40–2.46 (3H; m; cyclohexane-H), 2.51 (4H; brs; piperazine-H2′, H6′), 3.45 (4H; brs; piperazine-H3′, H5′), 3.60 (2H; s; -CH2-), 4.65 (2H; s; ClCH2-), 6.55 (1H; s; H5), 9.21 (1H; brs; -OH); 13C-NMR (DMSO-d6, 400 MHz) δ ppm: 25.80, 26.25, 29.80, 41.59, 42.08, 45.42, 52.83, 53.50, 53.89, 113.11, 144.79, 148.08, 161.92, 174.03, 174.14; ESI-MS (m/z): 325 (100%), 326, 369 (M++H), 371 (M++H+2), 391 (M++Na).
Anticonvulsant activity
The compounds were tested for their anticonvulsant activity against maximal electroshock (MES)- and subcutaneous pentylenetetrazol (scPTZ)-induced seizure threshold tests. The acute neurological toxicity was determined in the rotorod test. All these tests were performed in male mice according to the phase-I tests of the Antiepileptic Drug Development (ADD) program which were developed by National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS). Stimulator (Grass S88, Astro-Med. Inc. Grass Instrument Division, W. Warwick, RI, USA), constant current unit (Grass CCU1A, Grass Medical Instrument, Quincy, Mass., USA), and corneal electrodes were used for the evaluation of anticonvulsant activity against MES-induced seizure test. All synthesized compounds were suspended in 30% aqueous of PEG 400 and administered to the mice intraperitoneally in a volume of 0.01 mL/g at body weight. Twelve Swiss albino male mice (20 ± 2 g) were used for each compound. Mice were purchased from the Hacettepe University Animal Farm according to the ADD-NINDS programCitation36. All the animals were acclimatized for a week before use. The animals were maintained in colony cages under a 12 h-light-and-12 h-dark cycle and kept under standard (hygienic) conditions at an ambient temperature of 22 ± 3°C and at a relative humidity between 50 to 60% and fed on standard laboratory diet and food and water was provided ad libitum except at the time they were brought out of the cage. All the experimental protocols were carried out with the permission from Hacettepe University, ‘Laboratory Animals Ethic Committee’ decision (02. 01. 2009 date 2008/ 80-4 number). Control animals received 30% aqueous PEG 400. Pentyleneterazol was administered subcutaneously (s.c.) on the back of the neck. The rotorod toxicity test was performed on a 1 inch diameter knurled wooden rod, rotating at 6 rpm (the rotorod used in phase-I test was made by Hacettepe University Technical Department). MES tests were elicited with a 60-cycle alternating current of 50 mA intensity (5–7 times more than that required to elicit minimal seizures) delivered for 0.2 sec via corneal electrodes. A drop of 0.9% saline was instilled into the eye prior to application of the electrodes in order to prevent the death of the animal. Abolition of the hind limb tonic extension component of the seizure was defined as protection. 85 mg/kg of pentyleneterazol (produces seizures in more than 95% of mice) was administered as a 0.5% solution s.c. into the posterior midline. The animal was observed for 30 min to decide whether the failure of the threshold seizure (a single episode of clonic spasms of at least 5 sec duration) could be defined as protection. The rotorod test was used to evaluate neurotoxicity. The animal was placed on a 1 inch diameter knurled wooden rod rotating at 6 rpm. Normal mice remain on a rod rotating at this speed indefinitely. Neurologic toxicity was defined as the failure of the animal to remain on the rod for 1 min.
Antitubercular activity
The strains Mycobacterium tuberculosis H37Rv (ATCC 27294; American Type Culture Collection) reference strain and M. avium (ATCC 15769) were maintained on Lowenstein–Jensen medium and subcultured on Middlebrook 7H11 agar (Becton Dickinson) resuspended in 7H9-S broth medium supplemented with 10% [OADC; 0.1% casitone, 0.5% glycerol, supplemented oleic acid, albumin, dextrose, and catalase], 0.2% glycerol and 0.1% Bacto casitone (Difco). Suspensions were prepared in 0.04% (vol/vol) Tween 80–0.2%+bovine serum albumin so that adjusted to McFarland tube number 1. This was diluted to 1:20 and 100 μl aliquot was used as inoculum. Reference antibacterial agents of were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and dissolved in dimethylsulphoxide (streptomycin), or in d-water (isoniazid, ethambutol). The stock solutions of the agents were prepared in medium according to the Clinical and Laboratory Standards Institute (CLSI M38-A2, formerly NCCLS)Citation37,Citation38. A stock solution of the resazurin sodium salt (Sigma) powder was prepared at 0.01% in sterile distilled water. It was filters-sterilized and kept at 4°C. One hundred microliters of Middlebrook 7H9 broth (0.1% casitone, 0.5% glycerol, and 10% OADC; Becton-Dickinson) was dispensed in each well of a sterile flat-bottom 96-well plate, and serial twofold dilutions (256–0.06 µg/mL) of each compound were prepared directly in the plate. One hundred microliters of inoculum was added to each well. A growth control and a sterile control were also included for each stain. The plate was covered, and incubated at 37°C under a normal atmosphere. After 7 days of incubation, 10 µg/mL of resazurin solution was added to each well, and the plate was reincubated overnight. A change in colour from blue (oxidized state) to pink (reduced) indicated the growth of bacteria, and the minimum inhibitory concentration (MIC) was defined as the lowest concentration of drug that prevented this change in color.
Antibacterial and antifungal activities
The compounds of 1–18 were dissolved in dimethylsulphoxide:ethanol (80:20) and sterilized by filtration using 0.22 µm Millipore (MA 01730, USA) and used as the stock solutions. Reference antibacterial agents were purchased from Sigma Chemical Co. (St. Louis, MO, USA) and dissolved in phosphate buffer solution (ampicillin, pH 8.0; 0.1 mol/mL), dimethylsulphoxide (ketoconazole), or in water (gentamicin, levofloxacin, fluconazole)Citation10,Citation11. Antibacterial activity test were carried out against standards; Gram negative standard strains of Escherichia coli ATCC 35218, Pseudomonas aeruginosa ATCC 10145, Proteus mirabilis ATCC 7002, Klebsiella pneumoniae RSKK 574, Acinetobacter baumannii RSKK 02026, and as Gram positive standard strains of Staphylococcus aureus ATCC 25923, Enterococcus faecalis ATCC 29212, Bacillus subtilis ATCC 6633 and their drug resistant isolates were used for the determination of antibacterial activity. Candida albicans ATCC 10231, C. parapsilosis ATCC 22019, C. tropicalis ATCC 13803 and C. krusei ATCC 6258 were used for the determination of antifungal activity. Mueller-Hinton Broth (MHB; Difco) and Mueller-Hinton Agar (MHA; Oxoid) were applied for growing and diluting of the bacteria suspensionsCitation39. The synthetic medium RPMI-1640 with L-glutamine was buffered to pH 7 with 3-[N-morpholino]-propansulfonic acid and culture suspensions were prepared as described previouslyCitation40. The broth microdilution method was employed for antibacterial and antifungal activity tests. Media were placed into each 96 wells of the microplates. Extract solutions at 512 μg/mL were added into first rows of microplates and twofold dilutions of the compounds (256–0.125 μg/mL) were made by dispensing the solutions to the remaining wells. The lowest concentration of the compounds that completely inhibit macroscopic growth was determined and MICs were reported as described previously studyCitation37,Citation38,Citation40.
Results and discussion
Chemistry
Treatment of kojic acid with thionyl chloride at room temperature yielded chlorokojic acid which after reduction with zinc and hydrochloric acid yielded allomaltol (). These three compounds provided basis for our research area. Mannich bases of several hydroxypyranones including kojic acid and pyromeconic acid (3-hydroxy-4H-pyran-4-one) were synthesized before by different researchersCitation41,Citation42. They react with amines and formaline like phenols to produce the Mannich base as a result of aminoalkylation of the ortho position of the −OH group.
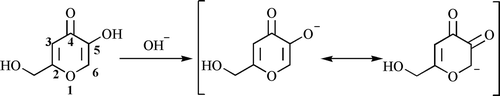
In an earlier study, it was reported that di-Mannich derivatives were obtained in an acidic medium from the reaction of kojic acid, formaline and aromatic amineCitation43. On the other hand, although there were two open nuclear positions of kojic acid (3- and 6-), because of its phenol-like properties, the reaction occurred only at 6-position and mono-Mannich derivatives were obtained in basic medium (). When the enolic hydroxyl group converted to an ether group, 6-position was deactivatedCitation41. The mechanisms of this Mannich reaction both in an acidic and a basic medium were investigated and found that, enhanced reactivity in a basic medium is due to increase of the electronegativity at 6-positionCitation42.
It is well known that chlorokojic acid is an important compound from the chemical point of view and as the chlorine atom in the structure readily undergoes nucleophilic substitutionCitation44, it has been used as starting or intermediate material in many reactions2–4,6–11.
General synthesis of compounds is given in . Chlorokojic acid was gained by commercially available kojic acid as described methods from the published literatureCitation8–11. In order to obtain chlorokojic acid; 2-hydroxymethyl moiety of kojic acid was chlorinated by using thionyl chloride at room temperature.
Mannich type reactions are three component condensation reactions involving carbonyl compounds which exist as keto-enol tautomeric forms, formaline and a secondary amine. The amino alkylation of aromatic substrates by Mannich reaction is of considerable importance for the design, synthesis and modification of biologically active molecules. It has advantages ranging from lower reaction times, increased reaction rates to higher yields and reproducibilityCitation45. In order to investigate the influence of secondary amines moieties such as piperidine, morpholine and piperazine in the structure of Mannich bases, all of them are used in our previous studies ()Citation4,Citation6–11. Herein the basic substituent was introduced at the 6-position of chlorokojic acid via a Mannich type reaction using formaline and an appropriate substituted piperazine.
The physicochemical parameters of the synthesized compounds including melting point, yield, calculated logP (clogP) and elemental analysis data are presented in . clogP refers to calculated hydrophobicity of the compounds, respectively, clogP have been calculated from ACD/ChemSketch, Product version 12.01. The thumb rules for clogP values, to a drug like molecule must be lower than “5” to by-pass the cell barrier.
Table 1. Physicochemical parameters of the synthesized compounds.
The structure of the compounds were clarified by IR, 1H-NMR and mass spectroscopy in experimental part. Also, with a view to analyse the structures of compounds 1, 6, 9, 14, 16 and 18 13C-NMR spectroscopy was used. The selected diagnostic bands of IR spectra of chlorokojic acid derivatives provide useful information for determining structures. All the compounds exhibited absorbtion bands about 1620 cm−1 due to ν (C=O) streching of pyranone ring. Because of hydroxymethyl moiety showing two hydrogen bondings both intra- and intermolecular, (C=O) streching gave signals at lower frequency. IR spectra of all compounds showed ν (C−Cl);at 732-766 cm−1, ν (C−O) at 1200-1195 cm−1 and ν (C = C) at 1447-1460 cm−1. The formation of the Mannich bases of chlorokojic acid was further confirmed with the 1H-NMR spectra. Assignments of the signals were based on the chemical shifts and intensity pattern. The 1H-NMR spectra of the compounds exhibited triplet peaks for -CH2- at piperazine between 2.2 and 3.6 ppm. J values of these peaks were from 4.4 to 5.0 Hz. Phenyl ring’s protons exhibite signals at 7.6 to 9.2 ppm. Compounds 7, 17 and 18 which had cyclic protons in their structure instead of phenyl ring, showed peaks ranging 0.78 to 2.17 ppm as multiplets. Characteristic H5 proton of the 4H-pyran-4-one ring were determined as singlet peaks between 6.52 and 6.57 ppm. Also, due to keto-enol tautomerisation of hydroxyl group on pyranone ring −OH peaks of compounds 2, 4, 5, 7, 9, 10, 11, 12 and 13 were not observed. 13C NMR spectra analysis were supported by DEPT and APT spectra which differentiate between −CH=, -CH2- and -CH3 groups. The 13C NMR signals of compounds 1, 6, 9, 14, 16 and 18 were in good agreement with proposed structures. Compounds displayed characteristic peaks of methylene group (ClCH2-) carbons at 41.02, 42.09, 41.43 41.31, 42.09 and 42.08 ppm, respectively. Carbonyl carbons of the 4H-pyran-4-one ring was found at a range of 161.06-161.93 ppm. Signals at 150.99-146.01 ppm were due to C3 of the pyranone ring whereas 112.08-113.12 ppm belong to C5. The distinctive signals of all compounds were observed in the mass spectra which followed the similar fragmentation pattern. The entire spectrums showed molecular ion peaks, M++23 (Na) peaks and isotope peaks owing to chlorine atom.
Anticonvulsant activity
Seizures that are arising from discharging lesions of the cerebral cortex often occur as part of an epileptic syndrome. It is a group of signs and symptoms that customarily occur together. Identification of the syndrome helps to determine the appropriate therapy and the prognosis. The MES-induced seizure test is a predictor of compounds that are active against tonic-clonic (grand mal) seizures. The scPTZ-induced seizure test is used to detect compounds useful in treating generalized absence (petit mal) seizuresCitation36.
According to our previous studies, when route A and C () were examined, it was seen that substituted phenylpiperazine derivatives bearing 3-trifluoromethyl, 4-fluoro, 2-methoxy, 2-chloro and 4-chloro moieties were the most protective compounds in the Mannich series against convulsions at all doses. Also, kojic acid and allomaltol derivatives that carry hydroxypiperidine moiety were significantly more protective against scPTZ and MES tests at all dosesCitation9 than other piperidine derivativesCitation6,Citation7.
Anticonvulsant activity tests were performed in male mice according to the phase-I tests of the ADD program which were developed by NIH and NINDSCitation36. Herein, the anticonvulsant activities of the synthesized compounds were evaluated by MES and scPTZ-induced seizure tests performed at 0.5 and 4 h after administration with 30, 100 and 300 mg/kg doses using male Swiss albino mice (20 ± 2 g). The acute neurological toxicity was determined in the rotorod test. The results are presented in .
Table 2. Anticonvulsant and neurotoxicity screening data of the synthesized compounds.
Chlorokojic acid was not found protective against scPTZ-induced seizure test; however, some series of chlorokojic acid achieved notable activity when their Mannich bases were examined. Compounds 12 and 14 showed protection at both 300 mg/kg and 100 mg/kg doses at 4 h. It may be speculated that this is probably because of increment in lipophilicity. Also, when halogen substitution to benzyl ring is examined, at all doses, 2,5-difluorobenzyl (compound 13) derivative was determined as the most active compound by scPTZ-induced seizure test at 4 h, but in contrast when 3,4-dichlorobenzyl derivative (compound 16) was tested instead of fluoro atom, scPTZ protection was failed. However this time, selective MES protection was observed at 300 and 100 mg/kg. None of the compounds 4, 5, 7, 10, 15, 17 and 18 showed anticonvulsant activity.
With respect to the results of the MES tests, chlorokojic acid showed protection at the 300 mg/kg dose at both at 0.5 and 4 h. The augmentation of this activity is aimed by preparing Mannich bases. Moreover, compounds 9 and 16 bearing 2-methylbenzyl and 2,4-dichlorobenzyl moiety, respectively, were found as the most effective molecules with selective protection at 100 and 300 mg/kg doses at 4 h in this series. Against the same test, compounds 1, 6 and 13 exhibited activity at the 300 mg/kg. At the same dose, compounds 2, 3, 6, 8 and 12 also showed protection against scPTZ-induced seizure test. None of the compounds showed neurotoxicity at any of the studied doses.
When the effect of aromaticity was examined, it was seen that, with the reduction of phenyl in compound 1 and benzyl group in compound 8 to change into cyclohexyl (compound 7) and cyclohexylmethyl (compound 17), respectively, their anticonvulsant activities disappeared. Also, compound 18 bearing cyclohexylcarbonyl was not protective. Finally, anticonvulsant activities of Mannich bases were increased in some compounds while some were decreased when compared to chlorokojic acid. When the results of this study were compared to our previous studiesCitation4,Citation8,Citation9 the expected increment in anticonvulsant activity of Mannich bases could not be observed. That shows us chlorokojic acid derivatives of Mannich bases have lower biological activities than kojic acid and allomaltol derivatives.
Antitubercular activity
The antitubercular activity of the compounds was performed as MICs against M. tuberculosis and M. avium by using Resazurin microplate assay procedure (REMA). Isoniazid, ethambutol and streptomycin were used as reference compounds ().
Table 3. Screening for antimicrobial activity against Gram positive bacteria and Mycobacterium (MIC in µg/mL).
Mannich bases of allomaltol derivatives including 3-methyl, 4-methyl and 3,5-dimethyl piperidine and 2-methoxyphenyl piperazine containing piperazine and piperidine structure which were firstly synthesized by our research group were found to have antimycobacterial effect against Mycobacterium smegmatisCitation46,Citation47. However the main cause of TB is M. tuberculosis. It divides extremely slower than the other bacteria but can be cultured in vitro whereas the others can only grow within the cells of a hostCitation48. M. avium is a part of an nontuberculous mycobacteria group which causes pulmonery diseases resembling TB. Herein, the in vitro antitubercular activity of the compounds against M. tuberculosis and M. avium was evaluated and MIC values are demonstrated in . All of the Mannich bases have antitubercular activity and have shown promising in vitro antitubercular activity against M. tuberculosis in a MIC range of 8-32 µg/mL. Among the entire series the most effective one was compound 3 carrying 4-methylphenyl piperazine structure against M. tuberculosis (MIC: 8 µg/mL). The antitubercular activity against M. avium was generally stronger with MIC values of 4-32 µg/mL. Compound 18 bearing carboxyphenyl piperazine moiety showed the highest antitubercular activity (MIC: 4 µg/mL) against M. avium and comparable results to reference drug ethambutol and stretomycin (MIC: 2 µg/mL). Generally, compounds showed greater inhibition activity over M. avium growth than M. tuberculosis whereas compounds 3 and 8-10 had the same MIC values against both microorganisms. The existing antimycobacterial activity of chlorokojic acid was improved by synthesizing their Mannich bases at only compounds 3 and 18. However when compared to reference drugs (isoniazid, ethambutol, streptomycin) antimycobacterial activity were not strong as expected and the scaffold should be developed in order to obtain more active compounds.
Antibacterial and antifungal activity
The newly synthesized compounds (1–18) were tested for their in vitro antibacterial and antifungal activities according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI)Citation10,Citation11. For antibacterial activity assessment, standard strains (Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, Acinetobacter baumannii, Staphylococcus aureus, Enterococcus faecalis and Bacillus subtilis) and their drug-resistant isolates were tested; and for antifungal activity Candida albicans and C. parapsilosis, C. tropicalis and C. krusei were used.
Ampicillin, gentamicin, levofloxacin for antibacterial, ketoconazole and fluconazole for antifungal assays were also tested under identical conditions. The results are demonstrated in and expressed as MIC values in comparison to reference drugs and chlorokojic acid.
Table 4. Screening for antimicrobial activity against Gram negative bacteria (MIC in µg/mL).
Table 5. Screening for antifungal activity of the compounds (MIC in µg/mL).
Some of the important antifungal agents (e.g. posaconazole, ketoconazole, itraconazole) that are being used for the treatment of fungal infections and antibiotics such as newly marketed linezolid, contain a piperazine and/or an azole ring in their structuresCitation11. Since the compounds we synthesized previously have remarkable antimicrobial activities, we aimed to assess the contribution of the substituents on the piperazine structure to the biological activity.
According to our previous studiesCitation10,Citation11, 6-(chloromethyl)-3-hydroxy-2-[(3,4-dichlorobenzylpiperazin-1-yl)methyl]-4H-pyran-4-one were significantly more active than 3,4-dichlorophenyl derivative. However, in the compound containing fluorine atom, no meaningful changes were observed in activity (). Our efforts for the synthesis of Mannich bases possessing antimicrobial activity led us to find out that compounds bearing chlorine atom were effective molecules. Also, to discover the effect of methylene linkage in compounds, we synthesized both the phenyl and the benzyl derivatives of the same substituentsCitation10,Citation11. In the light of these facts, we examined the activities of designed Mannich bases bearing benzyl piperazine.
The synthesized compounds showed a broad spectrum of antibacterial activity against Gram negative and Gram positive standard strains with MIC values between 4 and 64 μg/mL ( and ). In the meantime, the compounds showed activity against drug-resistant isolated both Gram positive and negative strains with MIC values of 16 to 128 μg/mL.
As observed for Gram negative bacteria, compounds 1 and 11, bearing phenyl piperazine and 3-trifluoromethylbenzyl moieties respectively, were the most active compounds with the same MIC values (8–16 µg/mL). Both of them were four-folds more active than chlorokojic acid against E. coli. In this case, against the other Gram negative bacteria, activity increased two-folds. Structural modifications were not effective on activity, because the other compounds (compounds 2–11; 12–18) had the same MIC values with chlorokojic acid (16–32 µg/mL).
As seen in , compound 12 having 4-trifluoromethylbenzyl in its structure was determined to have significantly high antibacterial potential against standard strains of Staphylococcus aureus and Bacillus subtilis with an inhibition between 4 and 8 µg/mL. In comparison of compound 12 with chlorokojic acid (MIC: 8-32 µg/mL) against both of mentioned bacteria, its activity increased four and two times, respectively. Moreover, compounds 1-12 and 13 also showed higher activity with MIC value 16 µg/mL than chlorokojic acid (MIC: 32 µg/mL) whereas compounds 14-16 and 18 had no difference in activity with chlorokojic acid. The least efficiency of the compounds among Gram positive bacteria was seen on Enterococcus facealis with a concentration of 32-64 µg/mL except compound 13 (MIC: 16 µg/mL). Among the series of compounds (14–16) bearing chlorobenzylpiperazine the expected increment, like in 3,4-dichloro derivatives (), was not observed in antimicrobial activity. Thus, it could be understood that within benzyl series the location of chlorine substituents affects the activity. As references ampicillin, gentamicin, and levofloxacin the tested compounds bearing slight activity against tested standard and their drug resistant isolates (E. coli, P. aeruginosa,. P. mirabilis,.K. pneumoniae, A. baumannii, S. aureus, E. faecalis, B. subtilis).
According to the obtained data (), compounds 1–3, having phenyl, 2- and 4-methylphenyl in their structures respectively, possessed significant antifungal activity against C. krusei with MIC value of 32 µg/mL, even more active than the reference drug, fluconazole and chlorokojic acid, while compounds 4–18 had the same (MIC: 64 µg/mL). The MIC values of compounds 1 and 12 were 8 µg/mL with showing the highest activity against C. albicans in this series. Also, compounds 3–11, 14 and 16 showed more remarkable antifungal activity with MIC value of 16 µg/mL than chlorokojic acid (MIC: 32 µg/mL). Compounds 1-3 inhibited the growth of C. tropicalis two-folds (MIC: 32 µg/mL) than chlorokojic acid and compounds 11, 15 and 16 (MIC: 64 µg/mL). Compared with reverences all tested compounds shows weak antifungal activity against Candida species (C. albicans, C. parapsilosis, C. tropicalis) except from C. krusei with a MIC values of fluconazole 64 µg/mL.
Conclusion
In present study, Mannich bases of chlorokojic acid were synthesized and screened for their biological activities. Compounds were designed in such a way that the pyranone nucleus was substituted with different piperazine derivatives containing phenyl, benzyl or cyclohexyl groups. The existing anticonvulsant activity of chlorokojic acid, which was active against MES-induced seizure test, was aimed to increase by preparing more lipophilic agents as Mannich derivatives. The results of anticonvulsant evaluation revealed that compound 13, bearing 2,4-difluorobenzyl moiety showed the highest protection against scPTZ-induced seizures. When antimicrobial activity was evaluated, compounds 1 and 11, bearing phenyl piperazine and 3-trifluoromethylbenzyl moieties respectively, were found as the most active compounds with MIC values of 8-16 µg/mL against Gram negative bacteria. Compound 12, carrying 4-trifluoromethylbenzyl group, was significantly effective among the synthesized compounds and chlorokojic acid against Gram positive bacteria. According to the antifungal activity results, the existing activity of chlorokojic acid increased, especially compound 1 was established as the most effective molecule against Candida spp. Beside this, compound 18 has been identified as a promising lead molecule for antimycobacterial activity with MIC value that is comparable to reference drugs. The endpoint in evaluation all of activity in piperazine containing Mannich bases of chlorokojic acid in this study is, these compounds have lower biological activities unexpectedly. However these compounds would represent a productive matrix for the development of new biologically important agents and deserve further investigation.
Acknowledgement
We would like to thank Professor Erhan Palaska for his help with the mass spectra analysis. The Ankara University Central Laboratory, Faculty of Pharmacy provided support for acquisition of the 1H and 13C NMR spectrometer and CHNS-932 (LECO) used in this work.
Declaration of interest
This project was supported as a M.Sc. thesis by Hacettepe University Scientific Research Fund (Project no. 09D01301002-4756). The authors state that there is no conflict of interest.
References
- Uher M, Konecny V, Rajniakova O. Synthesis of 5-Hydroxy-2-hydroxymethyl-4H-pyran-4-one derivatives with pesticide activity. Chem Pap 1994;48:282–284.
- Aytemir MD, Hider RC, Erol DD, Özalp M, Ekizoğlu M. Synthesis of new antimicrobial agents; amide derivatives of pyranones and pyrodinones. Turkish J Chem 2003;27:445–452.
- Aytemir MD, Erol DD, Hider RC, Özalp M. Synthesis and evaluation of antimicrobial activity of new 3-hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxamide derivatives. Turkish J Chem 2003;27:757–764.
- Aytemir MD, Calis U, Ozalp M. Synthesis and evaluation of anticonvulsant and antimicrobial activities of 3-hydroxy-6-methyl-2-substituted 4H-pyran-4-one derivatives. Arch Pharm (Weinheim) 2004;337:281–288.
- Brtko J, Rondahl L, Ficková M, Hudecová D, Eybl V, Uher M. Kojic acid and its derivatives: history and present state of art. Cent Eur J Public Health 2004;12 Suppl:S16–S18.
- Aytemir MD, Çalış Ü. Synthesis of some new hyroxypyranone derivatives and evaluation of their anticonvulsant activities. FABAD 2006;31:23–29.
- Aytemir MD, Çalış Ü. Synthesis of some novel Mannich bases derived from allomaltol, and evaluation of their anticonvulsant activities. H U J Fac Pharm 2007;27:1–10.
- Aytemir MD, Septioglu E, Calis U. Synthesis and anticonvulsant activity of new kojic acid derivatives. Arzneimittelforschung 2010;60:22–29.
- Aytemir MD, Calis U. Anticonvulsant and neurotoxicity evaluation of some novel kojic acids and allomaltol derivatives. Arch Pharm (Weinheim) 2010;343:173–181.
- Aytemir MD, Özçelik B. A study of cytotoxicity of novel chlorokojic acid derivatives with their antimicrobial and antiviral activities. Eur J Med Chem 2010;45:4089–4095.
- Aytemir MD, Özçelik B. Synthesis and biological activities of new mannich bases of chlorokojic acid derivatives. Med Chem Res 2011;20:443–452.
- Kasser JH, Kandioller W, Hartinger CG, Nazarov AA, Arion VB, Dyson PJ, Keppler BK. Mannich products of kojic acid and N-heterocycles and their Ru(II)–arene complexes: Synthesis, characterization and stability. J Organomet Chem 2010;695:875–881.
- Hryniewicz K, Stadnicka K, Adamski A, Pattek-Janczyk A. Crystal structure and the magnetic properties of tris(2-chloromethyl-4-oxo-4H-pyran-5-olato-κ2O5,O4)iron(III). J Coord Chem 2010;63:977.
- Weinberg ED. The mutual effects of antimicrobial compounds and metallic cations. Bacteriol Rev 1957;21:46–68.
- Burdock GA, Soni MG, Carabin IG. Evaluation of health aspects of kojic acid in food. Regul Toxicol Pharmacol 2001;33:80–101.
- Wang L. Role of epicardial QT intervals in the assessment of ventricular repolarisation dispersion. Gen Physiol Biophys 2003;22:213–220.
- Synytsya A, Blafková P, Synytsya A, Čopíková J, Spěváček J, Uher M. Conjugation of kojic acid with chitosan. Carbohydr Polym 2008;72:21–31.
- Veverka M, Kralovicova E. Synthesis of some biologically active derivatives of 2-hydroxymethyl-5-hydroxy-4H-pyran-4-one. Collect Czech Chem Commun 1990;55:833–840.
- Uchino K, Nagawa M, Tanasaki Y, Oda M, Fukuchi A. Kojic acid as an anti-speck agent. Agric Biol Chem 1988;52:2609–2610.
- Alverson J. Effects of mycotoxins, kojic acid and oxalic acid, on biological fitness of Lygus hesperus (Heteroptera: Miridae). J Invertebr Pathol 2003;83:60–62.
- Higa Y, Kawabe M, Nabae K, Toda Y, Kitamoto S, Hara T et al. Kojic acid -absence of tumor-initiating activity in rat liver, and of carcinogenic and photo-genotoxic potential in mouse skin. J Toxicol Sci 2007;32:143–159.
- Xiong X, Pirrung MC. Modular synthesis of candidate indole-based insulin mimics by Claisen rearrangement. Org Lett 2008;10:1151–1154.
- Alam O, Mullick P, Verma SP, Gilani SJ, Khan SA, Siddiqui N et al. Synthesis, anticonvulsant and toxicity screening of newer pyrimidine semicarbazone derivatives. Eur J Med Chem 2010;45:2467–2472.
- Aoyagi N, Kimura R, Murata T. Studies on passiflora incarnata dry extract. I. Isolation of maltol and pharmacological action of maltol and ethyl maltol. Chem Pharm Bull 1974;22:1008–1013.
- Kimura R, Matsui S, Ito S, Aimoto T, Murata T. Central depressant effects of maltol analogs in mice. Chem Pharm Bull 1980;28:2570–2579.
- Ferkany JW, Andree TH, Clarke DE, Enna SJ. Neurochemical effects of kojic amine, a GABAmimetic and its interaction with benzylamine oxidase. Neuropharmacology 1981;20:1177–1182.
- Martin GE, Bendesky RJ. Further evidence for a GABA-like action on kojic amine. Neurosci Lett 1981;27:37–40.
- Gupta UD, Katoch VM. Animal models of tuberculosis. Tuberculosis (Edinb) 2005;85:277–293.
- Lew JM, Kapopoulou A, Jones LM, Cole ST. TubercuList–10 years after. Tuberculosis (Edinb) 2011;91:1–7.
- Khoshneviszadeh M, Edraki N, Javidnia K, Alborzi A, Pourabbas B, Mardaneh J et al. Synthesis and biological evaluation of some new 1,4-dihydropyridines containing different ester substitute and diethyl carbamoyl group as anti-tubercular agents. Bioorg Med Chem 2009;17:1579–1586.
- Tateishi Y, Hirayama Y, Ozeki Y, Nishiuchi Y, Yoshimura M, Kang J et al. Virulence of Mycobacterium avium complex strains isolated from immunocompetent patients. Microb Pathog 2009;46:6–12.
- Sano C, Tatano Y, Shimizu T, Yamabe S, Sato K, Tomioka H. Comparative in vitro and in vivo antimicrobial activities of sitafloxacin, gatifloxacin and moxifloxacin against Mycobacterium avium. Int J Antimicrob Agents 2011;37:296–301.
- Kamal A, Shetti RV, Azeeza S, Swapna P, Khan MN, Khan IA et al. Anti-tubercular agents. Part 6: synthesis and antimycobacterial activity of novel arylsulfonamido conjugated oxazolidinones. Eur J Med Chem 2011;46:893–900.
- Saquib M, Husain I, Sharma S, Yadav G, Singh VK, Sharma SK et al. 2,3-dideoxy hex-2-enopyranosid-4-uloses as promising new anti-tubercular agents: design, synthesis, biological evaluation and SAR studies. Eur J Med Chem 2011;46:2217–2223.
- Nascimento GGF, Locatelli J, Freitas PC, Silva GL. Antibacterial activity of plant extracts and phytochemicals on antibiotic resistant bacteria. Braz J Microbiol 2000;31:247–256.
- Krall RL, Penry JK, White BG, Kupferberg HJ, Swinyard EA. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978;19:409–428.
- CLSI (formerly NCCLS), approved stand. M27-A, 15, 10, NCCLS, VA Med. Center, Tuscon, 1996.
- CLSI (formerly NCCLS), NCCLS document M100-S12, 940 West Valley Road, Wayne, Pennsylvania 19087, 2002.
- Özçelik B, Orhan I, Toker G. Antiviral assessment againts Herpes simplex virus and Parainfluenza-3 virus in Vero cell line and Madin-Darby bovine kidney Cells and Antimicrobial activity of some flavonoids. Z Naturforsch 2006;61c:632–638.
- Ozçelik B, Gürbüz I, Karaoglu T, Yesilada E. Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Microbiol Res 2009;164:545–552.
- O’Brien G, Patterson JM, Meadow JR. Amino derivatives of kojic acid. J Org Chem 1960;25:86–89.
- Ichimoto I, Ueda H, Tatsumi C. Studies on kojic acid and its related γ-Pyrone compounds. Part VII. The alkylation of Kojic Acid and Pyromeconic Acid through their Mannich Base. Agric Biol Chem 1965;1:94–98.
- WOODS LL. A new derivative of kojic acid. J Am Chem Soc 1946;68:2116.
- Uher M, Szymonska J, Korenova A, Tomasik P. Re-examination of nucleophilic substitution in chlorokojic acid. Monatsh Chem 2000;131:301–307.
- Ashok M, Holla BS, Poojary B. Convenient one pot synthesis and antimicrobial evaluation of some new Mannich bases carrying 4-methylthiobenzyl moiety. Eur J Med Chem 2007;42:1095–1101.
- Us D, Berk B, Gürdal E, Aytekin N, Kocagöz T, Çağlayan B, Aksan Kurnaz I, Demir Erol D. Mannich base derivatives of 3-hydroxy-6-methyl-4H-pyran-4-one with antimicrobial activity. Turkish J Chem 2010;34:447–456.
- Us D, Gürdal E, Berk B, Öktem S, Kocagöz T, Çağlayan B, Aksan Kurnaz I, Demir Erol D. 4H-Pyran-4-one derivatives: leading molecule for preparation of compounds with antimycobacterial potential. Turkish J Chem 2009;33:803–812.
- Parish T, Stoker NG. Mycobacteria: bugs and bugbears (two steps forward and one step back). Mol Biotechnol 1999;13:191–200.
- American Thoracic Society. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med 1997;156 (2 Pt 2): S1–25.