Abstract
Synthesis of carbazole substituted chalcone urea derivatives and their polyphenol oxidase enzyme activity effects on the diphenolase activity of banana tyrosinase were evaluated. Tyrosinase has been purified from banana on an affinity gel comprised of Sepharose 4B-l-tyrosine-p-aminobenzoic acid. The results showed that most of the compounds (3,4,5a,5d–h) inhibited and some of them (5c,5i–l) activated the tyrosinase enzyme activity. The molecular calculations were performed using Gaussian software for the synthesized compounds to explain the experimental results.
Introduction
Polyphenol oxidase (PPO), sometimes referred to as phenol oxidase, catecholase, phenolase, catechol oxidase, or even tyrosinase, is considered to be an o-dipenolCitation1. PPO (EC 1.14.18.1), a multifunctional copper containing enzyme, is widely distributed in nature. It catalyzes two distinct reactions of melanin synthesis: a hydroxylation of monophenols to o-diphenols (monophenolase activity) and an oxidation of o-diphenols to o-quinones (diphenolase activity), both using molecular oxygenCitation2. PPO is responsible, not only for melanisation in animals, but also for browning in plant. The unfavourable enzymatic browning of fruits and vegetables generally results in a loss of nutritional and commercial valueCitation3. An important group is constituted by compounds structurally analogous to phenolic substrate, which generally show competitive inhibition with respect to this substrate, although it can vary depending on enzyme source and substrate usedCitation4.
Chalcones are one of the major classes of natural products with widespread distribution in fruits, vegetables, spices, tea and soy based foodstuff. They have recently received a great deal of attention due to their interesting pharmacological activitiesCitation5. Some natural prenylated chalcones showed potent tyrosinase inhibitory activity. Three chalcones derivatives that are licuraside, isoisoliquritin, and Licochalcone A were isolated from the roots of the Glycyrrhiza species and competitively inhibited the monophenolase activity of mushroom tyrosinaseCitation6. Many biological activities have been attributed to this group, such as anticancer and antioxidantCitation7, antifungalCitation8, antimicrobialCitation9 and antibacterialCitation10 activities. A number of chalcone derivatives, have also been found to inhibit several important enzymes in cellular systems, including xanthine oxidaseCitation11, heme oxygenaseCitation12, protein tyrosine kinaseCitation13, quinone reductaseCitation14 and tyrosinaseCitation15,Citation16.
Nitrogen heterocycles have received a great deal of attention in the literature because of biological properties. Especially, among these heterocyclic systems, pyridine containing compounds have been the subject of expanding research efforts in heteroaromatic and biological chemistryCitation17. The pyrido [2,3-d] pyrimidine heterocycles, which are those annelated to a pyrimidine ring, are important because of their wide range of biologicalCitation18 and pharmaceutical applications (i.e. bronchodilators, vasodilators) and their anti-allergic, cardiotonic, antihypertensive, and hepatoprotective activitiesCitation19.
In the present study, we have synthesized derivatives of carbazole substituted chalcone urea for evaluation as potential inhibitors of PPO that could be beneficial in the prevent enzymatic browning.
Materials and methods
General procedure of the carbazole substituted chalcone urea derivatives
Melting points were taken on a Yanagimoto micro-melting point apparatus and are uncorrected. IR spectra were measured on a SHIMADZU Prestige-21 (200 VCE) spectrometer. 1H and 13C NMR spectra were measured on spectrometer at VARIAN Infinity Plus 300 and at 75 Hz, respectively. 1H and 13C chemical shifts are referenced to the internal deuterated solvent. The elemental analysis was carried out with a Leco CHNS-932 instrument Flash column chromatography was performed using Merck silica gel 60 (230–400 mesh ASTM). All chemicals was purchased from Merck, Alfa Easer, Sigma-Aldrich and Fluka.
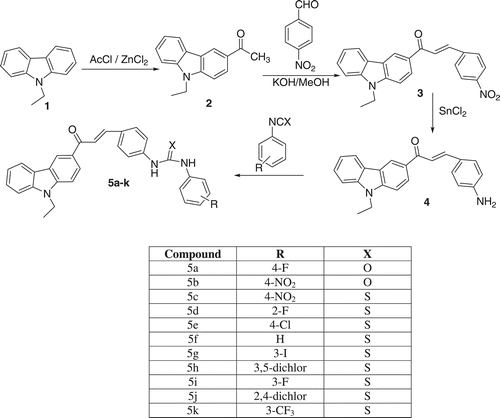
Synthesis of carbazole substituted chalcone urea derivatives was prepared according to .
Acetylation of 9-ethylcarbazole (2)
3-Acetyl-9-ethylcarbazole (2) necessary for the subsequent Claisen condensation was synthesizedCitation20 in one step starting from 9-ethyl-carbazole (1) (7.80 g, 40 mmol) and acetyl chloride (7 mL, 100 mmol). The Friedel – Crafts acylation is commonly catalyzed by BiCl3 (20.50 g, 65 mmol).
General procedure for the synthesis of chalcones (3)
The most useful method used to prepare chalcones is the condensation of acetophenones with benzaldehydes. Equimolar portions of 3-acetyl-9-ethylcarbazole (3.97 g, 16.77 mmol) and 4-nitrobenzaldehyde (2.48 g, 16.41 mmol) were dissolved in 30 mL of methanol. A 30 mL aliquot of 20% aqueous potassium hydroxide solution was then slowly added dropwise to the reaction mixture. The reaction solution was allowed to stir at room temperature for 24 h. The solution was extracted with AcOEt with an aqoues phase. Organic phase washed three times with water, dried with anhydrous magnesium sulphate and evaporated under reduced pressure.
1-(9-Ethyl-9H-carbazol-3-yl)-3-(4-nitro-phenyl)-propenone (3)
Recrystallized from ether to give as light yellow crystals. Yield 69.2%, m.p. 231°C; IR (KBr, υ, cm–1): 3070 (C=C, aromatic), 1660 (C=O), 1589 (NO2); 1HNMR (300 MHz, CDCl3, δ, ppm): 8.85 (1H, s, = CH), 7.81-7.82 (1H, d, = CH), 7.33–8.30 (10H, m, - Ar-H), 7.27–7.31 (1H, t, = CH), 4.39–4.46 (2H, q, - CH2), 1.46–1.61 (3H, t, - CH3). Anal. Calcd. for C23H18N2O3: C, 74.58; H, 4.90; N, 7.56. Found: C, 74.40; H, 4.974; N, 7.492.
General procedure for the synthesis of amino chalcones (4)
A mixture of 3 (1.1 g, 3.24 mmol), SnCl2 (3.66 g, 16.22 mmol) and tetrahydrofuran (30 mL) was stirred under reflux for 4 h. When the reaction completed, the solvent was evaporated under reduced pressure and the reaction mixture was diluted with water (15 mL), adjusted to pH = 10 with 1M sodium hydroxide solution, extracted with ethyl acetate (3 × 25 mL). The organic phase was dried over MgSO4 and filtered.
3-(4-Amino-phenyl)-1-(9-ethyl-9H-carbazol-3-yl)-propenone (4)
Recrystallized from ether to give as dark red crystals. Yield 50%, m.p. 152°C; IR (KBr, υ, cm–1): 1589 (- NH2), 3052 (C=C, aromatic), 1624 (C=O); 1HNMR (300 MHz, CDCl3, δ, ppm): 9.17 (1H, s, = CH), 8.39-8.42 (1H, d, = CH), 8.30-8.33 (1H, d, = CH), 7.92–7.97 (1H, d, = CH), 7.32–7.80 (5H, m, - Ar-H), 7.51–7.56 (1H, t, = CH), 7.30–7.35 (1H, t, = CH), 6.71–6.74 (2H, d, = CH), 4.48–4.50 (2H, q, -CH2), 4.47 (2H, s, -NH2), 1.33–1.36 (3H, t, -CH3). Anal. Calcd. for C23H20N2O: C, 81.15; H, 5.92; N, 8.23. Found: C, 79.25; H, 6.03; N, 7.56.
General procedure for synthesis of ureas and thioureas
A solution of 3-(4-amino-phenyl)-1-(9-ethyl-9H-carbazol-3-yl)-propenone (4) (0.19 g, 0.559 mmol) and isocyanate or isothiocyanate derivatives (0.096 g, 0.704 mmol) in dry DMF (2 mL) was stirred at 40°C for 6 h, quenched with water and filtered.
1-{4-[3-(9-Ethyl-9H-carbazol-3–yl)-3-oxo-propenyl]-phenyl}-3-(4-fluoro-phenyl)-urea (5a)
Recrystallized from ethyl acetate/hexane to give as light brown powder. Yield 88.89%, m.p. 285.3°C; IR (KBr, υ, cm–1): 3292 (-NH), 1707 (C=O), 1178 (C-F), 831 (C-H, aromatic); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 8.67 (1H, s, -NH), 8.35 (1H, s, -NH), 8.14 (1H, s, = CH), 8.03-,Ar-H), 4.21–4.28 (2H, q, - CH2), 1.26–1.31 (3H, t, - CH3). Anal. Calcd. for C30H24FN3O2: C, 75.46; H, 5.07; N, 8.80. Found: C, 74.06; H, 4.67; N, 7.90.
1-{4-[3-(9–Ethyl-9H-carbazol-3-yl)-3-oxo-propenyl]-phenyl}-3-(4-nitro-phenyl)-urea (5b)
Recrystallized from eter/acetone/hexane to give as brown powder. Yield 85.9%, m.p. 172.4°C; IR (KBr, υ, cm–1): 3252 (-NH), 3050 (C=C, aromatic), 1707 (C=O), 1501 (NO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 9.56 (1H, s, -NH), 9.26 (1H, s, -NH), 9.16 (1H, s, = CH), 8.37–8.40 (1H, d, = CH), 8.30–8.31 (1H, d, = CH), 8.27–8.28 (1H, d, = CH), 8.21–8.24 (1H, d, = CH), 7.95–8.10 (1H, d, = CH), 7.57–7.91 (9H, m, - Ar-H), 7.30–7.33 (1H, t, = CH), 7.52–7.54 (1H, t, =CH), 4.52–4.54 (2H, q, - CH2), 1.34–1.39 (3H, t, - CH3). Anal. Calcd. for C30H24N4O4 : C, 71.42; H, 4.79; N, 11.10. Found: C, 70.40; H, 4.19; N, 10.25.
1-{4-[3-(9-Ethyl-9H-carbazol-3-yl)-3–oxo-propenyl]-phenyl}–3 -(4-nitro-phenyl)-thiourea (5c)
Recrystallized from acetone/hexane to give as light brown powder. Yield 85.3%, m.p. 215.5°C; IR (KBr, υ, cm–1,): 3225 (-NH), 3058 (C=C, aromatic), 1179 (C=S), 1591 (NO2); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 10.12 (1H, s, -NH), 10.01 (1H, s, -NH), 8.80 (1H, s, = CH), 8.07–8.16 (4H, m, - Ar-H), 7.81–7.84 (1H, d, = CH), 7.21–7.74 (11H, m, - Ar-H), 4.35–4.38 (2H, q, - CH2), 1.35–1.40 (3H, t, - CH3). Anal. Calcd. for C30H24N4O3S: C, 69.21; H, 4.65; N, 10.76; S, 6.16. Found : C, 68.18; H, 4.35; N, 10.75; S, 5.19.
1-{4-[3-(9-Ethyl-9H-carbazol-3-yl)-3-oxo-propenyl]-phenyl}-3-(2-fluoro-phenyl)-thiourea (5d)
Recrystallized from ethyl acetate/hexane to give as light brown powder. Yield 88.6%, m.p. 252°C; IR (KBr, υ, cm–1): 3329 (-NH), 3019 (C=C, aromatic), 1194 (C=S), 1178 (C-F); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 10.24 (1H, s, -NH), 9.69 (1H, s, -NH), 9.16 (1H, s, = CH), 8.14–8.18 (1H, d, = CH), 7.30–8.37 (15H, m, - Ar-H), 4.50–4.52 (2H, q, - CH2), 1.33–1.36 (3H, t, - CH3). Anal. Calcd. for C30H24FN3OS: C, 73.00; H, 4.90; N, 8.51; S, 6.50. Found : C, 71.89; H, 4.45; N, 8.15; S, 5.29.
1-(4-Chloro-phenyl)-3-{4-[3-(9-ethyl-9H-carbazol-3-yl)-3-oxo-propenyl]-phenyl}-thiourea (5e)
Recrystallized from acetone/hexane to give as orange powder. Yield 77.6%, m.p. 163.9°C; IR (KBr, υ, cm–1): 3262 (-NH), 2981 (C=C, aromatic), 2260 (-N=C=S), 748 (C-Cl); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 10.16 (1H, s, -NH), 10.09 (1H, s, -NH), 9.16 (1H, s, = CH), 8.37–8.40 (1H, d, = CH), 8.31–8.32 (1H, d, = CH), 8.28–8.29 (1H, d, = CH), 7.92–7.95 (1H, d, =CH), 7.30–7.80 (12H, m, - Ar-H), 4.45–4.54 (2H, q, - CH2), 1.31–1.38 (3H, t, - CH3). Anal. Calcd. for C30H24ClN3OS: C, 70.64; H, 4.74; N, 8.24; S, 6.29. Found : C, 70.04; H, 4.24; N, 7.24; S, 5.19.
1-{4-[3-(9-Ethyl-9H-carbazol-3-yl)-3–oxo-propenyl]-phenyl}-3-phenyl-thiourea (5f)
Recrystallized from eter/ethyl acetate/hexane to give as orange powder. Yield 91%, m.p. 171.8°C; IR (KBr, υ, cm–1): 3327 (-NH), 3053 (C=C, aromatic), 2187 (-N=C=S); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 10.07 (1H, s, -NH), 10.01 (1H, s, -NH), 9.15 (1H, s, = CH), 8.13–8.17 (1H, d, = CH), 7.16–8.38 (16H, m, - Ar-H), 4.50–4.52 (2H, q, - CH2), 1.33–1.36 (3H, t, - CH3). Anal. Calcd. for C30H25N3OS: C, 75.76; H, 5.30; N, 8.84; S, 6.74. Found : C, 75.06; H, 4.50; N, 7.94; S, 5.74.
1-{4-[3-(9-Ethyl-9H–carbazol-3-yl)-3-oxo-propenyl]-phenyl}-3-(3-iodo-phenyl)-thiourea (5g)
Recrystallized from acetone/hexane to give as brown powder. Yield 76.9%, m.p. 207.8°C; IR (KBr, υ, cm–1): 3307 (-NH), 3048 (C=C, aromatic), 1192 (C=S), 622 (C-I); 1HNMR (300MHz, DMSO-d6, δ, ppm): 10.22 (1H, s, -NH), 10.07 (1H, s, -NH), 9.16 (1H, s, = CH), 8.37–8.40 (1H, d, = CH), 8.29-8.31 (1H, d, = CH), 8.14–8.19 (1H, d, = CH), 7.50–7.99 (11H, m, - Ar-H), 7.30–7.35 (1H, t, = CH), 7.13–7.19 (1H, t, = CH), 4.51–4.53 (2H, q, - CH2), 1.34–1.38 (3H, t, -CH3). Anal. Calcd. for C30H24IN3OS: C, 59.90; H, 4.02; N, 6.99; S, 5.33. Found : C, 58.73; H, 3.48; N, 6.598; S, 4.18.
1-(3,5-Dichloro-phenyl)-3-{4-[3-(9-ethyl-9H-carbazol-3-yl)-3-oxo-propenyl]-phenyl}-thiourea (5h)
Recrystallized from acetone/hexane to give as brown powder. Yield 64.1%, m.p. 201.4°C; IR (KBr, υ, cm–1): 3294 (-NH), 3077 (C=C, aromatic), 2039 (-N=C=S), 750 (C-Cl); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 9.47 (1H, s, -NH), 9.41 (1H, s, -NH), 8.71 (1H, s, =CH), 6.99–8.10 (15H, m, - Ar-H), 4.23–4.30 (2H, q, - CH2), 1.28–1.34 (3H, t, - CH3). Anal. Calcd. for C30H23Cl2N3OS: C, 66.18; H, 4.26; N, 7.72; S, 5.89. Found : C, 65.28; H, 3.56; N, 6.92; S, 4.89.
1-{4-[3-(9-Ethyl-9H-carbazol-3-yl)-3-oxo-propenyl]-phenyl}-3-(3–fluoro-phenyl)-thiourea (5i)
Recrystallized from ethyl acetate/hexane to give as dark orange powder. Yield 71.9%, m.p. 184.2°C; IR (KBr, υ, cm–1): 3319 (-NH), 2037 (-N=C=S), 1192 (C-F), 748 (C-H, aromatic); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 10.17 (1H, s, -NH), 10.12 (1H, s, -NH), 10.06 (1H, s, = CH), 9.13 (1H, s, = CH), 8.28–8.29 (1H, d, = CH), 8.25–8.26 (1H, d, = CH), 8.10–8.15 (1H, d, = CH), 7.89–7.93 (1H, d, = CH), 6.91–7.74 (11H, m, - Ar-H), 4.46–4.53 (2H, q, - CH2), 1.31–1.36 (3H, t, - CH3). Anal. Calcd. for C30H24FN3OS: C, 73.00; H, 4.90; N, 8.51; S, 6.50. Found : C, 68.71; H, 4.07; N, 8.45; S, 6.79.
1-(2,4-Dichloro-phenyl)–3-{4-[3-(9-ethyl-9H-carbazol-3-yl)-3-oxo-propenyl]-phenyl}-thiourea (5j)
Recrystallized from ether/acetone/hexane to give as brown powder. Yield 83.6%, m.p. 193.8°C; IR (KBr, υ, cm–1): 3329 (-NH), 3038 (C=C, aromatic), 1194 (C=S), 789 (C-Cl); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 10.34 (1H, s, -NH), 9.69 (1H, s, -NH), 9.16 (1H, s, = CH), 8.37–8.40 (1H, d, = CH), 8.30–8.32 (1H, d, = CH), 8.14–8.19 (1H, d, = CH), 7.95–7.97 (1H, d, = CH), 7.30–7.89 (11H, m, - Ar-H), 4.51–4.53 (2H, q, - CH2), 1.33–1.36 (3H, t, - CH3). Anal. Calcd. for C30H23Cl2N3OS: C, 66.18; H, 4.26; N, 7.72; S, 5.89. Found : C, 64.80; H, 3.84; N, 7.15; S, 4.70.
1-{4-[3-(9-Ethyl-9H–carbazol–3- yl)–3-oxo-propenyl]-phenyl}-3-(3-trifluoromethyl-phenyl)-thiourea (5k)
Recrystallized from acetone/hexane to give as orange powder. Yield 73.7%, m.p.199.4°C; IR (KBr, υ, cm–1): 3316 (-NH), 3058 (C=C, aromatic), 1175 (C=S), 1106 (C-CF3); 1HNMR (300 MHz, DMSO-d6, δ, ppm): 10.30 (1H, s, -NH), 10.21 (1H, s, -NH), 9.15 (1H, s, = CH), 8.37–8.39 (1H, d, = CH), 8.31–8.32 (1H, d, = CH), 8.28–8.29 (1H, d, = CH), 7.49–8.19 (13H, m, - Ar-H), 7.30–7.35 (1H, t, = CH), 4.51–4.54 (2H, q, - CH2), 1.34–1.39 (3H, t, - CH3). Anal. Calcd. for C31H24F3N3OS: C, 68.49; H, 4.45; N, 7.73; S, 5.90. Found : C, 69.06; H, 4.37; N, 7.55; S, 5.12.
Purification of PPO
All purification steps were carried out at 25°C. The extraction procedure was adopted from Wesche-Ebeling & MontgomeryCitation21. The bananas were washed with distilled water three times to prepare the crude extract, 50 g of bananas were cut quickly into thin slices and homogenized in a Waring blender for 2 min using 100 ml of 0.1 M phosphate buffer, pH:7.3 containing 5% poly(ethylene glycol) and 10 mM ascorbic acid. After filtration of the homogenate through muslin, the filtrate was centrifuged at 15,000g for 30 min, and the supernatant was collected. A crude protein precipitate was made by adding (NH4)2SO4 to 80% saturation. The resulting precipitate was suspended in a minimum volume of 5 mM phosphate buffer and then dialyzed against 5 the same buffer overnight. The enzyme solution was then applied to the Sepharose 4B-tyrosine-p-amino benzoic acid affinity columnCitation22, pre equilibrated with 5 mM phosphate buffer, pH 5.0. The affinity gel was extensively washed with the same buffer before the banana PPO (BPPO) was eluted with 1M NaCl, 5 mM phosphate, pH 7.0.
BPPO activity
Enzyme activity was determined; using catechol, by measuring the increase in absorbance at 420 nmCitation23, in a Biotek automated recording spectrophotometer. Enzyme activity was calculated from the linear portion of the curve. One unit of PPO activity was defined as the amount of enzyme that causes an increase in absorbance of 0.001 unit’s min−1 for 1 ml of enzyme at 25°C.
Inhibition of BPPO activity
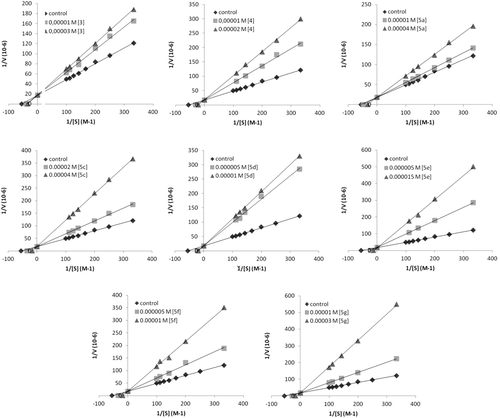
The inhibition type of diarylureas was determined by Lineweaver-Burk plots of 1/V vs. 1/S at two inhibitor concentrations. The inhibition constant, Ki, was deduced from the points of interception of the plots.
Results and discussion
Carbazole containing amino, nitro and urea chalcone derivatives were synthesized and examined effect on polyphenol oxidase enzyme. N-ethyl-3-acetylcarbazole (2) was prepared with N-ethylcarbazole and acetylchloride by ZnCl2 ctalayst. Carbazole containing nitro (3) and amino (4) chalcones prepared by the condensing various acetophenones and 4-nitrobenzaldehyde with NaOH as base, were reducted with tin (II) chloride in ethanol.The 4-aminochalcones were reacted with isocyanates and isothiocyanates to get the products (5a–l) at high yields.
The prepared compounds were characterized by 1H NMR, 13C NMR, IR and elemental analysis. The hydrogens attached to the nitrogen rezonans synthesized urea and thiourea compounds between 8.50 and 10.40 ppm and was indicated from the 1H NMR spectra. The signals for aromatic hydrogens and vinylic protons are between 6.55 and 8.15 ppm. From the 13C NMR spectra,chalcone and urea carbonyl are seen at around 188 and 150 ppm respectively. In the infrared spectra of compounds 5a–k, it was possible to observe the absorptions between 3250 and 3450 cm−1 relating to NH stretch, absorptions in 1600–1650 cm−1 from α, β-unsaturated carbonyl moiety stretch and absorptions in 1650–1750 cm–1 from urea carbonyl moiety stretching.
For evaluating the tyrosinase inhibitory activity, all the prepared compounds were subjected to tyrosinase inhibition assay with catechol as substrate. The results showed that most of the compounds (3, 4, 5a, 5c–g) inhibited and some of them (5b, 5h–k) activated the tyrosinase enzyme activity. Ki values were calculated from inhibition curves obtained with ureas (). Ki values of 19.97, 14.96, 24.96, 29.91, 7.47, 9.96, 7.48, 19.95 µM were obtained for ((3), (4), (5a), (5c), (5d), (5e), (5f), and (5g) respectively (). According to Ki values, (5d) was the most effective inhibitor for BPPO.
Table 1. Ki inhibition constants, dipol moment and ΔEHOMO-LUMO (eV) values of the compounds.
Several compound reported as PPO inhibitors were also shown to have inhibitors effect on the BPPO. Among the tested anti-browning reagent, the most effective ones were dithiotreithol and sodium metabisulfiteCitation24. The action of sulfite in the prevention of enzymatic browning can usually be explained by several processes. One is the action on o-quinones. The formation of quinone-sulfite complexes prevents the quinone polymerization. The action of sulfite in the prevention of enzymatic browning can usually be explained by several processes. One is the action on o-quinones. The formation of quinone-sulfite complexes prevents the quinone polymerizationCitation25. The enzyme also seemed to be sensitive to thiourea since PPO contains copper as a co-factor, the irreversible inactivation of this enzyme can be effected by substances (such as thiol compounds thiourea, -hydroxyquinoline, etc.), which remove copper from the active site of the enzymeCitation26. Chilaka et al. reported that thiourea was a good inhibitor of PPO, with low Ki values of 0.15 mM and inhibition of PPO was uncompetitiveCitation27. We were determined that (3), (4), (5a), (5c), (5d), (5e), (5f), and (5g) was a better inhibitor of PPO according to thiourea. Gulcin et al. reported that sodium diethyl dithiocarbamate was the most effective inhibitor (Ki: 1.79 nM) on nettle PPOCitation28. It was reported that the enzyme inhibited with diarylureasCitation15 and phenylthioureaCitation29, propanoic acidCitation30, alkyldithiocarbonatesCitation31 and coumarin schiff-basesCitation32, n-alkyl dithiocarbamate compoundsCitation33, sodium diethyl dithiocarbamateCitation28.
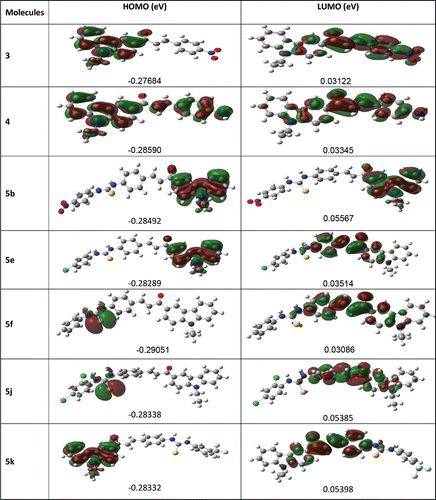
In order to understand and explain the experimental results obtained, molecular calculations were performed using Gaussian softwareCitation34a,b, for the synthesized compounds and some of the molecular structures are shown in . Some of the prepared compounds (5b, 5h–5k) did not inhibit tyrosinase at 50 µM, as well as show activator effects on tyrosinase enzyme. The other compounds (3,4, 5a, 5c–5g) showed enzyme inhibitory effects. Carbazole substituted chalcones (3 and 4) are precursor for the final products which are carbazole substituted chalcone urea derivatives. The compounds (3) and (4) are good inhibitory effect on tyrosinase enzyme. That can be explained either HOMO-LUMO energy differences or electron releasing and electron withdrawing groups on the phenyl ring. The compound (4) is better inhibitory effect than (3) because HOMO-LUMO energy differences of 4 (0.25245 eV) higher than 3 (0.24562 eV). In addition to this, amine group on the phenyl ring donates electron to the carbonyl group of chalcone and higher electron density binds copper ions more effectively in the active cite of enzyme. Urea derivatives (5a, 5c–5g) have enzyme inhibitory effect due to HOMO-LUMO energy differences. Generally, differentiation between HOMO and LUMO reflects the intensity of electron affinity, and lower differentiation suggests higher electron affinityCitation35. The calculated HOMO-LUMO energy differences of the compounds((5a, 5c–5g) showed a linear relationship for higher inhibitory effects with increasing ΔEHOMO-LUMO values which are higher than 0.23eV and the compounds (5b, 5h–5k) which have ΔEHOMO-LUMO values lower than 0.23eV showed enzyme activity effects. Among the urea compounds, 5d (Ki, 7.47 µM and ΔEHOMO-LUMO, 0.27405 eV) is the most inhibition effect. 5c (Ki, 29.91 µM and ΔEHOMO-LUMO, 0.23055 eV) is the least inhibition effect. This relation probably to be explained that the higher ΔEHOMO-LUMO differences of the molecule increases enzyme inhibition activity for possibility of binding to copper ions in the active cite of enzyme. Moreover, the dipole moments of the molecules are higher than 4.0 shows enzyme inhibitory effect. The results are shown in .
Declaration of interest
This work was supported by Research Fund of the Sakarya University. Project Number: 2012-02-04-033.
References
- Okot-Kotber M, Liavoga A, Yong KJ, Bagorogoza K. Activation of polyphenol oxidase in extracts of bran from several wheat (Triticum aestivum) cultivars using organic solvents, detergents, and chaotropes. J Agric Food Chem 2002;50:2410–2417.
- Yan Q, Cao R, Yi W, Yu L, Chen Z, Ma L et al. Synthesis and evaluation of 5-benzylidene(thio)barbiturate-beta-D-glycosides as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett 2009;19:4055–4058.
- Friedman M. Food browning and its prevention: An overview. J Agric Food Chem 1996;44:631–653.
- Vámos-Vigyázó L. Polyphenol oxidase and peroxidase in fruits and vegetables. Crit Rev Food Sci Nutr 1981;15:49–127.
- Di Carlo G, Mascolo N, Izzo AA, Capasso F. Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci 1999;65:337–353.
- Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci 2009;10:2440–2475.
- Anto RJ, Sukumaran K, Kuttan G, Rao MN, Subbaraju V, Kuttan R. Anticancer and antioxidant activity of synthetic chalcones and related compounds. Cancer Lett 1995;97:33–37.
- Lahtchev KL, Batovska DI, Parushev SP, Ubiyvovk VM, Sibirny AA. Antifungal activity of chalcones: a mechanistic study using various yeast strains. Eur J Med Chem 2008;43:2220–2228.
- Go ML, Wu X, Liu XL. Chalcones: an update on cytotoxic and chemoprotective properties. Curr Med Chem 2005;12:481–499.
- Batovska D, Parushev S, Stamboliyska B, Tsvetkova I, Ninova M, Najdenski H. A series of 3-unsubstituted/substituted-4,5-diphenyl-2-oxo-3 H-1,3-oxazole derivatives. Europ J Med Chem 2009;44:2211–2218.
- Sogawa S, Nihro Y, Ueda H, Miki T, Matsumoto H, Satoh T. Protective effects of hydroxychalcones on free radical-induced cell damage. Biol Pharm Bull 1994;17:251–256.
- Lee SH, Seo GS, Kim HS, Woo SW, Ko G, Sohn DH. 2′,4′,6′-Tris(methoxymethoxy) chalcone attenuates hepatic stellate cell proliferation by a heme oxygenase-dependent pathway. Biochem Pharmacol 2006;72:1322–1333.
- Nerya O, Musa R, Khatib S, Tamir S, Vaya J. Chalcones as potent tyrosinase inhibitors: the effect of hydroxyl positions and numbers. Phytochemistry 2004;65:1389–1395.
- Miranda CL, Aponso GL, Stevens JF, Deinzer ML, Buhler DR. Prenylated chalcones and flavanones as inducers of quinone reductase in mouse Hepa 1c1c7 cells. Cancer Lett 2000;149:21–29.
- Demir D, Gençer N, Arslan O, Genç H, Zengin M. In vitro inhibition of polyphenol oxidase by some new diarylureas. J Enzyme Inhib Med Chem 2012;27:125–131.
- Sonmez F, Sevmezler S, Atahan A, Ceylan M, Demir D, Gencer N et al. Evaluation of new chalcone derivatives as polyphenol oxidase inhibitors. Bioorg Med Chem Lett 2011;21:7479–7482.
- Quiroga J, Trilleras J, Abonía R, Insuasty B, Nogueras M, Cobo J, Torrec JM. 4-aminopyridine-5-carbaldehydes as intermediates in a friedlander type synthesis of 7-arylpyrido[2,3-D]pyrimidines. arkivoc 2009;xiv:9–27.
- Tu S, Li, C, Shi, F, Zhou, D, Shao, Q, Cao, L, Jiang, B. An efficient chemoselective synthesis of pyrido[2,3-d]pyrimidine derivatives under microwave irradiation. Synthesis 2008;3:369–372.
- Devi, I, Borah, HN, Bhuyan, PJ. Studies on uracils: a facile one-pot synthesis of oxazino[4,5-d]-,pyrano[2,3-d]-, pyrido[2,3-d]- and pyrimido[4,5-d]pyrimidines using microwave irradiation in the solid state. Tetrahedron Lett. 2004;45:2405–2410.
- Tang, H, Zhang, Z, Cong, C, Zhang, K. Potential application as optoelectronic material Russ J. of Org. Chem. 2009;45:559–563.
- Wesche-Ebeling P, Montgomery MW. Strawberry polyphenol oxidase: extraction and partial characterization. J Food Sci 1990;55:1320–1325.
- Arslan O, Erzengin M, Sinan S, Ozensoy O. Purification of mulberry (Morus alba L.) polyphenol oxidase by affinity chromatography and investigation of its kinetic and electrophoretic properties. Food Chem 2004;88:479–484.
- Espin JC, Morales M, Varon R, Tudela J, Garcia-Canovas F. A continuous spectrophotometric method for determining the monophenolase and diphenolase activities of apple polyphenol oxidase. Anal Biochem 1995;43:2807–2812.
- Sayaverde-Soto LA, Montgomery MW. Inhibition of polyphenol oxidase by sulfite. J. Food Sci. 1986;51:1531–1535.
- Embs RJ, Markakis P. The mechanism of sulphite inhibition of browning caused by polyphenol oxidase. J. Food Sci. 1965;30:753–758.
- Schwimmer S, Schwimmer S. (ed) Source Book of Food Enzymology. AVI Publishing, Westport. 1981;274:267
- Chilaka FC, Eze S, Anyadiegwu C, Uvere PO. Browning in processed yams: peroxidase or polyphenol oxidase? J. of the Science of Food and Agriculture 2002;82:899–903.
- Güllçin I, Küfrevioglu OI, Oktay M. Purification and characterization of polyphenol oxidase from nettle (Urtica dioica L.) and inhibitory effects of some chemicals on enzyme activity. J Enzyme Inhib Med Chem 2005;20:297–302.
- Ryazanova AD, Alekseev AA, Slepneva IA. The phenylthiourea is a competitive inhibitor of the enzymatic oxidation of DOPA by phenoloxidase. J Enzyme Inhib Med Chem 2012;27:78–83.
- Gheibi N, Saboury AA, Haghbeen K, Rajaei F, Pahlevan AA. Dual effects of aliphatic carboxylic acids on cresolase and catecholase reactions of mushroom tyrosinase. J Enzyme Inhib Med Chem 2009;24:1076–1081.
- Alijanianzadeh M, Saboury AA, Mansuri-Torshizi H, Haghbeen K, Moosavi-Movahedi AA. The inhibitory effect of some new synthesized xanthates on mushroom tyrosinase activities. J Enzyme Inhib Med Chem 2007;22:239–246.
- Gacche RN, Gond DS, Dhole NA, Dawane BS. Coumarin Schiff-bases: as antioxidant and possibly anti-inflammatory agents. J Enzyme Inhib Med Chem 2006;21:157–161.
- Gheibi N, Saboury AA, Mansuri-Torshizi H, Haghbeen K, Moosavi-Movahedi AA. The inhibition effect of some n-alkyl dithiocarbamates on mushroom tyrosinase. J Enzyme Inhib Med Chem 2005;20:393–399.
- (a) Gaussian 09, Revision A.1, Frisch, M. J, Trucks, G. W, Schlegel, H. B, Scuseria, G. E, Robb, M. A, Cheeseman, J. R, Scalmani, G, Barone, V, Mennucci, B, Petersson, G. A, Nakatsuji, H, Caricato, M, Li, X, Hratchian, H. P, Izmaylov, A. F, Bloino, J, Zheng, G, Sonnenberg, J. L, Hada, M, Ehara, M, Toyota, K, Fukuda, R, Hasegawa, J, Ishida, M, Nakajima, T, Honda, Y, Kitao, O, Nakai, H, Vreven, T, Montgomery, J. A. Jr, Peralta, J. E, Ogliaro, F, Bearpark, M, Heyd, J. J, Brothers, E, Kudin, K. N, Staroverov, V. N, Kobayashi, R, Normand, J, Raghavachari, K, Rendell, A, Burant, J. C, Iyengar, S. S, Tomasi, J, Cossi, M, Rega, N, Millam, J. M, Klene, M, Knox, J. E, Cross, J. B, Bakken, V, Adamo, C, Jaramillo, J, Gomperts, R, Stratmann, R. E, Yazyev, O, Austin, A. J, Cammi, R, Pomelli, C, Ochterski, J. W, Martin, R. L, Morokuma, K, Zakrzewski, V. G, Voth, G. A, Salvador, P, Dannenberg, J. J, Dapprich, S, Daniels, A. D, Farkas, O, Foresman, J. B, Ortiz, J. V, Cioslowski, J, Fox, D. J, Gaussian, Inc, Wallingford CT, 2009. (b) GaussView, Version 5, Dennington, R, Keith, T, Millam, J, Semichem. Inc., Shawnee Mission KS, 2009.
- Ohno S, Matsumoto N, Watanabe M, Nakajin S. Flavonoid inhibition of overexpressed human 3beta-hydroxysteroid dehydrogenase type II. J Steroid sBiochem Mol Biol 2004;88:175–182.