Abstract
A newly series of water-soluble 1-alkyl-3-(4-methyl-7, 8-dihydroxy-2H-chromen-2-one) benzimidazolium chloride salts (3a-j) were synthesized and their inhibitory effects on the activity of purified human carbonic anhydrase (hCA) I and II were evaluated. hCA I and II from human erythrocytes were purified by a simple one step procedure by using Sepharose 4B-L-tyrosine-sulphanilamide affinity column. The result showed that all the synthesized compounds were inhibited the CA isoenzymes activity. Among them, 3g and 3j were found to be most active (IC50 = 22.09 µM and 20.33 µM) for hCA I and hCA II, respectively.
Introduction
Coumarin derivatives were reported as a novel class of inhibitor of metalloenzyme carbonic anhydrase (CA)Citation1,Citation2. Benzimidazole consists of the fusion of benzene and imidazole. The most known benzimidazole compound in nature is N-ribosyl-dimethylbenzimidazoleCitation3, which serves as an axial ligand for cobalt in vitamin B12. Benzimidazole has been used widely as carbon skeleton for preparation N-heterocyclic carbenes (NHC). Benzimidazoles and their NHC’s are usually used as ligand for transition metal complexesCitation4. Besides using on synthesis of NHC’s, antimicrobial activities of benzimidazolium salts were reportedCitation5.
The metalloenzyme CA (EC 4.2.1.1) catalyzes a very simple but critically important physiological reaction: the involvement of the CA enzyme family, which catalyzes the physiological hydration of CO2 to yield bicarbonate and a proton, in many physiological/pathological processes open up widespread opportunities for the development of diverse, specific inhibitors for clinical applicationCitation6–9.
The active site of most CAs contains a zinc ion (Zn2+), which is essential for catalysis. The CA reaction is involved in many physiological and pathological processes, including respiration and transport of CO2 and bicarbonate between metabolizing tissues and lungs; pH and CO2 homeostasis; electrolyte secretion in various tissues and organs; biosynthetic reactions such as gluconeogenesis, lipogenesis and ureagenesis; bone resorption; calcification; and tumorigenicityCitation10–18. Many of the CA isoenzymes involved in these processes are important therapeutic targets with the potential to be inhibited to treat a range of disorders including edema, glaucoma, obesity, cancer, epilepsy and osteoporosisCitation19–24. Given the physiological importance of the CA, the metabolic impact of chemicals for crop production should receive greater study.
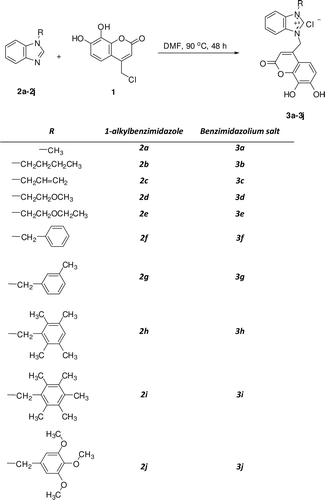
In this study, series of 10 new water-soluble 1-alkyl-3-(4-methyl-7, 8-dihydroxy-2H-chromen-2-one) benzimidazolium chloride salts (3a-j) derivatives were synthesized and their inhibitory effects on the activity of purified human carbonic anhydrase (hCA) I and II were evaluated.
Materials and methods
All reactions for the preparation of benzimidazolium salts were carried out under argon, flame-dried glassware using Standard Schlenk techniques. Chemicals were obtained by Sigma Aldrich. DMF used as solvent on synthesis of benzimidazolium salts was dried by P2O5.
Melting points were determined by Electrothermal-9200 melting point apparatus. FT-IR spectra were recorded on ATR unit in the range 400–4000 cm−1 with Perkin Elmer Spectrum 100 Spectrophotometer. 1H-NMR and 13C-NMR were recorded using a Bruker AC300P FT spectrometer operating at 300.13 MHz (1H), 75.47 MHz (13C). Chemical shifts are given in ppm relative to TMS, coupling constants (J) in Hz. Elemental Analyses were performed by IBTAM (Inonu University Scientific and Technological Research Central).
Synthesis of 4-chloromethyl-7, 8-dihydroxy-2H-chromen-2-one (1)
4-chloromethyl-7, 8-dihydroxy-2H-chromen-2-one (1) was synthesized by the procedure of Gumus et al.Citation25. Crude product purified by column chromatography (acetone/hexane 3/2 solvent system). Yield (yellow): 58%, melting point: 216–217°CCitation26. FT-IR (cm−1): 3542 and 3434 (Ar–OH), 3088, 1668 (C=O), 1611 (C=C). 1H-NMR (δH, DMSO-d6):10.27 (s, 1H, -OH), 9.40 (s, 1H, -OH), 7.20-7.17 (d, J = 9 Hz, 1H, Ar-H), 6.87-6.84 (d, J = 9 Hz, 1H, Ar-H), 6.42 (s, 1H, -C=C-H), 4.93 (s, 2H, -CH2). 13C-NMR (δC, DMSO-d6): 160.6, 151.9, 150.2, 144.1, 132.9, 115.9, 112.8, 111.4, 110.6, 41.9.
General procedure for the preparation of the 1-alkylbenzimidazole compounds (2a-2j)
1-alkylbenzimidazole compounds were synthesized by procedure of Ozdemir et al.Citation27
General procedure for synthesis of benzimidazolium salts (3a-3j)
10 mmol from 1-alkylbenzimidazole (2a-j) was solved in dried DMF (5 mL) and 10 mmol 4-chloromethyl-7, 8-dihydroxy-2H-chromen-2-one was added into the solution and the mixture heated 48 h at 90°C. After 48 h, diethyl ether was added to mixture and precipitates were collected by filtration. Crude product was washed with acetone and ethanol so that dried under reduced pressure.
1-methyl-3-(4-methyl-7, 8-dihydroxy-2H-chromen-2-one) benzimidazolium chloride (3a)
Yield: 80%, mp: 306–308°C. Anal. cal. for. C18H15ClO4N2 C:60.26, H:4.21, N:7.81; found C:60.31, H:4.24, N: 7.87. FT-IR (cm−1): 1561 (C-N), 1683 (C=O), 3250 (OH): 1H-NMR (δH, DMSO-d6,): 10.61 (s, 1H, -OH), 9.93 (s, 1H, NCHN), 9.5 (s, 1H, -OH), 7.00-8.12 (m, 6H, ArH), 6.14 (s, 2H, -CH2), 5.73 (s, 1H, -C=C-H), 4.14 (s, 3H, -CH3). 13C-NMR (δC, DMSO-d6): 160.3, 150.6, 149.8, 144.4, 143.8, 133.1, 132.5, 131.5, 127.4, 127.2, 115.3, 114.4, 114.0, 113.1, 110.3, 108.8, 46.8, 34.1.
1-(n-butyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one)benzimidazolium chloride (3b)
Yield: 68%. mp: 269.6°C. Anal. cal. for. C21H21ClO4N2 C:62.92 H:5.28, N:6.99; found: C:62.93, H:5.28, N: 6.96. FT-IR (cm−1): 1566 (C-N), 1710 (C=O), 3320 (OH), 1H-NMR (δH, DMSO-d6): 10.58 (s, 1H, -OH), 10.00 (s, 1H, NCHN), 9.5 (s, 1H, -OH), 6.96-8.19 (m, 6H, ArH), 6.10 (s, 2H, -CH2), 5.75 (s, 1H, -C=C-H), 4.52-4.57 (t, 2H, J = 7 Hz, -CH2CH2CH2CH3), 1.89-1.99 (quint, 2H, J = 7 Hz, -CH2CH2CH2CH3), 1.33-1.41 (six, 2H, J = 7 Hz, -CH2CH2CH2CH3), 0.91-0.96 (t, 3H, J = 7 Hz, -CH2CH2CH2CH3). 13C-NMR (δC, DMSO-d6): 160.3, 150.6, 149.6, 143.8, 143.7, 133.1, 131.8, 131.7, 127.5, 127.3, 115.3, 114.5, 114.2, 113.1, 110.3, 109.1, 56.5, 47.3, 30.8, 19.6, 13.9.
1-allyl-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one)benzimidazolium chloride (3c)
Yield: 82%. mp: 296–298°C. Anal. cal. for. C20H17ClO4N2 C:62.42, H:4.45, N: 7.28; found: C: 62.45, H: 4.46, N: 7.25. FT-IR (cm−1): 1566 (C-N), 1712 (C=O), 3210 (OH). 1H-NMR (δH, DMSO-d6): 10.53 (s, 1H, -OH), 9.91 (s, 1H, NCHN), 9.51 (s, 1H, -OH), 6.96-8.06 (m, 6H, ArH), 6.18 (ddt, 1H, -CH2-CH=CH′H″, JCH2-CH = 5.88 Hz, JH-H′ = 10.31 Hz, JH-H″ = 17.17 Hz ), 6.12 (s, 2H, -CH2), 5.74 (s, 1H, -C=C-H), 5.52-5.43 (dd, 2H, -CH2-CH=CH′H″, JH′-H″ = 1.20 Hz, JH-H″ = 15.60 Hz), 5.23 (d, 2H, -CH2-CH=CH′H″, JCH2-CH = 5.91 Hz). 13C-NMR (δC, DMSO-d6):160.3, 150.6, 149.6, 143.9, 143.8, 133.1, 131.8, 131.7, 131.4, 127.5, 127.4, 121.3, 115.3, 114.6, 114.3, 113.1, 110.4, 108.9, 56.5, 49.7.
1-(2-methoxyethyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one)benzimidazolium chloride (3d)
Yield: 25%. mp: 251–254°C. Anal. cal. for. C20H19ClO5N2 C:59.63 H:4.75 N:6.95; found: C: 59.68, H: 4.80, N: 6.88. FT-IR (cm−1): 1560 (C-N), 1708 (C=O), 3380 (OH). 1H-NMR (δH, DMSO-d6): 10.58 (s, 1H, -OH), 9.96 (s, 1H, NCHN), 9.51 (s, 1H, -OH), 6.98-8.20 (m, 6H, ArH), 6.16 (s, 2H, -CH2), 5.64 (s, 1H, -C=C-H), 4.75-4.78 (t, 2H, J = 4 Hz, -CH2CH2OCH3), 3.82-3.85 (t, 2H, J = 4 Hz, -CH2CH2OCH3), 3.28 (s, 3H, -CH2CH2OCH3). 13C-NMR (δC, DMSO-d6): 160.3, 150.6, 149.8, 144.2, 143.8, 133.1, 131.9, 131.5, 127.5, 127.3, 115.3, 114.7, 114.2, 113.1, 110.3, 108.6, 69.3, 58.7, 47.4, 46.98.
1-(2-ethoxyethyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one)benzimidazolium chloride (3e)
Yield: 17%. mp: 219–223°C. Anal. cal. for. C21H21ClO5N2 C:60.51 H: 5.08 N:6.72; found: C: 60.61, H: 5.15, N:6.63. FT-IR (cm−1): 1564 (C-N), 1709 (C=O), 3270 (OH). 1H-NMR (δH, DMSO-d6): 10.6 (s, 1H, OH), 9.94 (s, 1H, NCHN), 9.51 (s, 1H, OH), 6.98-8.21 (m, 6H, ArH), 6.17 (s, 2H, -CH2), 5.61 (s, 1H, C=C-H), 4.75-4.78 (t, 2H, J = 5 Hz, -CH2CH2OCH2CH3), 3.85-3.88 (t, 2H, J = 5 Hz, -CH2CH2OCH2CH3), 3.45-3.50 (q, 2H, J = 7 Hz, -CH2CH2OCH2CH3), 1.01-1.06 (t, 3H, J = 7 Hz, -CH2CH2OCH2CH3). 13C-NMR (δC, DMSO-d6, 300 MHz, ppm): 160.2, 150.7, 149.9, 144.1, 143.8, 133.1, 131.8, 131.6, 127.5, 127.3, 115.3, 114.7, 114.2, 113.1, 110.3, 108.6, 67.1, 66.1, 47.6, 47.0, 15.3.
1-benzyl-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one)benzimidazolium chloride (3f)
Yield: 70%, 288–289°C. Anal. cal. for. C24H19ClO4N2 C:66.29 H:4.40 N:6.44; found: C:66.33, H:4.42, N: 6.41, FT-IR (cm−1): 1564 (C-N), 1703 (C=O), 3220 (OH). 1H-NMR (δH, DMSO-d6): 10.60 (s, 1H, -OH), 10.12 (s, 1H, NCHN), 9.51 (s, 1H, -OH), 6.99-8.07 (m, 11H, ArH), 6.13 (s, 2H, -CH2), 5.83 (s, 2H,-CH2C6H5), 5.81 (s, 1H, -C=C-H). 13C-NMR (δC, DMSO-d6): 160.3, 150.6, 149.5, 144.1, 143.9, 134.2, 133.1, 131.9, 131.6, 129.5, 129.3, 129.0, 127.6, 127.4, 115.2, 114.6, 114.5, 113.2, 110.3, 109.2, 50.7, 47.2.
1-(3-methylbenzyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one)benzimidazolium chloride (3g)
Yield: 71%. mp: 241–245°C. Anal. cal. for C25H21ClO4N2 C: 66.89, H:4.72, N:6.24; found: C:66.95, H:4.74, N: 6.20. FT-IR (cm−1): 1567 (C- N), 1700 (C=O), 3450 (OH). 1H-NMR (δH, DMSO-d6): 10.58 (s, 1H, -OH), 10.06 (s, 1H, NCHN), 9.53 (s, 1H, -OH), 7.08-8.03 (m, 10H, ArH), 6.24 (s, 2H, -CH2), 5.79 (s, 1H, -C=C-H), 5.77 (s, 2H, -CH2C6H4(CH3)), 2.3 (s, 3H, Ar-CH3). 13C-NMR (δC, DMSO-d6): 160.3,150.6, 149.5, 144.0, 143.9, 138.9, 134.1, 133.1, 131.9, 131.5, 129.9, 129.4, 129.4, 127.6, 127.47, 126.01, 115.0, 114.6, 114.4, 113.1, 110.3, 109.3, 50.7, 47.2, 21.4.
1-(2,3,5,6-tetramethylbenzyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one) benzimidazolium chloride (3h)
Yield: 31%. mp: 288–289°C. Anal. cal. for. C28H27ClO4N2 C:68.50 H:5.54 N:5.71; found: C:69.56 H:5.55, N:5.77. FT-IR (cm−1): 1567 (C-N), 1731 (C=O), 3440 (OH). 1H-NMR (δH, DMSO-d6): 10.54 (s, 1H, -OH), 9.49 (s, 1H, NCHN), 9.26 (s, 1H, -OH), 6.94-8.31 (m, 7H, ArH), 6.02 (s, 2H, -CH2), 5.78 (s, 2H, -CH2C6H(CH3)4), 5.60 (s, 1H, -C=C-H), 2.48 (s, 6H, 2xAr-CH3), 2.18 (s, 6H, 2xAr-CH3). 13C-NMR (δC, DMSO-d6): 160.3, 150.6, 150.2, 143.7, 142.8, 134.9, 134.7, 133.0, 133.0, 132.3, 132.2, 128.8, 127.7, 127.4, 115.0, 114.6, 114.4, 113.1, 110.1, 107.9, 56.5, 47.23, 46.49, 20.6, 15.9.
1-(2,3,4,5,6-pentamethylbenzyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one) benzimidazolium chloride (3i)
Yield: 15%, mp: 272°C. Anal. cal. for. C29H29ClO4N2 C:68.97, H:5.79, N: 5.55; found: C:69.05, H:5.85, N:5.47. FT-IR (cm−1): 1570 (C-N), 1705 (C=O), 3350 (OH). 1H-NMR (δH, DMSO-d6): 10.52 (s, 1H, -OH), 9.49 (s, 1H, NCHN), 9.23 (s, 1H, -OH), 6.94-8.34 (m, 6H, ArH), 6.01 (s, 2H, -CH2), 5.77 (s, 2H, -CH2C6(CH3)5), 5.59 (s, 1H, -C=C-H), 2.25 (s, 3H, Ar-CH3), 2.23 (s, 12 H, Ar-CH3). 13C-NMR (δC, DMSO-d6): 160.3, 150.6, 150.3, 143.7, 142.7, 136.8, 134.3, 133.5, 133.0, 132.25, 132.2, 127.7, 127.4, 126.1, 115.0, 114.7, 114.4, 113.1, 110.3, 107.9, 65.4, 47.00, 17.47,, 17.2, 16.9.
1-(3,4,5-trimethoxybenzyl)-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one)benzimidazolium chloride (3j)
Yield: 86%. mp: 284–286°C. Anal cal. for. C27H25ClO7N2 C: 61.78, H: 4.80, N:5.34; found: C:61.82, H:4.81, N:5.30. FT-IR (cm−1): 1563 (C-N), 1730 (C=O), 3231 (OH). 1H-NMR (δH, DMSO-d6): 10.60 (s, 1H,-OH), 10.13 (s, 1H, NCHN), 9.51 (s, 1H, -OH), 6.98-8.20 (m, 8H, ArH), 6.14 (s, 2H, -CH2), 5.77 (s, 1H, -C=C-H), 5.70 (s, 2H, CH2C6H2(OCH3)3), 3.77 (s, 6H, Ar-OCH3), 3.65 (s, 3H, Ar-OCH3). 13C-NMR (δC, DMSO-d6): 162.9,160.3, 153.7, 150.6, 149.7, 143.9, 138.1, 133.1, 131.9, 131.6, 129.4, 127.6, 127.4, 115.2, 114.7, 114.4, 113.2, 110.4, 109.1, 107.0, 60.5, 56.5, 51.0, 47.09.
Preparation of haemolysate and purification from blood red cells
Blood samples (25 mL) were taken from healthy human volunteers. They were anticoagulated with acid-citrate-dextrose (ACD), centrifuged at 2000g for 20 min at 4°C and the supernatant was removed. The packed erythrocytes were washed three times with 0.9% NaCI and then haemolysed in cold water. The ghosts and any intact cells were removed by centrifugation at 2000g for 25 min at 4°C, and the pH of the haemolysate was adjusted to pH 8.5 with solid Tris-base. The 25 mL haemolysate was applied to an affinity column containing L-tyrosine-sulfonamide-Sepharose-4BCitation28 equilibrated with 25 mM Tris–HCl/0.1 M Na2SO4 (pH 8.5). The affinity gel was washed with 50 mL of 25 mM Tris–HCl/22 mM Na2SO4 (pH8.5). The human CA (hCA) isozymes were then eluted with 0.1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6), which recovered hCA I and hCA II, respectively. Fractions of 3 mL were collected and their absorbance measured at 280 nm.
CA enzyme assay
CA activity was measured by the Maren method which is based on determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 hydrationCitation29. The assay solution was 0.5 M Na2CO3/0.1 M NaHCO3 (pH 10.0) and Phenol Red was added as the pH indicator. CO2-hydratase activity (enzyme units (EU)) was calculated by using the equation t0-tc/tc where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
In vitro inhibition studies
For the inhibition studies of coumarin, different concentrations of these compounds were added to the enzyme. Activity percentage values of CA for different concentrations of each coumarin were determined by regression analysis using Microsoft Office 2000 Excel. CA enzyme activity without a coumarin solution was accepted as 100% activity.
Results and discussion
In this study, series of 10 new water-soluble 1-alkyl-3-(4-methyl-7,8-dihydroxy-2H-chromen-2-one) benzimidazolium chloride salts (3a-j) derivatives were synthesized () and their inhibitory effects on the activity of purified hCA I and II were evaluated. For the coumarin having an inhibition affect, the inhibitor concentration causing up to 50% inhibition (IC50 values) was determined from the graphs (Figure 1 at Supplementary material). The result showed that these compounds (3a-j) inhibited the CA enzyme activity. The IC50 values of 3a-j analogues against hCA I and II were summarized in . Among the compounds synthesized, 3g and 3j was found to be most active one (IC50 = 22.09 µM and 20.33 µM) for hCA I and hCA II, respectively.
Table 1. The IC50 values of coumarin compounds.
Coumarins/thiocoumarins may possess various tautomeric forms, such as the zwitterionic benzo (thio) pyrylium phenoxides, which may bind within the CA active site similarly to phenols (Chart 2, step A)Citation30, i.e. by anchoring to the zinc-bound water molecule/hydroxide ionCitation31. It was reported that the coumarin-binding site in CAs may interact with diverse compounds, such as the antiepileptic drug lacosamide, which inhibits mammalian CAs I-XV, with inhibition constants in range of 331 nM–4.56 µMCitation32.
The natural productCitation11,Citation33 coumarin 6-(1S-hydroxy-3-methylbutyl)-7-methoxy-2H-chromen-2-one, as well as structurally related, simple coumarin derivatives possessing various substitution patterns at the heterocyclic ringCitation11, were shown to be hydrolyzed within the CA active site with formation of 2-hydroxy-cinnamic acids, which possess significant CA inhibitory properties and bind in a completely unprecedented manner to the enzyme, not interacting with the Zn(II) ion as the sulphonamide/sulfamate/sulfamide type of inhibitorsCitation33.
It was reported that the coumarin lacks classical zinc binding functional groups and exhibits an unusual binding mode that may be exploited to design isoform selective CA inhibitors. The inhibitor exhibits an extended two-arm conformation that effectively plugs the entrance to the active siteCitation33. The present investigation reported that coumarin derivatives (3a-j) had potent inhibitory effects on the hCAI and hCA II.
It has been reported that an original sulphonamide derivative of coumarin, namely 2-(8-methoxycoumarine-3-carbamido)-1,3,4-thiadia-zole-5-sulfonamide was synthesized. This compound was showed to inhibition effect against the hydratase activities of hCA I, hCA II (IC50 values of 4.7, 9.6 and 25.3 µM) and dog CA, respectivelyCitation15,Citation34. Our inhibition values were closes theirs.
Gupta and Kumaran (2005) reported that uncharged compounds cannot be made selective for cytosolic or membrane-bound isozyme since in both the cases the compounds appear to follow the same mechanism of inhibition. However, for the charged compounds the polarizability of the molecule seems to greatly favour the inhibition of the membrane-bound enzyme, and hence, they can be made selective for this enzyme by enhancing their polarizability, which is found to play no role in the inhibition of cytosolic enzymesCitation35.
Barros et al. reported that reaction of 20 aromatic/heterocyclic sulphonamides containing a free amino, imino, hydrazino or hydroxyl group, with 8-quinoline-sulfonyl chloride afforded a series of water-soluble (as hydrochloride or triflate salts) compounds. The new derivatives were assayed as inhibitors of the zinc enzyme CA, and more precisely of three of its isozymes, CA I, II (cytosolic forms) and IV (membrane-bound form), involved in important physiological processes. Some of the best inhibitors synthesized were topically applied as 2% water solutions onto the eye of normotensive and glaucomatous albino rabbits, when strong and long-lasting intraocular pressure (IOP) lowering was observed with many of them. This new compound is quite water-soluble as hydrochloride salt, behaves as a strong CA II inhibitor, and fared better than the parent molecule in lowering IOP in experimental animalsCitation26. Therefore, our new water-soluble coumarins can effective inhibitor for CA isozymes.
In summary, enzyme inhibition is more important issue for drug design and biochemical applicationsCitation36–41. The results showed that new coumarin derivatives inhibited the hCA I and II enzyme activity. Therefore, our results suggested that new coumarin derivatives are likely to be adopted as candidates to treat glaucoma and may be taken for further evaluation in in vivo studies.
Declaration of interest
This work was financially supported by Inönü University Research Fund (Project no: 2010/31).
Supplementary Material
Download PDF (402.9 KB)Supplementary Material
Download MS Word (475 KB)References
- Maresca A, Temperini C, Vu H, Pham NB, Poulsen SA, Scozzafava A et al. Non-zinc mediated inhibition of carbonic anhydrases: Coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–3062.
- Maresca A, Temperini C, Pochet L, Masereel B, Scozzafava A, Supuran CT. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–344.
- Barker HA, Smyth RD, Weissbach H, Toohey JI, Ladd JN, Volcani BE. Isolation and properties of crystalline cobamide coenzymes containing benzimidazole or 5, 6-dimethylbenzimidazole. J Biol Chem 1960;235:480–488.
- Ozdemir I, Demir S, Cetinkaya B, Gourlaouen C, Maseras F, Bruneau C et al. Direct arylation of arene C-H bonds by cooperative action of NHcarbene-ruthenium(II) catalyst and carbonate via proton abstraction mechanism. J Am Chem Soc 2008;130:1156–1157.
- Hindi KM, Panzner MJ, Tessier CA, Cannon CL, Youngs WJ. The medicinal applications of imidazolium carbene-metal complexes. Chem Rev 2009;109:3859–3884.
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Thiry A, Dogné JM, Masereel B, Supuran CT. Targeting tumor-associated carbonic anhydrase IX in cancer therapy. Trends Pharmacol Sci 2006;27:566–573.
- McKiernan JM, Buttyan R, Bander NH, Stifelman MD, Katz AE, Chen MW et al. Expression of the tumor-associated gene MN: A potential biomarker for human renal cell carcinoma. Cancer Res 1997;57:2362–2365.
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Expert Opin Ther Pat 2006;16:1627–1664.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Smith KS, Ferry JG. Prokaryotic carbonic anhydrases. FEMS Microbiol Rev 2000;24:335–366.
- Stams T, Christianson DW. (2000). X-ray crystallographic studies of mammalian carbonic anhydrase isozymes. In: Chegwidden WR, Carter ND, Edwards YH, eds. X-ray crystallographic studies of mammalian carbonic anhydrase isozymes. The Carbonic Anhydrases: New Horizons. Boston: Birkhauser Verlag, 159–174.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Nishimori I, Minakuchi T, Onishi S, Vullo D, Cecchi A, Scozzafava A et al. Carbonic anhydrase inhibitors: Cloning, characterization, and inhibition studies of the cytosolic isozyme III with sulfonamides. Bioorg Med Chem 2007;15:7229–7236.
- Vullo D, Franchi M, Gallori E, Antel J, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of mitochondrial isozyme V with aromatic and heterocyclic sulfonamides. J Med Chem 2004;47:1272–1279.
- Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. J Med Chem 2005;48:7860–7866.
- Nishimori I, Minakuchi T, Onishi S, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. DNA cloning, characterization, and inhibition studies of the human secretory isoform VI, a new target for sulfonamide and sulfamate inhibitors. J Med Chem 2007;50:381–388.
- Vullo D, Voipio J, Innocenti A, Rivera C, Ranki H, Scozzafava A et al. Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isozyme VII with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 2005;15:971–976.
- Nishimori I. (2004). Acatalytic CAs: Carbonic anhydrase-related proteins. In: Supuran CT, Scozzafava A, Conway J, eds. Carbonic anhydrase—Its inhibitors and activators. Boca Raton: CRC Press, 25–43.
- Vullo D, Franchi M, Gallori E, Pastorek J, Scozzafava A, Pastorekova S et al. Carbonic anhydrase inhibitors: Inhibition of the tumor-associated isozyme IX with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 2003;13:1005–1009.
- Vullo D, Innocenti A, Nishimori I, Pastorek J, Scozzafava A, Pastoreková S et al. Carbonic anhydrase inhibitors. Inhibition of the transmembrane isozyme XII with sulfonamides-a new target for the design of antitumor and antiglaucoma drugs? Bioorg Med Chem Lett 2005;15:963–969.
- Lehtonen J, Shen B, Vihinen M, Casini A, Scozzafava A, Supuran CT et al. Characterization of CA XIII, a novel member of the carbonic anhydrase isozyme family. J Biol Chem 2004;279:2719–2727.
- Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of the transmembrane isozyme XIV with sulfonamides. Bioorg Med Chem Lett 2005;15:3828–3833.
- Ashida M, Brey PT. Role of the integument in insect defense: Pro-phenol oxidase cascade in the cuticular matrix. Proc Natl Acad Sci USA 1995;92:10698–10702.
- Gumus A, Karadeniz Ş, Uğraş Hİ, Bulut M, Çakır Ü, Gören AC. Synthesis, complexation, and biological activity studies of 4-aminomethyl-7,8-dihydroxy coumarines and their crown ether derivatives. J Heteroc Chem 2010;47:1127–1133.
- Borras J, Scozzafava A, Menabuoni L, Mincione F, Briganti F, Mincione G et al. Carbonic anhydrase inhibitors: Synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl moieties: Is the tail more important than the ring? Bioorg Med Chem 1999;7:2397–2406.
- Ozdemir İ, Şahin N, Gök Y, Demir S, Çetinkaya B. In situ generated 1-alkylbenzimidazole-palladium catalyst for the Suzuki coupling of aryl chlorides. J Molec Catal 2005;234:181–185.
- Arslan O, Nalbantoglu B, Demir N, Ozdemir H, Küfrevioglu OI. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Turk J Med Sci 1996;26:163–166.
- Maren TH. A simplified micromethod for the determination of carbonic anhydrase and its inhibitors. J Pharm Exp Ther 1960;130:2629–2634.
- Ebbesen P, Pettersen EO, Gorr TA, Jobst G, Williams K, Kieninger J et al. Taking advantage of tumor cell adaptations to hypoxia for developing new tumor markers and treatment strategies. J Enzyme Inhib Med Chem 2009;24 Suppl 1:1–39.
- Nair SK, Ludwig PA, Christianson DW. 2-site binding of phenol in the active-site of human carbonic-anhydrase-ii - structural implications for substrate association. J Am Chem Soc 1994;116:3659–3660.
- Temperini C, Innocenti A, Scozzafava A, Parkkila S, Supuran CT. The coumarin-binding site in carbonic anhydrase accommodates structurally diverse inhibitors: The antiepileptic lacosamide as an example and lead molecule for novel classes of carbonic anhydrase inhibitors. J Med Chem 2010;53:850–854.
- Vu H, Pham NB, Quinn RJ. Direct screening of natural product extracts using mass spectrometry. J Biomol Screen 2008;13:265–275.
- Bayram T, Arslan O, Ugras HI, Cakir U, Ozensoy O. Purification of carbonic anhydrase from dog erythrocytes and investigation ofin vitroinhibition by various compounds. J Enzyme Inhib Med Chem 2007;22:739–744.
- Gupta SP, Kumaran S. A quantitative structure-activity relationship study on some aromatic/heterocyclic sulfonamides and their charged derivatives acting as carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2005;20:251–259.
- Aydemir T, Kavrayan D. Purification and characterization of glutathione-s-transferase from chicken erythrocyte. Artif Cells Blood Substit Immobil Biotechnol 2009;37:92–100.
- Gencer N, Arslan O. In vitro effects of some pesticides on PON1Q192 and PON1R192 isoenzymes from human serum. Fresen Envir Bull 2011;20:590–596.
- Gençer N, Arslan O. Purification human PON1Q192 and PON1R192 isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J Chromatogr B Analyt Technol Biomed Life Sci 2009;877:134–140.
- Sinan S, Gencer N, Turan Y, Arslan O. In vitro inhibition of the carbonic anhydrase from saanen goat (capra hircus) with pesticides. Pest Biochem Phys 2007;88:307–311.
- Kiranoglu S, Sinan S, Gencer N, Köckar F, Arslan O. In vivoeffects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biol Pharm Bull 2007;30:1048–1051.
- Sinan S, Kockar F, Gencer N, Yildirim H, Arslan O. Amphenicol and macrolide derived antibiotics inhibit paraoxonase enzyme activity in human serum and human hepatoma cells (HepG2) in vitro. Biochemistry Mosc 2006;71:46–50.