Abstract
In this study, we have synthesised (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and a series of its derivatives (5, 13–16) and tested the ability of these compounds to inhibit two metalloenzyme human carbonic anhydrase (hCA, EC 4.2.1.1) isozymes, hCA I and hCA II. The synthesised compounds showed inhibitory effect on hCA I and hCA II isozymes. The results showed that synthesised compounds (5, 13–16) demonstrated the best inhibition activity against hCA I (IC50: 3.22–54.28 μM) and hCA II (IC50: 18.52–142.01 μM). The compound 14 showed the highest inhibiton effect against hCA I (IC50: 3.22 μM; Ki: 1.19 ± 1.4 μM). On the other hand, the compound 13 showed the highest inhibiton effect against hCA II (IC50: 18.52 μM; Ki: 3.25 ± 1.13 μM).
Introduction
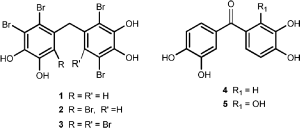
Bromophenols are abundantly found in marine life and they have important biological activitiesCitation1. It was reported that some of the bromophenols (1–3) exhibited enzyme inhibition, cytotoxicity, feeding deterrent, antioxidant and antimicrobial activitiesCitation2. AntioxidantCitation2 and carbonic anhydrase (CA) inhibitory propertiesCitation3 of the derivatives of compound 4 with Br and antioxidant propertiesCitation4 of the derivatives of compound 5 with Br were investigated.
Carbonic anhydrase (CA) is a zinc metalloenzyme that catalyses the reversible reactions of CO2 and water: ![]() . It classically participates in the maintenance of pH homeostasis in human body, catalysing the reversible hydration of CO2 in a two-step reaction to yield bicarbonate and protonsCitation5,Citation6. CA seems to be almost ubiquitously expressed in living organisms. At least threeCitation7 to fiveCitation8 genetically unrelated families (α, β, γ, δ and ξ) of CA isoforms exist. The α-CA family is the best studied group, although recent reviews indicate rapid advancement of knowledge about other CA familiesCitation9,Citation10. On the other hand, 16 isozymes have been described till now. These isoenzymes differ in their subcellular localisation, catalytic activity and susceptibility to different classes of inhibitors. Some of these isozymes are cytosolic (CA I, II, III, VII and XIII), others are membrane-bound (CA IV, IX, XII and XIV), two of them are mitochondrial (CA VA and CA VB) and other one is secreted in saliva (CA VI). It has been reported that CA XV isoform is not expressed in humans or in other primates, however, it is abundant in rodents and other higher vertebratesCitation11,Citation12. A critical problem in the design of CA inhibitors (CAIs) with pharmacological applications for the treatment and prevention of various diseases is related to the high number of these CA isoforms, the diffuse localisation of CAs in many tissues/organs and the lack of isozyme selectivity of the presently available inhibitorsCitation13.
. It classically participates in the maintenance of pH homeostasis in human body, catalysing the reversible hydration of CO2 in a two-step reaction to yield bicarbonate and protonsCitation5,Citation6. CA seems to be almost ubiquitously expressed in living organisms. At least threeCitation7 to fiveCitation8 genetically unrelated families (α, β, γ, δ and ξ) of CA isoforms exist. The α-CA family is the best studied group, although recent reviews indicate rapid advancement of knowledge about other CA familiesCitation9,Citation10. On the other hand, 16 isozymes have been described till now. These isoenzymes differ in their subcellular localisation, catalytic activity and susceptibility to different classes of inhibitors. Some of these isozymes are cytosolic (CA I, II, III, VII and XIII), others are membrane-bound (CA IV, IX, XII and XIV), two of them are mitochondrial (CA VA and CA VB) and other one is secreted in saliva (CA VI). It has been reported that CA XV isoform is not expressed in humans or in other primates, however, it is abundant in rodents and other higher vertebratesCitation11,Citation12. A critical problem in the design of CA inhibitors (CAIs) with pharmacological applications for the treatment and prevention of various diseases is related to the high number of these CA isoforms, the diffuse localisation of CAs in many tissues/organs and the lack of isozyme selectivity of the presently available inhibitorsCitation13.
Chemicals are generally known to activate or inhibit several enzymes in vivo and affect metabolic pathwaysCitation14,Citation15. The main classes of CAIs are known: the metal complexing anions, and the substituted sulfonamides and their bioesters such as sulfamates and sulfamides, which bind to the metal ion of the enzyme either by substituting for the nonprotein zinc ligand to generate a tetrahedral adduct or by participating in the metal coordination sphere to generate trigonal bipyramidal species. Phenols are also very important inhibitors that display competitive inhibitionCitation16–21. The Zn2+ ion in CAs is critical for the inhibition of these enzymes. Three mechanisms of inhibition have been proposed, one of which involves anchoring of the inhibitor to the Zn2+-bound solvent molecule such as water or hydroxide ion. Phenols and polyamines bind in this wayCitation12,Citation16–21.
In this study, we have synthesised the novel (3,4-dihydroxyphenyl) (2,3,4-trihydroxyphenyl)methanone and a series of its derivatives (5, 13–16) and evaluated their potency to be novel CA isoenzymes (hCA-I and hCA-II) inhibitors.
Results and discussion
Synthesis
After (3,4-dimethoxyphenyl)(2,3,4-trimethoxyphenyl)methanone (6) was obtained from the reaction of 3,4-dimethoxybenzoic acid and 1,2,3-trimethoxybenzene in polyphosphoric acid by the known methodCitation2,Citation22–24, bromination reactions of 6 with different equivalents (equiv.) of ceric ammonium nitrate (CAN)/LiBr at room temperature (RT) were performed. A monobromide 7 and a dibromide 8 were obtained from these reactions as the sole products ()Citation24.
Scheme 1. Reagents and conditions. (a) LiBr (1.1 equiv.)/CAN (1.1 equiv.), CH3CN, RT, 3 d, 97%; (b) LiBr (6.0 equiv.)/CAN (6.0 equiv.), CH3CN, RT, 6 d, 95%; (c) KOH-NH2NH2/(OHCH2)2, 110°C–190°C, 3 d, 83%; (d) LiBr (2.1 equiv.)/CAN (2.1 equiv.), CH3CN, RT, 3 d, 95%; (e) LiBr (1.1 equiv.)/CAN (1.1 equiv.), CH3CN, RT, 3 d; (f) BBr3, CH2Cl2, 96%.
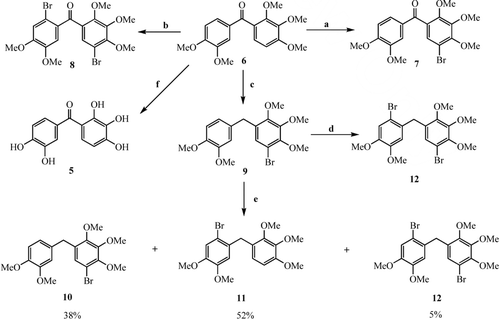
Wolff–Kishner reduction of ketone 6 gave diarylmethane derivative 9 in high yield (). Bromination of 9 with 1.1 equivalents of LiBr/CAN gave a mixture of three products, while its reaction with 2.2 equivalents of LiBr/CAN gave a product as sole product. From these isolated products, the product produced in the reaction as sole product was dibromide 12, and the products found in the mixture were bromides 10, 11 and 12 ()Citation4.
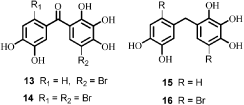
Demethylation reaction of 6 with BBr3 in CH2Cl2 at 0°C–25°C gave the phenol 5 (). Demethylation reactions of 7–9 and 12 with BBr3 in CH2Cl2 were similarly performed at 0°C–25°C. Bromophenol derivatives 13–16 were synthesised from these demethylation reactionsCitation4.
CA purification and assay
In this study, CA isoenzymes I and II (hCA I and hCA II) were purified from human erythrocytes (). The inhibitory effects of bromophenols were tested in vitro. The inhibitor concentrations that caused 50% inhibition (IC50) were determined from activity versus (%)-[bromophenols] plots, and the Ki values were calculated from Lineweaver–Burk plots ().
Table 1. Summary of purification procedure for human carbonic anhydrase isoenzymes (hCA I and hCA II) by a sepharose-4B-thyrosine-sulfanilamide affinity column chromatography.
Inhibition effects of CA isoenzymes
CA isoenzymes inhibitory effects of the synthesised (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and a series of its derivatives (5, 13–16) were tested under in vitro conditions, and IC50 values were calculated and given in
Table 2. Human carbonic anhydrase isonzymes (hCA I and hCA II) inhibition data with some synthesised bromophenols, by an esterase assay with 4-nitrophenylacetate as substrate.
In this study, we report the inhibitory effects of synthesised (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and a series of its derivatives (5, 13–16), and on the hydratase activity of hCA I and hCA II. The data in show the following regarding the inhibition of hCA I and hCA II by bromophenol derivatives. The strongest inhibitory activity has been observed with compounds 14 and 16, investigated in this study for the inhibition of the rapid cytosolic isozyme hCA I and hCA II (). According to two compounds, the derivative of 15 showed middle hCA I inhibitory activity with IC50 of 54.28 μM (), whereas the remaining other derivatives were quite effective hCA I inhibitors, with IC50’s in the range of 4.28–27.77 μM (). It is well known that phenolic compounds are effective for both hCA I and hCA IICitation17,Citation18 and the other CA isoenzymes (CA I–XVI)Citation19,Citation20. In the previous reports, other phenol derivatives such as salicylic acid and paracetamol were investigated using a stopped flow, CO2 hydration assay for monitoring CA inhibitionCitation19,Citation20. It was reported that the presence of one or three OH moieties in phenols seemed to influence little hCA I inhibitory activitiesCitation17. It demonstrated CA I and CA II inhibitory activities of p-hydroxybenzoic acid, p-coumaric acid, gallic, syringic acid, ferulic acid, quercetin and ellagic acid. It may be observed that small structural changes lead to impressive differences of activity. For example, ferulic acid is 2.55 times a better hCA II inhibitor compared with p-coumaric acid, of which it differs only by the presence of a supplementary methoxy moiety attached to the aromatic ring. The two isozymes showed rather different inhibition profiles with these compounds. However, hCA II was usually more easily inhibited by all the phenolic acids or polyphenolsCitation17.
In this study, all of new synthesised (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl) methanone and its derivatives (5, 13–16) had five −OH moieties in the both phenol rings. Bromine is electronegative rather than that of carbon atoms. The bromine group in phenolic ring pulls electrons from carbon atoms. Thus, proton abstraction ability of phenolic ring increases. This situation increases the CA isoenzyme inhibition effect of synthesised (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives compounds (5, 13–16).
The synthesised (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives (5, 13–16) have two phenolic rings. These synthesised phenolic compounds have been investigated as inhibitors of CA isoenzymes in this study. The rationale of investigating these compounds as CAIs lies in the fact that phenol has been shown to be the only competitive inhibitor with CO2 as the substrate for the main isoform of CA such as human CA I and IICitation25. In another study, Christianson and colleagues reported on the x-ray crystal structure for the adduct of hCA II with phenol, showing this compound to bind to CA by anchoring its OH moiety to the zinc-bound water/hydroxide ion of the enzyme active site through a hydrogen bond as well as to the NH amide of Thr199, an amino acid conserved in all α-CAs and critically important for the catalytic cycle of these enzymesCitation26. Recently, Senturk et al. investigated the effect of some monomeric and dimeric antioxidant phenolic compounds such as 2,6-dimethylphenol, 2,6-diisopropylphenol (propofol), 2,6-di-t-butylphenol, butylated hydroxytoluene, butylated hydroxyanisole, vanillin, guaiacol, di(2,6-dimethylphenol), di(2,6-diisopropylphenol) and di(2,6-di-t-butylphenol) on hCA I and II isozymesCitation12. It had been suggested that the phenolic compounds bind to enzyme active site through the phenolic hydroxyl group, which is anchored through a hydrogen bond network to the Zn(II)-bound water molecule and Thr199, the gate keeper residue of the enzymeCitation26,Citation27. In this study, the best inhibitory effect was found for di(2,6-di-t-butylphenol), with IC50 values of 37.5 μM (for hCA I) and 0.29 μM (for hCA II)Citation12.
CAIs are a class of pharmaceuticals used as antiglaucoma agents, diuretics, antiepileptics, in the management of mountain sickness, gastric and duodenal ulcers, neurological disorders or osteoporosis. Thus, the discovery of novel CAIs is of great importance for pharmacological and medicinal approaches, and many inhibitors have been designed and synthesised in the literatureCitation12. However, it is critically important to explore further classes of potent CAIs in order to detect compounds with a different inhibition profile when compared with sulfonamides and their bioisosteres and to find novel applications for the inhibitors of these widespread enzymes.
Conclusions
We have synthesised (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and a series of its derivatives (5, 13–16) and evaluated the ability of these compounds to inhibit hCA I and hCA II. All these molecules (5, 13–16) inhibited both the isoenzymes. The best inhibitors of hCA I were compounds 14 and 16, which exhibited IC50 values of 3.22 and 4.28 μM, respectively. On the other hand, the best inhibitor of hCA II was compound 13, with an IC50 value of 13.52 μM. These findings indicate that the synthesised phenolic compounds may be used as potent inhibitors of CA isoenzymes possibly targeting other isoforms, which have not been assayed yet for their interactions with such agents.
Experimental section
General information
All chemicals and solvents are commercially available and were used after distillation or treatment with drying agents. The 1H- and 13C-NMR spectra were recorded on a 200 (50) and 400 (100)-MHz Varian spectrometer; δ in ppm and Me4Si as the internal standard. All column chromatography was performed on silica gel (60-mesh, Merck). Preparative thick-layer chromatography (PLC) is 1 mm of silica gel 60 PF (Merck) on glass plates.
The synthesis of compounds (5, 13–16)
The synthesis procedure of compounds (5, 13–16) was reported previouslyCitation4,Citation24.
Purification of CA isozymes from human erythrocytes by affinity chromatography
CA isozymes were purified via a simple single-step method using Sepharose-4B-L tyrosine-sulfanilamide affinity gel chromatography, described previouslyCitation12. The purity of the enzymes was confirmed using SDS polyacrylamide gel electrophoresis. The running and stacking gels contained 10% and 3% acrylamide, respectively, and 0.1% SDS, according to the Laemmli procedureCitation28 described previouslyCitation14. A 20-mg sample was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coomassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then destained with several changes of the same solvent without the dye.
Hydratase activity assay
The hydratase activity of hCA I and hCA II was assayed by following the hydration of CO2 according to the method described by Wilbur and AndersonCitation29. The activity of CO2-hydratase in enzyme units (EU) was calculated by using the equation (to − tc/tc), where to and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
Esterase activity assay
CA activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (CHEBIOS UV–VIS) according to the method described by Verpoorte et al.Citation30 The enzymatic reaction contained 1.4 mL 0.05 M Tris-SO4 buffer (pH 7.4), 1 mL 3 mM 4-NPA, 0.5 mL H2O and 0.1 mL enzyme solution (total volume, 3.0 mL). A reference measurement was obtained by preparing the mixture without the enzyme solution. All measurements were made in triplicate. The Ki values were determined from a series of experiments using three different bromophenols concentrations and 4-NPA as the substrate at five different concentrations to construct Lineweaver–Burk curvesCitation31.
Protein determination
The yield of protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford methodCitation32, using bovine serum albumin as the standard.
Acknowledgements
The authors are indebted to the Department of Chemistry and Ataturk University for financial support of this work (Project No: BAP-2009/84). The authors report no conflicts of interest.
References
- Gribble GW. The diversity of naturally occurring organobromine compounds. Chem. Soc. Rev. 1999; 28: 335–346.
- Balaydin HT ,Gülçin Í ,Menzek A ,Göksu S ,Sahin E. Synthesis and antioxidant properties of diphenylmethane derivative bromophenols including a natural product. J Enzyme Inhib Med Chem 2010;25:685–695.
- Balaydin HT, Soyut H, Ekinci D, Göksu S, Beydemir S, Menzek A et al. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzyme Inhib Med Chem 2012; 27: 43–50.
- Çetinkaya Y, Göçer H, Menzek A, Gülçin I. Synthesis and antioxidant properties of (3,4-dihydroxyphenyl)(2,3,4-trihydroxyphenyl)methanone and its derivatives. Arch Pharm Chem Life Sci. Doi: 10.1002/ardp.201100272.
- Gülçin I, Beydemir S, Büyükokuroğlu ME. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol Pharm Bull. 2004; 27: 613–616.
- Beydemir S, Gülçin I. Effect of melatonin on carbonic anhydrase from human erythrocyte in vitro and from rat erythrocyte in vivo. J Enzyme Inhib Med Chem 2004; 19: 193–197.
- Hewett-Emmett D. Evolution and distribution of the carbonic anhydrase gene families. EXS 2000;90:29–76.
- Xu Y ,Feng L ,Jeffrey PD ,Shi Y ,Morel FM. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 2008;452:56–61.
- Rowlett RS. Structure and catalytic mechanism of the beta-carbonic anhydrases. Biochim Biophys Acta 2010;1804:362–373.
- Hisar O ,Beydemir S ,Gülçin I ,Küfrevioglu OI ,Supuran CT. Effects of low molecular weight plasma inhibitors of rainbow trout (Oncorhynchus mykiss) on human erythrocyte carbonic anhydrase-II isozyme activity in vitro and rat erythrocytes in vivo. J Enzyme Inhib Med Chem 2005;20:35–39.
- Cankaya M, Hernandez AM, Ciftci M, Beydemir S, Ozdemir H, Budak H et al. An analysis of expression patterns of genes encoding proteins with catalytic activities. BMC Genomics 2007;8:232.
- Sentürk M ,Gülçin I ,Dastan A ,Küfrevioglu OI ,Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–3211.
- Supuran CT ,Scozzafava A ,Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Coban TA ,Beydemir S ,Gülçin I ,Ekinci D. Morphine inhibits erythrocyte carbonic anhydrase in vitro and in vivo. Biol Pharm Bull 2007;30:2257–2261.
- Çoban TA, Beydemir Ş, Gülçin İ, Ekinci D. The inhibitory effect of ethanol on carbonic anhydrase isoenzymes: in vivo and in vitro studies. J Enzyme Inhib Med Chem 2008; 23: 266–270.
- Sentürk M, Gülçin I, Beydemir S, Küfrevioglu OI, Supuran CT. In vitro inhibition of human carbonic anhydrase I and II Isozymes with natural phenolic compounds. Chem Biol Drug Des 2011;77:494–499.
- Öztürk Sarıkaya SB, Gülçin İ,, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of phenolic acids. Chem Biol Drug Des. 2010; 75: 515–520.
- Oztürk Sarikaya SB ,Topal F ,Sentürk M ,Gülçin I, Supuran CT. In vitro inhibition of a-carbonic anhydrase isozymes by some phenolic compounds. Bioorg Med Chem Lett 2011;21:4259–4262.
- Innocenti A ,Beyza Oztürk Sarikaya S ,Gülçin I ,Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mammalian isoforms I-XIV with a series of natural product polyphenols and phenolic acids. Bioorg Med Chem 2010;18:2159–2164.
- Innocenti A ,Gülçin I ,Scozzafava A ,Supuran CT. Carbonic anhydrase inhibitors. Antioxidant polyphenols effectively inhibit mammalian isoforms I-XV. Bioorg Med Chem Lett 2010;20:5050–5053.
- Davis RA ,Innocenti A ,Poulsen SA ,Supuran CT. Carbonic anhydrase inhibitors. Identification of selective inhibitors of the human mitochondrial isozymes VA and VB over the cytosolic isozymes I and II from a natural product-based phenolic library. Bioorg Med Chem 2010;18:14–18.
- Akbaba Y, Balaydın HT, Göksu S, Şahin E, Menzek A. Total synthesis of the biologically active, naturally occurring 3,4-dibromo-5-[2-bromo-3,4-dihydroxy-6-(methoxymethyl)benzyl]benzene-1,2-diol and regioselective o-demethylation of aryl methyl ethers. Helv Chim Acta, 2010; 93: 1127–1135.
- Şahin E, Balaydın HT, Göksu S, Menzek A. 2,3-Dibromo-1-[4-(2,3-dibromo-4,5-dimethoxybenzyl)-2,5-dimethoxybenzyl]-4,5-dimethoxybenzene. Acta Cryst. 2010; E66: o3029–o3029.
- Çetinkaya Y, Menzek A, Sahin E, Balaydın HT. Selective O-Demethylation During Bromination of (3,4-dimethoxyphenyl)(2,3,4-trimethoxyphenyl)methanone. Tetrahedron 2011; 67: 3483–3489.
- Innocenti A ,Vullo D ,Scozzafava A ,Supuran CT. Carbonic anhydrase inhibitors: interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–1587.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Supuran CT ,Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Laemmli DK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227: 680–685.
- Wilbur KM ,Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–154.
- Verpoorte JA ,Mehta S ,Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934; 57: 685–693.
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976; 72: 248–254.
