Abstract
New series of quinazoline containing sulfonamide derivatives were prepared and screened for their antitumor activity. Four human cancer cell lines, namely, hepatoma cancer cell line (HepG2), breast cancer cell line (MCF-7), cervix cancer cell line (HeLa) and colon cancer cell line (HCT-8), were used to measure the cytotoxic activity. Compounds 8 and 21 exhibited remarkable antitumor activity almost similar to that of the standard drug (doxorubicin). Six compounds 16, 22, 23, 29, 30 and 33, showed considerable activity and few compounds were totally inactive.
Keywords::
Introduction
Tumor growth and metastasis depends on multiple factors, including the physiological process of angiogenesis. For example, elevated expression of vascular endothelial growth factor, epidermal growth factor, fibroblast growth factor and platelet derived growth factor are associated with tumor angiogenesis, metastases, survival and resistance to apoptosis. Therefore, these growth factors have represented potential molecular targets for inhibition of tumor growth and progressionCitation1–4. In addition, data accumulate that significant levels of cross talk exists within and between signalling networks in most tumours, necessitating the combined inhibition of multiple targets in order to establish efficient tumour growth inhibition. In that light, cell signalling network models indicate that partial inhibition of a number of targets is more effective than the complete inhibition of a single targetCitation5. Consequently, various approaches were pursued to inhibit multiple signalling pathways. The use of a combination of different compounds can introduce adverse effects related to pharmacokinetics, toxicity and patient compliance. Alternatively, the use of multi-targeted agents can circumvent most of the problems due to combination therapyCitation6.
Therefore, our interest was to develop a series of novel small molecules as potential antitumor agents that can block biologically relevant molecular targets. In this application, we prepared certain quinazoline containing sulfonamide pharmacophores and/or their isosteres. The quinazoline core has been shown to be a suitable carrier that allows for an effective DNA intercalation. In that context, several quinazoline derivatives have been shown to exhibit high affinity for receptor tyrosine kinases, as well as other oncogenic targetsCitation5,Citation6.
Many new sulfonamide derivatives, such as E7070 (indisulam), have shown substantial antitumor activity via different mechanismsCitation7–17. It was also reported to selectively accumulate within cancerous cells. Therefore, in this work, we synthesized somequinazoline containing sulfonamide derivatives and studied their activity against some selected human tumour cell lines.
Results and discussion
Chemistry
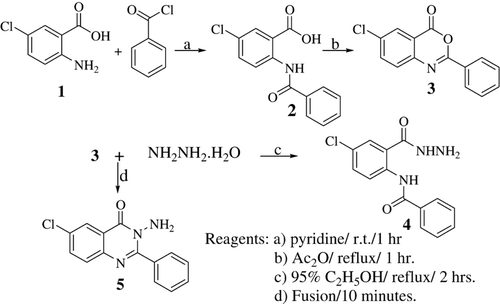
The synthetic strategies adopted for the synthesis of the intermediates and target compounds are depicted in . In , 5-chloroanthranilic acid was allowed to react with benzoyl chloride in pyridine to afford the amide derivative 2 that underwent dehydrative cyclization through boiling in acetic anhydride to afford the corresponding 3H-benzoxazin-4-one 3. Treating the latter compound with hydrazine hydrate in 95% ethanol afforded the diamide derivative 4. However, hydrazinolysis under the condition of fusion yielded the 3-amino derivative 5.
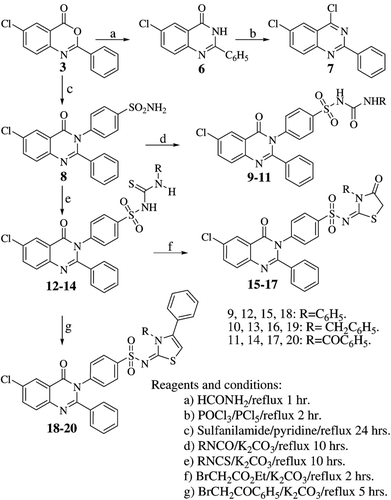
comprised the synthesis of 3H-quinazolin-4-one 6, which on chlorination using a mixture of phosphorus oxychloride and phosphorus pentachloride afforded the 4-chloroquinazoline derivative 7. The first target sulfonamide derivative 8 was obtained by allowing the 3H-benzoxazin-4-one derivative 3 to condense with sulfanilamide.
The substituted sulfonylureas 9–11 were successfully obtained by condensing the sulfonamide 8 with the appropriate isocyanate in the presence of anhydrous potassium carbonate as a mild basic catalyst. The IR spectra of compounds 9–11 were characterized by a urea carbonyl band in addition to that due to the quinazoline at around 1680 cm−1. Similarly, the sulfonylthiourea derivatives 12–14 were obtained by condensing compound 8 with the corresponding isothiocyanate. The IR spectra of these compounds revealed a characteristic C=S band at 1140 cm−1. The latter compounds, in turn, were further utilized for the preparation of some thiazole ring systems. Thus, cyclization of the sulfonylthioureido derivatives 12–14 with ethyl 2-bromoacetate in the presence of anhydrous sodium acetate afforded the corresponding thiazolidin-4-ones 15–17. The IR spectra of these compounds were characterized by two carbonyl bands, whereas, their 1H NMR spectra revealed a new singlet at around δ 4.0 ppm attributed to the oxothiazolidine C-5 two protons. Similarly, reacting the same derivatives 12–14 with phenacyl bromide under similar reaction conditions resulted in the formation of 1,3-thiazolinones 18–20. Their IR spectra showed one C=O band at around 1685 cm−1 and the 1H NMR spectra showed a new singlet at around δ 6.30 ppm due to the 1,3-thiazoline C-5 proton.
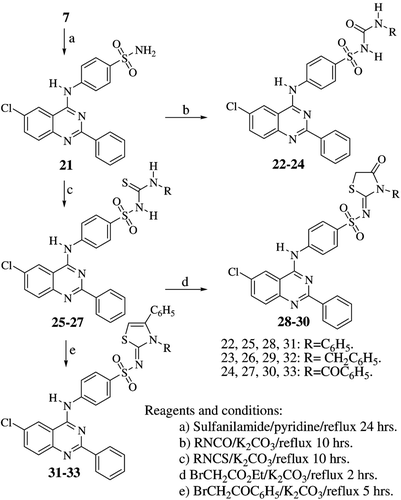
In the same vein, represents condensation of compound 7 with sulfanilamide to afford the corresponding N4-(2-phenyl-6-chloroquinazolin-4-yl)-sulfanilamide 21, which was also converted to the corresponding substituted sulfonylureas 22–24 and sulfonylthioureas 25–27 by condensation with the appropriate isocyanate or isothiocyanate. The thiourea derivatives were also condensed with ethyl 2-bromoacetate in the presence of anhydrous sodium acetate to afford the corresponding thiazolidin-4-ones 28–30. Similarly, the 1,3-thiazolines 31–33 were obtained by reacting derivatives 25–27 with phenacyl bromide under alkaline conditions.
Pharmacology
Based on the results assembled in , it can be generally seen that the quinazoline derivatives 19–33, with the benzene sulfonamide moiety at position 4, were more active than those derivatives 8–18 with that moiety at position 3 of the quinazoline core. Also, compounds 8 and 21 with free SO2NH2 group were more effective antitumor agents than their substituted derivatives. In addition, the urea derivatives proved to be more active than the thioisosteres and the 1,3-thiazolidinone derivatives 15–17 and 28–30 were more potent antitumor agents than theirthiazoline congeners 18–20 and 31–33.
Table 1. IC50 of synthesized compounds against cancer cell lines.
Concerning the spectrum of activity, two compounds 8 and 21 exhibited remarkable anti-proliferative activity. In our study, they showed activity comparable to that of the standard drug (with IC50 of 0.012 µM) against the four selected cell lines. Free sulfonamide group was a common feature in these two compounds; such moiety was reported to play a crucial role in CA inhibition and antitumor activityCitation7–15. Condensing the sulfonamide moiety with different isocyanates produced the disubstituted sulfonylureido derivatives 9–11 and 22–24 of which compounds 10, 22 and 23 exhibited good antitumor activity against the tested cell lines, but they were less active than the parent sulfonamide derivative; compound 16 displayed moderate antitumor activity against hepatoma cancer cell line and good activity against the other cell lines, compound 17 was active against human beast as well as colon cancer cell lines and moderate activity against human cervix cell line. However, compound 24 was active only against colon cancer cell line. On the other hand, converting the sulfonamide moiety to the substituted sulfonylthioureido derivatives, 12–14 and 25–27, led to inactive products. This observation may reflect the importance of the ureido functionality rather than the thioureido in modifying this type of compounds. Incorporating the thioureido moiety in a partly rigid structure resulted in four active compounds. Compounds 16 and 17 showed moderate activity, whereas compounds 29 and 30 retained remarkable activity comparable to that of the standard drug. Thus derivatization with isocyanate led to some reduction in the extent and spectrum of activity when compared to that of the parent compound.
Conclusion
The present work led to the development of novel hybrids of quinazoline containing sulfonamide pharmacophore that exhibited remarkable antitumor activities. Four tumour cell lines, human breast cancer cell line (MCF-7), human cervix cell line (HeLa), human hepatoma cell line (HepG2) and human colon cancer cell line (HCT-8), were used to measure cytotoxic activity of the proposed quinazoline sulfonamides. All the tested compounds showed varying degrees of activity against the four selected cell lines. Few compounds were ineffective against some of the tested cell lines. Two compounds, 8 and 21, exhibited antitumor activity comparable to that of the standard drug (doxorubicin) based on their easy synthesis. Six compounds, 16, 22, 23, 29, 30 and 33, showed considerable activity and some were totally inactive. Finally, the remarkable activity obtained from the present series of compounds is worthy of more interest and warrants further studies to reveal the exact mechanism of action of the active compounds and to design more active and selective antitumor agents.
Experimental protocols
Chemistry
Melting points (°C, uncorrected) were determined in open capillaries on a Gallenkamp melting point apparatus (Sanyo Gallenkamp, Southborough, UK) and were uncorrected. Infra red spectra were recorded in KBr discs using IR-470 Shimadzu spectrometer (Shimadzu, Tokyo, Japan). 1H NMR spectra were recorded on Bruker AC-300 Ultra Shield NMR spectrometer (Bruker, Flawil, Switzerland, δ ppm) at 300 MHz for 1H and 75 MHz for 13C, using tetramethylsilane as internal standard. Electron impact Mass Spectra were recorded on a Shimadzu GC-MS-QP 5000 instrument (Shimadzu, Tokyo, Japan). Elemental analyses were performed, on Carlo Erba 1108 Elemental Analyzer (Heraeus, Hanau, Germany), at the Microanalytical Unit, Faculty of Science, Cairo University, Cairo, Egypt, and the found results were within ±0.4% of the theoretical values.
Preparation of target compounds
Compounds 1–7 were prepared according to a reported procedureCitation16.
6-Chloro-3-(4-aminosulfonylphenyl)-2-phenyl-quinazolin-4-one 8
A mixture of the benzoxazone 3 (1.28 g, 0.005 mol) and sulfanilamide (0.96 g, 0.0056 mol) in dry pyridine was heated under reflux for 6 h. The solvent was removed under reduced pressure and the residue was triturated with 10% hydrochloric acid, the precipitated crude product was filtered, washed with water, dried and crystallized.
8:Yield: 65%, mp: 196–198°C (ethanol). IR (cm−1): 3318, 3231 (NH2), 1670 (C=O), 1352, 1160 (SO2).1H NMR (δ, ppm): 7.47–8.16 (m, 12H, Ar-H), 8.52 (s, 2H, NH2). 13C NMR (δ, ppm): 119.6, 120.5, 123.4, 125.9, 127.5, 128.4, 129.0, 130.2, 131.8, 133.7, 135.1, 136.3, 149.5 (Ar-C), 156.0, 165.4 (C=O). MS (EI): m/z 413 [M+ + 2, 13%], 411 [M+, 38%]. Anal. Calcd for C20H14ClN3O3S (411.86): C, 58.32; H, 3.43; N, 10.20; S, 7.79. Found: C, 58.49; H, 3.29; N, 9.98; S, 7.98.
1-Substituted-3-[6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl-(4-benzenesulfonyl)]urea 9–11
A mixture of the sulfonamide derivative 8 (2.06 g, 0.005 mol) and anhydrous potassium carbonate (2.8 g, 0.02 mol) in n-butanol (25 mL) was heated under reflux with the appropriate isocyanate (0.005 mol) for 10 h. The solvent was removed under reduced pressure, and the residue was triturated with 10% hydrochloric acid, the precipitated crude product was filtered, washed with water, dried and crystallized.
1-Phenyl-3-[(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-benzenesulfonyl]urea 9:Yield: 58%, mp: 136–138°C (ethanol). IR (cm−1): 3317, 3202 (2NH), 3099 (CH, arom.), 1687, 1655 (2C=O), 1317, 1154 (SO2).1H NMR (δ, ppm): 7.45–8.09 (m, 17H, Ar-H), 8.95 (s, 1H, NH exchangeable), 10.53 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 118.2, 120.6, 122.0, 124.1, 126.4, 128.0, 129.2, 130.5, 132.7, 133.9, 135.5, 137.5, 144.9, 157.0, 159.3 (Ar-C), 164.4, 166.2 (2C=O). MS (EI): m/z 533 [M+ + 2, 11%], 531 [M+, 31%]. Anal. Calcd for C27H19ClN4O4S (530.98): C, 61.07; H, 3.61; N, 10.55; S, 6.04. Found: C, 60.93; H, 3.40; N, 10.19; S, 5.79.
1-Benzyl-3-[(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-benzenesulfonyl]urea 10:Yield: 55%, mp: 143–145°C (ethanol). IR (cm−1): 3310, 3208 (2NH), 3072 (CH arom), 2986 (CH, aliph.), 1670, 1640 (2C=O), 1322, 1165 (SO2).1H NMR (δ, ppm): 4.25 (s, 2H, CH2), 7.45–8.09 (m, 17H, Ar-H), 8.89 (s, 1H, NH exchangeable), 9.95 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 49.8 (CH2), 120.0, 121.5, 123.1, 124.7, 126.0, 127.1, 128.2, 129.1, 130.2, 132.5, 133.8, 135.2, 136.4, 137.5, 141.5, 146.5, 156.2 (Ar-C), 161.5, 164.3 (2C=O). MS (EI): m/z 547 [M+ + 2, 4%], 545 [M+, 14%]. Anal. Calcd for C28H21ClN4O4S (545.01): C, 61.71; H, 3.88, N, 10.28; S, 5.88. Found: C, 62.03; H, 4.09; N, 9.95; S, 6.19.
1-Benzoyl-3-[(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-benzenesulfonyl]urea 11:Yield: 60%, mp: 159–161°C (dioxane). IR (cm−1): 3307, 3222 (2NH), 3075 (CH, arom.), 1682, 1670, 1658 (3C=O), 1304, 1157 (SO2).1H NMR (δ, ppm): 7.45–8.09 (m, 17H, Ar-H), 9.94 (s, 1H, NH exchangeable), 10.58 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 118.3, 120.6, 122.5, 124.7, 126.3, 128.1, 129.0, 130.4, 132.4, 134.1, 135.6, 138.0, 145.0 (Ar-C), 155.3, 158.7, 161.5 (3C=O), 163.8 (C=N). MS (EI): m/z 561 [M+ + 2, 2.7%], 559 [M+, 8.5%]. Anal. Calcd for C28H19ClN4O5S (558.99): C, 60.16; H, 3.43; N, 10.02; S, 5.74. Found: C, 59.86; H, 3.72; N, 9.70; S, 5.46.
1-Substituted-3-[(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-benzene-sulfonyl]thiourea 12–14
To a solution of the appropriate isothiocyanate (0.011 mol) in dry acetone (5 mL) was added a mixture of the sulfonamide 8 (2.06 g, 0.005 mol) and anhydrous potassium carbonate (2.8 g, 0.02 mol) in dry acetone (25 mL), the reaction mixture was heated under reflux for 10 h. The solvent was removed under reduced pressure and the residue was triturated in 10% hydrochloric acid. The obtained crude product was filtered, washed with water, dried and crystallized.
1-Phenyl-3-[(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-benzene-sulfonyl]thiourea 12:Yield: 68%, mp: 130–132°C (dioxane). IR (cm−1): 3327, 3212 (2NH), 3092 (CH, arom.), 1681 (C=O), 1315, 1149 (SO2), 1147 (C=S). 1H NMR (δ, ppm): 7.43–8.12 (m, 17H, Ar-H), 8.93 (s, 1H, NH exchangeable), 10.70 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 119.9, 120.2, 121.6, 123.0, 125.3, 127.5, 128.3, 129.9, 132.6, 134.0, 135.6, 137.8, 145.3, 156.7 (Ar-C), 163.8 (C=O), 174.0 (C=S). MS (EI): m/z 549 [M+ + 2, 12.4%], 547 [M+, 37.5%]. Anal. Calcd for C27H19ClN4O3S2 (547.05): C, 59.28; H, 3.50; N, 10.24; S, 11.72. Found: C, 58.93; H, 3.74; N, 9.92; S, 12.01.
1-Benzyl-3-[(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-benzene-sulfonyl]thiourea 13:Yield: 65%, mp: 139–141°C (ethanol). IR (cm−1): 3351, 3200 (2NH), 3087 (CH arom.), 2969 (CH, aliph.), 1682 (C=O), 1308, 1230 (SO2), 1136 (C=S). 1H NMR (δ, ppm): 4.39 (s, 2H, CH2), 7.20–7.95 (m, 17H, Ar-H), 8.37 (s, 1H, NH exchangeable), 10.06 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 48.3 (CH2), 121.1, 122.4, 125.6, 126.8, 127.4, 129.0, 130.4, 132.5, 133.9, 135.9, 140.6, 149.2 (Ar-C), 159.5 (C=O), 165.2 (C=N), 176.3 (C=S). MS (EI): m/z 563 [M+ + 2, 19.6%], 561 [M+, 60.9%]. Anal. Calcd for C28H21ClN4O3S2 (561.07): C, 59.94; H, 3.77; N, 9.99; S, 11.43. Found: C, 60.28; H, 3.46; N, 10.25; S, 11.60.
1-Benzoyl-3-[(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-4-benzene-sulfonyl]thiourea 14:Yield: 68%, mp: 153–155°C (dioxane). IR (cm−1): 3321, 3199 (2NH), 3085 (CH, arom.), 1667, 1649 (2C=O), 1349, 1225 (SO2), 1134 (C=S). 1H NMR (δ, ppm): 7.35–8.11 (m, 17H, Ar-H), 8.90 (s, 1H, NH exchangeable), 10.56 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 120.6, 121.5, 122.4, 124.6, 126.1, 127.4, 129.0, 130.5, 132.8, 134.3, 135.5, 138.0, 144.9, 154.9(Ar-C), 163.0, 165.5 (C=O), 178.0 C=S). MS (EI): m/z 577 [M+ + 2, 8.5%], 575 [M+, 26.2%]. Anal. Calcd for C28H19ClN4O4S2 (575.06): C, 58.48; H, 3.33; N, 9.74; S, 11.15. Found: C, 58.14; H, 3.05; N, 9.43; S, 10.81.
2-[4-(6-Chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-phenylsulfonylimino]-3-substituted-thiazolidin-4-one 15–17
To a solution of the appropriate sulfonylthioureido derivatives 12–14 (0.005 mol) in n-butanol (20 mL), ethyl bromoacetate (1 g, 0.006 mol) and anhydrous sodium acetate (0.82 g, 0.01 mol) were added and the reaction mixture was heated under reflux for 2 h. The mixture was then poured into ice-cold water (30 mL), and the solid product thus formed was filtered, washed with water, dried and crystallized.
2-[4-(6-Chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-phenylsulfonylimino]-3-phenyl-thiazolidin-4-one 15:Yield: 66%, mp: 155–157°C (ethanol/toluene; 1:1). IR (cm−1): 3088 (CH arom.), 2985 (CH aliph.), 1687, 1654 (2C=O), 1328, 1234 (SO2). 1H NMR (δ, ppm): 4.05 (s, 2H, thiazol-CH2), 7.34–7.95 (m, 17H, Ar-H). 13C NMR (δ, ppm): 31.0 (thiazol-CH2), 119.8, 120.0, 121.4, 122.7, 124.6, 126.8, 127.9, 129.5, 131.7, 133.8, 136.3, 137.9, 146.3, 158.0 (Ar-C), 163.9, 164.7 (2C=O). MS (EI): m/z 589 [M+ + 2, 3.6%], 587 [M+, 11.0%]. Anal. Calcd for C29H19ClN4O4S2 (587.07): C, 59.33; H, 3.26; N, 9.54; S, 10.92. Found: C, 59.70; H, 3.51; N, 9.26; S, 11.28.
2-[4-(6-Chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-phenylsulfonylimino]-3-benzyl-thiazolidin-4-one 16:Yield: 64%, mp: 163–165°C (ethanol/toluene; 1:1). IR (cm−1): 3088 (CH arom.), 2974 (CH aliph.), 1673, 1655 (2C=O), 1343, 1225 (SO2). 1H NMR (δ, ppm): 4.02 (s, 2H, thiazol-CH2), 4.35 (s, 2H, benzyl-CH2), 7.35–7.85 (m, 17H, Ar-H). 13C NMR (δ, ppm): 30.0 (thiazol-CH2), 38.5 (benzyl-CH2), 120.1, 121.3, 122.4, 123.1, 124.0, 126.5, 127.7, 129.5, 132.0, 133.9, 135.8, 138.2, 147.0 (Ar-C), 155.5, 167.1 (2C=O), 169.1 (C=N). MS (EI): m/z 603 [M+ + 2, 1.7%], 601 [M+, 5.0%]. Anal. Calcd for C30H21ClN4O4S2 (601.10): C, 59.94; H, 3.52; N, 9.32; S, 10.67. Found: C, 60.17; H, 3.80; N, 9.19; S, 10.98.
2-[4-(6-Chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-phenylsulfonylimino]-3-benzoyl-thiazolidin-4-one 17:Yield: 66%, mp: 170–172°C (ethanol/toluene; 1:1). IR (cm−1): 3088 (CH arom.), 2974 (CH aliph.), 1698, 1671, 1655 (3C=O), 1340, 1227 (SO2). 1H NMR (δ, ppm): 3.94 (s, 2H, thiazol-CH2), 7.34–7.87 (m, 17H, Ar-H). 13C NMR (δ, ppm): 30.0 (thiazol-C), 120.0, 121.2, 122.1, 123.0, 124.5, 126.1, 127.6, 130.5, 132.4, 134.0, 135.6, 138.3, 146.4, 158.6 (Ar-C), 164.2, 165.8, 169.5 (3C=O), 171.4 (C=N). MS (EI): m/z 617 [M+ + 2, 7.7%], 615 [M+, 24.0%]. Anal. Calcd for C30H19ClN4O5S2 (615.08): C, 58.58; H, 3.11; N, 9.11; S, 10.43. Found: C, 58.80; H, 2.82; N, 9.37; S, 10.71.
3-Substituted-4-phenyl-2-[4-(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-phenyl-sulfonylimino]-1,3-thiazoline 18–20
A solution of the appropriate sulfonyl-thioureido derivatives 12–14 (0.005 mol) in absolute ethanol (20 mL) was refluxed with phenacyl bromide (1.1 g, 0.0055 mol) and anhydrous sodium acetate (0.82 g, 0.01 mol) for 5 h during which the solid product was partially crystallized out. The mixture was left to cool to room temperature, filtered, washed with water, dried and crystallized.
3,4-Diphenyl-2-[4-(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-phenyl-sulfonylimino]-1,3-thiazoline 18:Yield: 66%, mp: 142–144°C (ethanol/toluene; 1:1). IR (cm−1): 3088 (CH arom.), 1687 (C=O), 1328, 1234 (SO2). 1H NMR (δ, ppm): 6.15 (s, 1H, thiazol-H), 7.34–7.95 (m, 22H, Ar-H). 13C NMR (δ, ppm): 107.5 (thiazol-C), 118.6.0, 120.0, 121.0, 122.2, 123.1, 124.4, 125.9, 127.4, 129.7, 131.9, 134.1, 135.8, 139.0, 146.7, 148.0, 152.4 (Ar-C), 165.8, (C=O). MS (EI): m/z 649 [M+ + 2, 5.5%], 647 [M+, 19.4%]. Anal. Calcd for C35H23ClN4O3S2 (647.17): C, 64.96; H, 3.58; N, 8.66; S, 9.91. Found: C, 65.30; H, 3.31; N, 8.25; S, 10.14.
3-Benzyl-4-phenyl-2-[4-(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-phenyl-sulfonylimino]-1,3-thiazoline 19:Yield: 67%, mp: 152–154°C (ethanol/toluene; 1:1). IR (cm−1): 3086 (CH arom.), 2980 (CH aliph.), 1679 (C=O), 1351, 1225 (SO2). 1H NMR (δ, ppm): 3.79 (s, 2H, benzyl-CH2), 6.24 (s, 1H, thiazol-H), 7.17–7.92 (m, 22H, Ar-H). 13C NMR (δ, ppm): 45.1 (benzyl-C), 110.0 (thiazol-C), 118.7.0, 119.8, 121.0, 122.2, 123.4, 124.6, 125.9, 127.2, 129.0, 131.8, 133.5, 135.9, 138.5, 147.7, 150.8, 152.0 (Ar-C), 164.9, (C=O). MS (EI): m/z 663 [M+ + 2, 11.5%], 661 [M+, 35.8%]. Anal. Calcd for C36H25ClN4O3S2 (661.19): C, 65.39; H, 3.81; N, 8.47; S, 9.70. Found: C, 65.25; H, 4.10; N, 8.34; S, 9.98.
3-Benzoyl-4-phenyl-2-[4-(6-chloro-4-oxo-2-phenylquinazolin-3(4H)-yl)-phenyl-sulfonylimino]-1,3-thiazoline 20:Yield: 62%, mp: 168–170°C (ethanol/toluene; 1:1). IR (cm−1): 3088 (CH arom.), 1684, 1655 (2C=O), 1354, 1220 (SO2). 1H NMR (δ, ppm): 4.01 (s, 2H, benzyl-CH2), 6.44 (s, 1H, thiazol-H), 7.13–7.88 (m, 22H, Ar-H). 13C NMR (δ, ppm): 111.8 (thiazol-C), 121.2, 122.4, 123.5, 126.1, 126.8, 127.2, 128.0, 128.8, 129.3, 130.2, 131.8, 132.5, 133.7, 134.7, 135.3, 137.0, 139.2, 143.9, 150.0, 151.2, 153.6 (Ar-C), 161.5, 164.5 (2C=O). MS (EI): m/z 677 [M+ + 2, 10.0%], 675 [M+, 32.5%]. Anal. Calcd for C36H23ClN4O4S2 (675.18): C, 64.04; H, 3.43; N, 8.30; S, 9.50. Found: C, 63.85; H, 3.73; N, 8.39; S, 9.72.
4-(6-Chloro-2-phenylquinazolin-4-yl-amino)-benzenesulfonamide 21
A mixture of compound 7 (0.825 g, 0.003 mol) and sulfanilamide (0.516 g, 0.003 mol) in acetone (12 mL) in presence of anhydrous potassium carbonate (0.5 g) was heated under reflux for 5 h. The solvent was then evaporated under vacuum and the separated solid was filtered, washed with water, dried and crystallized from ethanol.
21:Yield: 61%, mp: 180–182°C (ethanol). IR (cm−1): 3337, 3274, 3219 (NH, NH2), 3085 (CH arom.), 1352, 1160 (SO2).1H NMR (δ, ppm): 7.47–8.16 (m, 12H, Ar-H), 8.95 (s, 2H, NH2), 10.17 (s, 1H, NH). 13C NMR (δ, ppm): 115.2, 116.5, 118.9, 126.8, 128.3, 129.1, 130.1, 131.0, 131.7, 132.6, 133.3, 134.0, 146.2, 148.5, 155.4, 1593.0 (Ar-C). MS (EI): m/z 413 [M+ + 2, 14%], 411 [M+, 45%]. Anal. Calcd for C20H15ClN4O2S (410.88): C, 58.46; H, 3.68; N, 13.64; S, 7.80. Found: C, 58.69; H, 3.39; N, 13.79; S, 8.10.
1-Substituted-3-[4-(6-chloro-2-phenylquinazolin-4-yl)-aminophenylsulfonyl]urea 22–24
A mixture of the sulfonamide derivative 8 (2.06 g, 0.005 mol) and anhydrous potassium carbonate (2.8 g, 0.02 mol) in dry acetone (25 mL) was heated under reflux with the appropriate isocyanate (0.005 mol) for 24 h. The solvent was removed under reduced pressure and the residue was triturated with 10% hydrochloric acid, and then the precipitated crude product was filtered, washed with water, dried and crystallized.
1-Phenyl-3-[4-(6-chloro-2-phenylquinazolin-4-yl)-aminophenylsulfonyl]-urea 22:Yield: 58%, mp: 162–164°C (ethanol). IR (cm−1): 3320, 3281, 3209 (3NH), 3075 (CH, arom.), 1670 (C=O), 1315, 1151 (SO2). 1H NMR (δ, ppm): 7.13–8.50 (m, 17H, Ar-H), 8.74 (s, 1H, NH exchangeable), 9.88 (s, 1H, NH exchangeable), 10.52 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 117.1, 117.7, 118.5, 121.9, 123.6, 126.1, 127.8, 128.0, 129.0 129.1, 129.4, 129.8, 130.2, 130.8, 131.2, 133.3, 136.0, 144.5, 147.1, 155.4, 157.2, 158.6(Ar-C), 166.2 (C=O). MS (EI): m/z 532 [M+ + 2, 8.3%], 530 [M+, 25%]. Anal. Calcd for C27H20ClN5O3S (530.0): C, 61.19; H, 3.80; N, 13.21; S, 6.05. Found: C, 60.92; H, 3.45; N, 13.18; S, 6.20.
1-Benzyl-3-[4-(6-chloro-2-phenylquinazolin-4-yl)-aminophenylsulfonyl]urea 23:Yield: 57%, mp: 143–145°C (ethanol). IR (cm−1): 3310, 3238, 3200 (3NH), 3077 (CH arom.), 2965 (CH, aliph.), 1658 (C=O), 1321, 1160 (SO2).1H NMR (δ, ppm): 4.32 (s, 2H, CH2), 7.25–8.16 (m, 17H, Ar-H), 8.50 (s, 1H, NH exchangeable), 9.64 (s, 1H, NH exchangeable), 10.40 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 48.1 (CH2), 117.2, 117.8, 118.6, 121.5, 124.0, 126.3, 127.5, 128.2, 128.7, 129.0, 129.4, 129.8, 130.3, 130.9, 132.0, 133.5, 135.7, 142.8, 147.6, 154.8, 159.0 (Ar-C), 166.0 (C=O). MS (EI): m/z 546 [M+ + 2, 5.3%], 544 [M+, 14%]. Anal. Calcd for C28H22ClN5O3S (544.02): C, 61.82; H, 4.08; N, 12.87; S, 5.89 Found: C, 61.70; H, 3.92; N, 13.04; S, 5.65.
1-Benzoyl-3-[4-(6-chloro-2-phenylquinazolin-4-yl)-aminophenylsulfonyl]-urea 24:Yield: 60%, mp: 159–161°C (dioxane). IR (cm−1): 3315, 3222, 3180 (3NH), 3065 (CH, arom.), 1674, 1641 (2C=O), 1311, 1154 (SO2).1H NMR (δ, ppm): 7.42–8.13 (m, 17H, Ar-H), 8.55 (s, 1H, NH exchangeable), 9.90 (s, 1H, NH exchangeable), 10.46 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 117.1, 117.6, 119.8, 128.0, 128.8, 129.6, 130.0, 130.6, 131.1, 131.8, 132.5, 133.0, 133.6, 134.8, 146.5, 148.2, 156.3, 158.5 (Ar-C), 161.4, 165.0 (2C=O). MS (EI): m/z 560 [M+ + 2, 1.9%], 558 [M+, 5.9%]. Anal. Calcd for C28H20ClN5O4S (558.01): C, 60.27; H, 3.61; N, 12.55; S, 5.75. Found: C, 59.97; H, 3.82; N, 12.79; S, 5.93.
1-Substituted-3-[(6-chloro-2-phenylquinazolin-3yl)-4-benzenesulfonyl]thiourea 25–27
A mixture of the sulfonamide derivative 8 (2.06 g, 0.005 mol) and anhydrous potassium carbonate (2.8 g, 0.02 mol) in dry acetone (25 mL) was heated under reflux with the appropriate isothiocyanate (0.005 mol) for 24 h. The solvent was removed under reduced pressure and the residue was triturated with 10% hydrochloric acid, the precipitated crude product was filtered, washed with water, dried and crystallized.
1-Phenyl-3-[(6-chloro-2-phenylquinazolin-3yl)-4-benzenesulfonyl]thiourea 25:Yield: 58%, mp: 162–164°C (ethanol). IR (cm−1): 3312, 3275, 3205 (3NH), 3071 (CH, arom.), 1316, 1147 (SO2), 1125 (C=S). 1H NMR (δ, ppm): 7.18–8.21 (m, 17H, Ar-H), 8.93 (s, 1H, NH exchangeable), 9.80 (s, 1H, NH exchangeable), 10.15 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 117.0, 117.4, 118.5, 121.2, 123.4, 126.0, 127.2, 128.3, 128.7 129.1, 129.5, 129.8, 130.3, 130.8, 131.2, 133.5, 135.2, 144.2, 146.8, 152.4, 157.5 (Ar-C), 181.0 (C=S). MS (EI): m/z 548 [M+ + 2, 8.3%], 546 [M+, 25%]. Anal. Calcd for C27H20ClN5O2S2 (546.1): C, 59.39; H, 3.69; N, 12.83; S, 11.74. Found: C, 59.54; H, 3.95; N, 12.64; S,11.98.
1-Benzyl-3-[(6-chloro-2-phenylquinazolin-3yl)-4-benzenesulfonyl]thiourea 26:Yield: 55%, mp: 143–145°C (ethanol). IR (cm−1): 3325, 3251, 3201 (3NH), 3072 (CH arom.), 2963 (CH, aliph.), 1321, 1160 (SO2), 1119 (C=S). 1H NMR (δ, ppm): 4.20 (s, 2H, CH2), 7.21–8.17 (m, 17H, Ar-H), 8.85 (s, 1H, NH exchangeable), 9.96 (s, 1H, NH exchangeable), 10.45 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 48.3 (CH2), 117.1, 117.7, 118.5, 121.3, 123.5, 126.0, 127.2, 128.1, 128.5, 129.1, 129.5, 129.8, 130.3, 131.0, 131.9, 133.4, 135.5, 142.5, 147.0, 153.8, 157.4 (Ar-C), 161.9, 185.0 (C=S). MS (EI): m/z 562 [M+ + 2, 5.3%], 560 [M+, 14%]. Anal. Calcd for C28H22ClN5O2S2 (560.1): C, 60.04; H, 3.96; N, 12.50; S, 11.45. Found: C, 59.77; H, 3.70; N, 12.25; S, 11.19.
1-Benzoyl-3-[(6-chloro-2-phenylquinazolin-3yl)-4-benzenesulfonyl]thiourea 27:Yield: 60%, mp: 159–161°C (dioxane). IR (cm−1): 3317, 3240, 3185 (3NH), 3071 (CH, arom.), 1674 (C=O), 1314, 1156 (SO2), 1120 (C=S). 1H NMR (δ, ppm): 7.42–8.36 (m, 17H, Ar-H), 8.84 (s, 1H, NH exchangeable), 9.91 (s, 1H, NH exchangeable), 10.50 (s, 1H, NH exchangeable). 13C NMR (δ, ppm): 116.5, 117.3, 119.5, 128.0, 128.4, 129.6, 130.1, 130.5, 131.2, 131.8, 132.4, 133.0, 133.7, 134.7, 146.4, 148.1, 158, 162.5 (Ar-C), 166.0 (C=O), 184.0 (C=S). MS (EI): m/z 576 [M+ + 2, 2.9%], 574 [M+, 9%]. Anal. Calcd for C28H20ClN5O3S2 (574.07): C, 58.58; H, 3.51; N, 12.20; S, 11.17. Found: C, 57.33; H, 3.80; N, 12.06; S, 10.95.
2-[4-(6-Chloro-2-phenylquinazolin-4-yl)-aminophenylsulfonylimino]-3-substituted-thiazolidin-4-one 28–30
To a solution of the appropriate sulfonylthioureido derivatives 25–27 (0.005 mol) in n-butanol (20 mL), ethyl bromoacetate (1 g, 0.006 mol) and anhydrous sodium acetate (0.82 g, 0.01 mol) were added. The reaction mixture was heated under reflux for 2 h, poured into ice-cold water (30 mL) and the separated solid was filtered, washed with water, dried and crystallized.
2-[4-(6-Chloro-2-phenylquinazolin-4-yl)-aminophenylsulfonylimino]-3-phenylthiazolidin-4-one 28:Yield: 60%, mp: 155–157°C (ethanol/toluene; 1:1). IR (cm−1): 3327 (NH), 3089 (CH arom.), 2985 (CH aliph.), 1667 (C=O), 1325, 1231 (SO2). 1H NMR (δ, ppm): 4.17 (s, 2H, thiazol-CH2), 7.23–7.95 (m, 17H, Ar-H). 13C NMR (δ, ppm): 31.5 (thiazol-CH2), 117.3, 117.9, 120.1, 121.9, 124.8, 128.1, 128.9, 129.3, 129.8, 130.5, 130.9, 131.8, 132.5, 134.6, 136.0, 146.8, 149.5 (Ar-C), 162.2, 167.0 (2C=N), 169.4 (C=O). MS (EI): m/z 588 [M+ + 2, 4.6%], 586 [M+, 15.0%]. Anal. Calcd for C29H20ClN5O3S2 (586.08): C, 59.43; H, 3.44; N, 11.95; S, 10.94. Found: C, 59.71; H, 3.60; N, 12.18; S, 10.85.
2-[4-(6-Chloro-2-phenylquinazolin-4-yl)-aminophenylsulfonylimino]-3-benzylthiazolidin-4-one 29:Yield: 63%, mp: 163–165°C (ethanol/toluene; 1:1). IR (cm−1): 3338 (NH), 3090 (CH arom.), 2958 (CH aliph.), 1677 (C=O), 1340, 1223 (SO2). 1H NMR (δ, ppm): 3.91 (s, 2H, thiazol-CH2), 4.35 (s, 2H, benzyl-CH2), 6.98–7.99 (m, 17H, Ar-H). 13C NMR (δ, ppm): 29.8 (thiazol-CH2), 39.7 (benzyl-CH2), 117.2, 117.8, 119.5, 126.5, 127.1, 127.6, 128.5, 128.9, 129.2, 129.5, 130.5, 131.0, 131.6, 132.4, 134.7, 142.5, 145.0, 149.2 (Ar-C), 162.0, 164.2 (2C=N), 168.1 (C=O). MS (EI): m/z 602 [M+ + 2, 2.7%], 600 [M+, 7.2%]. Anal. Calcd for C30H22ClN5O3S2 (600.11): C, 60.04; H, 3.70; N, 11.67; S, 10.69. Found: C, 60.15; H, 3.53 N, 11.85; S, 10.83.
2-[4-(6-Chloro-2-phenylquinazolin-4-yl)-aminophenylsulfonylimino]-3-benzoylthiazolidin-4-one 30:Yield: 66%, mp: 170–172°C (ethanol/toluene; 1:1). IR (cm−1): 3328 (NH), 3088 (CH arom.), 1670, 1658 (2C=O), 1334, 1230 (SO2). 1H NMR (δ, ppm): 3.88 (s, 2H, thiazol-CH2), 6.96–7.97 (m, 17H, Ar-H). 13C NMR (δ, ppm): 30.2 (thiazol-C), 117.0, 117.9, 119.8, 126.3, 127.0, 127.6, 128.3, 128.8, 129.1, 129.7, 130.5, 131.1, 131.6, 132.7, 134.5, 142.3, 149.0 (Ar-C), 160.0, 162.4, 164.1 (3C=N), 169.0, 171.5 (2C=O). MS (EI): m/z 616 [M+ + 2, 9.5%], 614 [M+, 29.0%]. Anal. Calcdfor C30H20ClN5O4S2 (614.09): C, 58.68; H, 3.28; N, 11.40; S, 10.44. Found: C, 58.47; H, 3.25; N, 11.61; S, 10.64.
3-Substituted-4-phenyl-2-[4-(6-chloro-2-phenylquinazolin-4yl)-aminophenylsulfonylimino]-1,3-thiazoline 31–33
A solution of the appropriate sulfonyl-thioureido derivatives 12–14 (0.005 mol) in absolute ethanol (20 mL) was refluxed with phenacyl bromide (1.1 g, 0.006 mol) and anhydrous sodium acetate (0.82 g, 0.01 mol) for 5 h during which the solid product was partially crystallized out. The mixture was left to cool, filtered, washed with water, dried and crystallized.
3,4-Diphenyl-2-[4-(6-chloro-2-phenylquinazolin-4yl)-aminophenylsulfonylimino]-1,3-thiazoline 31:Yield: 66%, mp: 142–144°C (ethanol/toluene; 1:1). IR (cm−1): 3319 (NH), 3088 (CH arom.), 1328, 1234 (SO2). 1H NMR (δ, ppm): 6.15 (s, 1H, thiazol-H), 7.34–7.95 (m, 22H, Ar-H). 13C NMR (δ, ppm): 107.5 (thiazol-C), 118.6.0, 120.0, 121.0, 122.2, 123.1, 124.4, 125.9, 127.4, 129.7, 131.9, 134.1, 135.8, 139.0, 146.7 (Ar-C), 163.0, 167.4 (2C=N). MS (EI): m/z 648 [M+ + 2, 5.5%], 646 [M+, 19.4%]. Anal. Calcd for C35H24ClN5O2S2(646.18): C, 65.06; H, 3.74; N, 10.84; S, 9.92. Found: C, 65.30; H, 3.31; N, 10.85; S, 10.14.
3-Benzyl-4-phenyl-2-[4-(6-chloro-2-phenylquinazolin-4yl)-aminophenylsulfonylimino]-1,3-thiazoline 32:Yield: 60%, mp: 152–154°C (ethanol/toluene; 1:1). IR (cm−1): 3325 (NH), 3086 (CH arom.), 2980 (CH aliph.), 1351, 1225 (SO2). 1H NMR (δ, ppm): 3.79 (s, 2H, benzyl-CH2), 6.24 (s, 1H, thiazol-H), 7.17–7.92 (m, 22H, Ar-H). 13C NMR (δ, ppm): 45.1 (benzyl-C), 110.0 (thiazol-C), 118.7.0, 119.8, 121.0, 122.2, 123.4, 124.6, 125.9, 127.2, 129.0, 131.8, 133.5, 135.9, 138.5, 147.7 (Ar-C), 160.8, 164.0 (2C=N). MS (EI): m/z 662 [M+ + 2, 11.5%], 660 [M+, 35.8%]. Anal. Calcd forC36H26ClN5O2S2 (660.21): C, 65.49; H, 3.97; N, 10.61; S, 9.71. Found: C, 65.25; H, 4.10; N, 10.34; S, 9.98.
3-Benzoyl-4-phenyl-2-[4-(6-chloro-2-phenylquinazolin-4yl)-aminophenylsulfonylimino]-1,3-thiazoline 33:Yield: 52%, mp: 168–170°C (ethanol/toluene; 1:1). IR (cm−1): 3321 (NH), 3088 (CH arom.),1678 (C=O), 1354, 1220 (SO2). 1H NMR (δ, ppm): 4.01 (s, 2H, benzyl-CH2), 6.44 (s, 1H, thiazol-H), 7.13–7.88 (m, 22H, Ar-H). 13C NMR (δ, ppm): 111.8 (thiazol-C), 121.2, 122.4, 123.5, 126.1, 126.8, 127.2, 128.0, 128.8, 129.3, 130.2, 131.8, 132.5, 133.7, 134.7, 135.3, 137.0, 139.2, 143.9, 150.0 (Ar-C),161.4, 162.6 (2C=N),164.5 (C=O). MS (EI): m/z 676 [M+ + 2, 10.0%], 674 [M+, 32.5%]. Anal. Calcd forC36H24ClN5O3S2 (674.19): C, 64.13; H, 3.59; N, 10.39; S, 9.51. Found: C, 63.89; H, 3.73; N, 10.09; S, 9.72.
Pharmacology
Materials
Doxorubicin was supplied by LC Laboratories® (Woburn, MA). All other chemicals were obtained from Sigma Chemical Company (St. Louis, MO). The human hepatoma cell line (HepG2), human breast cancer cell line (MCF-7) human cervix cancer cell line (HeLa) and human colon cancer cell line (HCT-8) were obtained from the American Type Culture Collection (Rockville, MD). Tissue culture plates and flasks were purchased from Costar (Milan, Italy).
Evaluation of cellular cytotoxicity
The newly synthesized compounds (8–33) were initially screened at single concentration of 20 µg/mL using the colorimetric MTT (3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyl-tetrazolium bromide) assay to test their in vitro cytotoxicity against HepG2, MCF-7, HeLaand HCT-8. The clinically used anticancer drug doxorubicin was used as standard for comparative purposesCitation17.
The stock solutions of the tested compounds were prepared in DMSO and were used for serial dilutions in culture medium. The tested cell lines were grown in RPMI-1640 medium supplemented with 10% calf serum. For growth assays, exponentially growing cells were suspended in the above-mentioned medium at a density of 103–105 cells/well, seeded onto 96-well plates (200 µL/well), and incubated at 37°C in a humidified 5% CO2 atmosphere for 24 h. After that, the cell medium in test wells was changed to new culture medium containing the required concentrations of the tested compounds, while the cell medium in control wells was changed to new culture medium containing an equivalent volume of solvent. After incubation at 37°C in a humidified 5% CO2 atmosphere for 3 days, 100 µL of MTT (0.5 µg/mL) in serum-free medium was added to each well and incubated at 37°C for an additional 4 h. Then, 200 µL of DMSO was added to each well and mixed thoroughly to dissolve the resulting formazan product. The cell viability was evaluated by measuring the optical densities at 544 nm using a Microelisa Reader. The percentage of cell growth inhibition was calculated as follows:
% Inhibition = (Mean ODcontrol−Mean ODtest)/Mean ODcontrol × 100%, where OD is the optical density. The dose-response relationships of the compounds effecting ≥ 50% inhibition in one dose (20 µg/mL) prescreening for each cell line were measured using concentrations of 10, 0.1 and 0.01 µg/mL, and the concentration causing 50% cell growth inhibition (IC50) was calculated. The results are shown in .
Declaration of interest
This work was supported by a grant from King Abdulaziz city for science and technology (KACST; Grant No.: ARP-29–265), and the National Plan of Science, Technology and Innovation (Grant No. 10-MED1188-02) King Saud University, Riyadh, Saudi Arabia.
References
- Hasan J, Jayson GC. VEGF antagonists. Expert Opin Biol Ther 2001;1:703–718.
- Hamby JM, Showalter HD. Small molecule inhibitors of tumor-promoted angiogenesis, including protein tyrosine kinase inhibitors. Pharmacol Ther 1999;82:169–193.
- Aggarwal BB, Danda D, Gupta S, Gehlot P. Models for prevention and treatment of cancer: problems vs promises. Biochem Pharmacol 2009;78:1083–1094.
- Csermely P, Agoston V, Pongor S. The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci 2005;26:178–182.
- Garofalo A, Goossens L, Baldeyrou B, Lemoine A, Ravez S, Six P et al. Design, synthesis, and DNA-binding of N-alkyl(anilino)quinazoline derivatives. J Med Chem 2010;53:8089–8103.
- Park J, Ahn KS, Bae EK, Kim BS, Kim BK, Lee YY et al. Blockage of interleukin-6 signaling with 6-amino-4-quinazoline synergistically induces the inhibitory effect of bortezomib in human U266 cells. Anticancer Drugs 2008;19:777–782.
- Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Anticancer and antiviral sulfonamides. Curr Med Chem 2003;10:925–953.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Neri D, Supuran CT.Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–777.
- Owa T, Yoshino H, Okauchi T, Yoshimatsu K, Ozawa Y, Sugi NH et al. Discovery of novel antitumor sulfonamides targeting G1 phase of the cell cycle. J Med Chem 1999;42:3789–3799.
- Van Kesteren C, Beijnen JH, Schellens JH. E7070: a novel synthetic sulfonamide targeting the cell cycle progression for the treatment of cancer. Anticancer Drugs 2002;13:989–997.
- Minakuchi T, Nishimori I, Vullo D, Scozzafava A, Supuran CT. Molecular cloning, characterization, and inhibition studies of the Rv1284 beta-carbonic anhydrase from Mycobacterium tuberculosis with sulfonamides and a sulfamate. J Med Chem 2009;52:2226–2232.
- Dubois L, Lieuwes NG, Maresca A, Thiry A, Supuran CT, Scozzafava A et al. Imaging of CA IX with fluorescent labelled sulfonamides distinguishes hypoxic and (re)-oxygenated cells in a xenograft tumour model. Radiother Oncol 2009;92:423–428.
- Gitto R, Agnello S, Ferro S, De Luca L, Vullo D, Brynda J et al. Identification of 3,4-Dihydroisoquinoline-2(1H)-sulfonamides as potent carbonic anhydrase inhibitors: synthesis, biological evaluation, and enzyme–ligand X-ray studies. J Med Chem 2010;53:2401–2408.
- Huang Z, Lin Z, Huang J. A novel kind of antitumour drugs using sulfonamide as parent compound. Eur J Med Chem 2001;36:863–872.
- Alafeefy AM, Kadi AA, Al-Deeb OA, El-Tahir KE, Al-Jaber NA. Synthesis, analgesic and anti-inflammatory evaluation of some novel quinazoline derivatives. Eur J Med Chem 2010;45:4947–4952.
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990;82:1107–1112.