Abstract
A novel series of 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-8-yl)-1H-indole-2-carboxamides (3c–3g) were synthesized. The present study was undertaken to investigate the possible antihyperlipidemic effect of these novel compounds on hyperlipidemic rats. Hyperlipidemia was induced by a single intraperitoneal injection of Triton WR-1339 (300 mg/kg). The tested animals were divided into normal control (NCG), hyperlipidemic control (HCG), compounds 3c-, 3d-, 3e-, 3f-, 3g- and bezafibrate (BF)-treated groups. At a dose of 15 mg/kg, compounds 3c–3g and BF (100 mg/kg) significantly (p < 0.0001) reduced elevated plasma triglycerides levels after 12 and 24 h compared to the hyperlipidemic control group. However, only compounds 3e and 3g obviously showed a significant (p < 0.0001) reduction in plasma total cholesterol levels after 12 and 24 h. Moreover, high-density lipoprotein cholesterol levels were significantly increased in all treated groups. The current study demonstrates that 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-8-yl)-1H-indole-2-carboxamides (3c–3g) have a definite antihyperlipidemic potential and these beneficial activities may contribute to their cardioprotective and antiatherosclerotic role.
Introduction
Cardiovascular diseases continue to be a leading cause of morbidity and mortality among adults worldwideCitation1. Elevated levels of cholesterol, low-density lipoprotein cholesterol (LDL-C) and triglycerides (TG) contribute significantly to the development of atherosclerosis and coronary heart diseasesCitation2. It is widely acknowledged that dietary and drug therapy interventions could reduce serum lipids and dramatically decrease the risk of cardiovascular diseases (CVDs) and strokeCitation3.
Fibrates and their derivatives including a well known commercially available drug bezafibrate are widely used to decrease hyperlipidemia. Bezafibrate significantly reduces serum triglyceride and free fatty acid levelsCitation4. Fibrates act mainly on increasing the hydrolysis of TG by the induction of lipoprotein lipase and the reduction of apolipoprotein C-III synthesisCitation5.
Triton WR-1339, a nonionic detergent (oxyethylated tertiary octylphenol formaldehyde polymer) has been widely used to produce acute hyperlipidemia in animal models in order to screen natural or chemical drugsCitation6. The accumulation of plasma lipids by this detergent takes place by blocking the uptake of lipoprotein from the circulation by extrahepatic tissues, resulting in an increase in the level of circulatory lipoproteinsCitation7.
In recent years, there has been burgeoning interest in developing new pharmacologically active hypolipidemic drugs to overcome the efficacy problems of the current medications and the adverse effects.
During the last decade, a lot of attention has been given to studies focused on the synthesis of indole containing agents and their pharmacological activities. From these studies, which include our previous published data, it was found that the compounds containing the indole ring or anthraquinone moiety have a promising potential as lipid-lowering agentsCitation8–15. Previous work by our group revealed that N-(benzoylphenyl)-5-fluoro-1H-indole-2-carboxamide derivatives exhibited significant hypolipidemic effectCitation13.
Given the importance in correcting hyperlipidemia to decrease the risk of developing cardiovascular diseases, the present study focuses on the synthesis and pharmacological evaluation of a novel derivatives of 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-8-yl)-1H-indole-2-carboxamides; Compounds 3c–3g will be screened as models for their lipid-lowering effect ().
Materials and methods
Chemical studies
Infrared (IR) spectra were recorded on an Avatar Thermo Nicolet Impact 400 FT–IR spectrophotometer using Smart Omni-Transmission software; all samples were prepared as potassium bromide (Acros, Geel, Belgium) disks. 1H and 13C nuclear magnetic resonance (NMR) spectra were measured on a Bruker Ultra Shield 300 MHz instrument (Bruker, Bremen, Germany) operating at 300 MHz (1H) and 75 MHz (13C), respectively. Elemental analysis of C, H, and N was performed on a Euro elemental analyzer (model EA3000 A; Milan, Italy). High-resolution mass spectra (HRMS) were measured in positive ion mode using electrospray ion trap (ESI) technique by collision-induced dissociation on a Bruker Apex-4 (Tesla) instrument (Bremen, Germany). The analytical results for the elements were within ±0.4% of the theoretical values.
All starting materials were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. Experiments were performed in purified solvents.
Synthesis of 5-fluoro-1H-indole-2-carbonyl chloride (2)
A mixture of 5-fluoro-1H-indole-2-carboxylic acid (1.25 g, 7 mmol) and thionyl chloride (SOCl2) (2.5 mL, 34 mmol) in 40 mL of dry dichloromethane (DCM) was stirred under reflux for 6 h. After cooling to room temperature, DCM and the excess SOCl2 were evaporated under reduced pressure. The solid residue was suspended in hexane and the suspension was evaporated to dryness to afford 1.55 g (95%) of the solid residue which was used without further purification. 1H-NMR: (300 MHz, CDCl3) δ (ppm): 6.85–7.61 (4H, Ar), 9.81 (1H, NH).
Synthesis of 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-1-yl)-1H-indole-2-carboxamide (3c)
1-Aminoanthraquinone (0.87 g, 3.9 mmol) was added to a suspended solution of (0.098 g, 3.9 mmol) of sodium hydride (NaH) in dry N,N-dimethylformamide (DMF) and stirred about 30 min at room temperature before the addition of 5-fluoro-1H-indole-2-carbonyl chloride (0.91 g, 3.9 mmol). The reaction mixture was refluxed for 24 h, and then cooled to room temperature. DMF was evaporated under reduced pressure and the residue was stirred for 10 min in chloroform (CHCl3) and flashed through a short chromatography column (chloroform:methanol) in a ratio of (993:7). The solvent was removed under reduced pressure and the product was dried in vacuo. Yield: 35%; IR (KBr, cm−1): 3382, 3329, 1655, 1589, 1570; 1H-NMR: (300 MHz, DMSO-d6) δ (ppm): 11.51 and 11.82 (s, 1H, CONH, rotamers), 9.43 (s, 1H, H-1 indole), 7.35–7.41 (m, 2H, Ar-H), 7.65–7.71 (m, 2H, Ar-H), 7.88–7.92 (m, 3H, Ar-H), 8.03–8.10 (m, 2H, Ar-H), 8.21–8.39 (m, 2H, Ar-H); MS (ESI, +ve): m/z (M + H)+ = 407.0817, (C23H13FN2NaO3 requires 407.0808); analytically calculated for C23H13FN2O3: C, 71.87; H, 3.41; N, 7.29. Found: C, 71.42; H, 3.79; N, 6.92%.
Synthesis of N-(1-chloro-9,10-dihydro-9,10-dioxoanthracen-5-yl)-5-fluoro-1H-indole-2-carboxamide (3d)
1-Amino-5-chloroanthraquinone (0.93 g, 3.9 mmol) was added to a suspended solution of (0.098 g, 3.9 mmol) of NaH in dry N,N-dimethylformamide (DMF) and stirred about 30 min at room temperature before the addition of 5-fluoro-1H-indole-2-carbonyl chloride (0.91 g, 3.9 mmol). Workup of the reaction mixture as described for 3c above produced the compound 3d. Yield: 39%; IR (KBr, cm−1): 3379, 3320, 1664, 1584, 1571; 1H-NMR: (300 MHz, DMSO-d6) δ (ppm): 11.73 (s, 1H, CONH), 9.44 (s, 1H, H-1 indole), 7.51–7.61 (m, 5H, Ar-H), 7.49 (m, 1H, 7′-H), 7.31 (d, J = 8.0 Hz, 8′-H), 7.08-7.25 (m, 3H, Ar-H); MS (ESI, +ve): m/z (M + H)+ = 441.0419, (C23H12ClFN2NaO3 requires 441.0418); analytically calculated for C23H12ClFN2O3: C, 65.96; H, 2.89; N, 6.69. Found: C, 65.49; H, 3.18; N, 6.20%.
Synthesis of 5-fluoro-N-(9,10-dihydro-1-hydroxy-9,10-dioxoanthracen-4-yl)-1H-indole-2-carboxamide (3e)
1-Amino-4-hydroxyanthraquinone (0.93 g, 3.9 mmol) was added to a suspended solution of (0.18 g, 3.9 mmol) of NaH in dry N,N-dimethylformamide (DMF) and stirred about 30 min at room temperature before the addition of 5-fluoro-1H-indole-2-carbonyl chloride (0.91 g, 3.9 mmol). Workup of the reaction mixture as described for 3c above produced the compound 3e except that the acidification of the resulting solution by diluted HCl solution was conducted before the evaporation of the DMF. Yield: 23%; IR (KBr, cm−1): 3382, 3328, 3199, 1656, 1579, 1570; 1H-NMR: (300 MHz, DMSO-d6) δ (ppm): 13.69 (s, 1H, OH), 11.50 and 11.83 (s, 1H, CONH, rotamers), 9.43 (s, 1H, H-1 indole), 8.28–7.87 (m, 5H, Ar-H), 7.39–7.35 (m, 2H, Ar-H), 7.29–7.24 (m, 1H, Ar-H), 6.91–7.10 (m, 2H, Ar-H); MS (ESI, +ve): m/z (M + H)+ = 423.0761, (C23H13FN2NaO4 requires 423.0757); analytically calculated for C23H13FN2O4: C, 69.00; H, 3.27; N, 7.00. Found: C, 68.51; H, 3.77; N, 6.53%.
Synthesis of N-(1-bromo-9,10-dihydro-3-methyl-9,10-dioxoanthracen-4-yl)-5-fluoro-1H-indole-2-carboxamide (3f)
1-Amino-4-bromo-2-methylanthraquinone (1.23 g, 3.9 mmol) was added to a suspended solution of (0.098 g, 3.9 mmol) of NaH in dry N,N-dimethylformamide (DMF) and stirred about 30 min at room temperature before the addition of 5-fluoro-1H-indole-2-carbonyl chloride (0.90 g, 3.9 mmol). Workup of the reaction mixture as described for 3c above produced the compound 3f. Yield: 33%; IR (KBr, cm−1): 3375, 3311, 1663, 1589, 1574; 1H-NMR: (300 MHz, DMSO-d6) δ (ppm): 11.51 and 11.83 (s, 1H, CONH, rotamers), 9.42 (s, 1H, H-1 indole), 8.27–7.86 (m, 4H, Ar-H), 7.39–7.36 (m, 2H, Ar-H), 7.30–7.24 (m, 1H, Ar-H), 6.91–7.11 (m, 2H, Ar-H), 2.45 (s, 3H, CH3); MS (ESI, +ve): m/z (M + H)+ = 499.0069, (C24H14BrFN2NaO3 requires 499.0069); analytically calculated for C24H14BrFN2O3: C, 60.40; H, 2.96; N, 5.87. Found: C, 60.01; H, 3.37; N, 5.51%.
Synthesis of 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-3-yl)-1H-indole-2-carboxamide (3g)
2-Aminoanthraquinone (0.87 g, 3.9 mmol) was added to a suspended solution of (0.099 g, 3.9 mmol) of NaH in dry N,N-dimethylformamide (DMF) and stirred about 30 min at room temperature before the addition of 5-fluoro-1H-indole-2-carbonyl chloride (0.90g, 3.9 mmol). Workup of the reaction mixture as described for 3c above produced the compound 3g. Yield: 56%; IR (KBr, cm−1): 3383, 3325, 1651, 1589, 1573; 1H-NMR: (300 MHz, DMSO-d6) δ (ppm): 12.01 (br s, 1H, CONH), 10.95 (br s,1H, H-1 indole), 8.68 (m, 1H, Ar-H), 8.37–8.41 (m, 1H, Ar-H), 8.10–8.23 (m, 3H, Ar-H), 7.80–7.92 (m, 3H, Ar-H), 7.45–7.59 (m, 2H, Ar-H), 7.08–7.30 (m, 1H, Ar-H) ppm; MS (ESI, +ve): m/z [M + Na]+ 407.0817 (C22H13FN2NaO3, requires 407.0808); analytically calculated for C23H13FN2O3: C, 71.87; H, 3.41; N, 7.29. Found: C, 71.41; H, 3.69; N, 7.02%.
Animals and treatments
Forty eight adult male Wistar rats, weighing 180–250 g, bred in the animal care centre of Faculty of Pharmacy, Al-Zaytoonah University, Amman, Jordan, were provided ad libitum access only to tap water throughout the experimental duration. Rats were maintained in a 12 h light–dark cycle under constant humidity (55 ± 15%) and (22 ± 2°C). All experiments were performed in accordance with the Guidelines for Animal Welfare Committee of Al-Zaytoonah University.
Triton model of hyperlipidemia
Triton WR-1339 was dissolved in (dimethyl sulfoxide (DMSO)) and administered intraperitoneally to the rats (300 mg/kg body weight) in order to induce hyperlipidemia.
Pharmacological experimental design
Overnight fasted rats were randomly divided into eight groups of six animals each. The first group, serving as normal control group (NCG) received an intraperitoneal administration of normal saline; the second hyperlipidemic group (HCG) received an intraperitoneal injection of Triton 2% DMSO. In the third, fourth, fifth, sixth and seventh groups, rats were intraperitoneally injected with Triton, followed by an intragastric administration of (1 mL) of compounds 3c, 3d, 3e, 3f and 3g (15 mg/kg body weight) dissolved in 2% DMSO. The last group (BF) was also intraperitoneally injected with Triton and intragastrically treated with bezafibrate (100 mg/kg body weight) dissolved in 2% DMSOCitation16,Citation17. After 12 and 24 h of treatments, animals were anaesthetized with diethyl ether and blood was collected. The blood samples were immediately centrifuged (3000 rpm for 10 min) and the plasma was used for lipid analysis by an enzymatic method with an automatic analyzer (Model Erba XL-300, Mannheim, Germany).
LD50 determination of compounds 3c, 3d, 3e, 3f and 3g
Healthy rats of six animals per group and weighing 180–250 g were used for acute toxicity study and determination of LD50. Rats had free access of water and food, except for a short fasting period before treatment with the single oral dose of compounds 3c, 3d, 3e, 3f and 3g or the solvent. Compounds were formulated with DMSO.
An approximate LD50 was initially determined in a pilot study by a so called “staircase method” using a small number of animals (two each dose) and increasing doses of tested compounds. Six doses were used (200, 400, 600, 800, 1000, and 1200 mg/kg) and given to six groups of rats (six in each group). One additional group of rats was given equal amounts of DMSO orally and served as control group.
After administration of the tested compounds, the rats were observed for toxic effects during 72 h. The toxicological effects were observed in terms of mortality expressed as LD50 therefore the number of deceased animals within 24 h was noted. The LD50 of the test compound was determined by Litchfield and Wilcoxon methodCitation18.
Dose-response study of compounds 3c, 3d, 3e, 3f and 3g
The dose of the tested compounds were determined by a preliminary study using five different groups of rats of 10 rats each as shown in ().
Statistical analysis
Statistical analysis was performed using analysis of variance ANOVA and a least significant difference (LSD) post hoc test was used to compare individual means. The results were expressed as the mean ± SD of six values in each group, and a statistical probability of p < 0.05, p < 0.0001 was considered to be significant.
Results
Synthesis
5-Fluoro-1H-indole-2-carbonyl chloride (2) was prepared in good yield by reaction of 5-fluoro-1H-indole-2-carboxylic acid (1) with an excess thionyl chloride in dried dichloromethane solvent under reflux ().
In the search for new indole derivatives that may display antihyperlipidemic activities, a new series of 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-8-yl)-1H-indole-2-carboxamides (3c–3g) () was prepared and tested for their biological activities.
The target compounds were synthesized by the coupling reaction of 5-fluoro-1H-indole-2-carbonyl chloride and an excess of the corresponding aminoanthraquinone (c–g) in the presence of sodium hydride as a strong base in dried DMF under reflux. Hydride anion was employed as a catalyst to increase the rate of the nucleophilic substitution reaction (consequently the yield), since the aromatic aminoanthraquinones are weak nucleophiles. It is worth mentioning that the direct solid reaction of 2 with the corresponding amine (without solvent) could be also applied.
The reaction progress was monitored by thin layer chromatography (TLC) which was based on the disappearance of the carbonyl chloride (2) spot. Due to the vigorous experimental conditions, the purification of the final products by the column chromatography is an essential step. The IR, MS, NMR spectral data for the new compounds is in accordance with the assigned structures; details were given in the experimental part.
Pharmacological activity
Induction of hyperlipidemia by Triton WR-1339
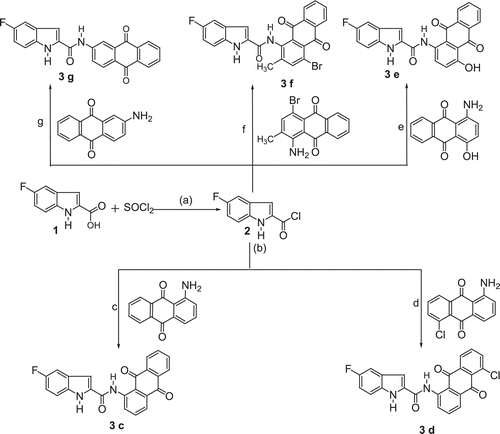
The plasma total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) levels of all groups treated for 12 and 24 h are shown in ( and ), respectively. The acute injection of Triton WR-1339 caused a significant increase in plasma cholesterol, low-density lipoprotein cholesterol and triglyceride (p < 0.0001) levels in hyperlipidemic control group (HCG) either 12 or 24 h after Triton injection in comparison with the normal control group (NCG). In fact, the increases of plasma TC levels in the (HCG) were 81 and 294% after 12 and 24 h, respectively, as compared to the (NCG). At the same time, LDL-C levels in the (HCG) were also elevated by 121 and 103% after 12 and 24 h, respectively ().
Figure 1. Effect of Triton-WR-1339 on lipid profile after (a) 12 h and (b) 24 h. Values are means ± SD from six animals in each group. NCG, control group; HCG, hyperlipidemic control group; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. HCG is compared to NCG. *p < 0.0001.
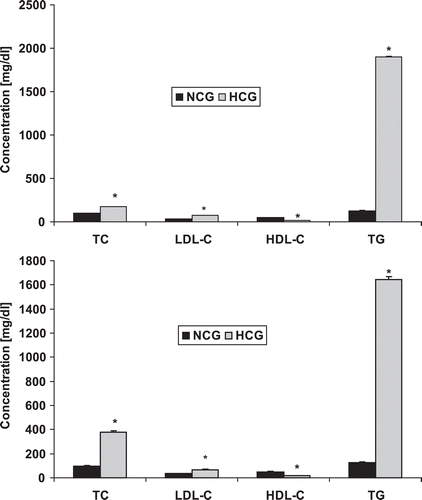
Figure 2. Dose-response study of the tested compounds. 3c, 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-1-yl)-1H-indole-2-carboxamide treated group; 3d, N-(1-chloro-9,10-dihydro-9,10-dioxoanthracen-5-yl)-5-fluoro-1H-indole-2-carboxamide treated group; 3e, 5-fluoro-N-(9,10-dihydro-1-hydroxy-9,10-dioxoanthracen-4-yl)-1H-indole-2-carboxamide; 3f, N-(1-bromo-9,10-dihydro-3-methyl-9,10-dioxoanthracen-4-yl)-5-fluoro-1H-indole-2-carboxamide; 3g, 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-3-yl)-1H-indole-2-carboxamide.
The triglyceride levels in the (HCG) were markedly increased by more than 15 and 12 times after 12 and 24 h, respectively, as compared to the (NCG).
Triton WR-1339 caused a significant decrease in HDL cholesterol levels (p < 0.0001) in the hyperlipidemic control (HCG), at both 12 and 24 h after Triton administration in comparison with the NCG. In fact, the decreases of plasma HDL-C levels in the HCG were 73 and 68% after 12 and 24 h, respectively, as compared to the NCG ().
Effect of compounds 3c, 3d, 3e, 3f, 3g and bezafibrate on rat plasma lipid profile
The plasma total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL-C) and low-density lipoprotein (LDL-C) levels of compounds 3c-, 3d-, 3e-, 3f-, 3g- and bezafibrate (BF)-treated rats at 12 and 24 h are shown in , respectively. Importantly, the elevated plasma TG levels produced by the acute injection of Triton WR-1339 were significantly (p < 0.0001) suppressed in compounds 3c by (73, 22%), in 3d by (74, 24%), in 3e by (86, 33%), in 3f by (75, 23%), in 3g by (80, 33%) and in BF by (88, 52%) after 12 and 24 h, respectively, with respect to hyperlipidemic control HCG.
Table 1. Effect of compounds 3c, 3d, 3e, 3f, 3g and bezafibrate on plasma lipid levels in Triton WR-1339-induced hyperlipidemic rats after 12 h and 24 h.
With the exception of bezafibrate, compounds 3c, 3d, 3e, 3f and 3g showed a significant (p < 0.0001) 33, 37, 46, 40 and 20% reduction in plasma cholesterol level, respectively, after 12 h of Triton administration compared to the HCG.
However, after 24 h of Triton administration only compounds 3f and 3g managed to maintain this reduction of plasma cholesterol level.
The HDL-cholesterol levels were significantly increased after 12 h of Triton administration (+40, +48, +148, +36, +114 and +219%, p < 0.0001) in 3c, 3d, 3e, 3f, 3g and BF, respectively, compared to HCG (). The increase in HDL-cholesterol levels after 24 h in compounds 3c, 3g and BF were maintained (p < 0.0001). The increase in HDL-C levels were not considered highly significant (+7, +38 and +11%, p < 0.05) in 3c, 3e and 3f, respectively, compared to HCG ().
Compounds 3c, 3d, 3e, 3f and 3g show similar trend on meditating plasma LDL-C level after 12 h of Triton administration compared to HCG. In fact, compounds 3c, 3d, 3e, 3f and 3g significantly (p < 0.0001) reduced LDL-C level by (39, 41, 50, 38 and 26%), respectively, compared to HCG.
However, only compounds 3e and 3g maintained this reduction in LDL-C level after 24 h of Triton injection compared to HCG.
Neither after 12 h nor after 24 h did bezafibrate group significantly decreased LDL-C level compared to HCG.
The median lethal dose (LD50) in rats of the active test compounds was evaluated by a single intra-gastric administration. The LD50 (mean ± SD) of the active compounds 3c, 3d, 3e, 3f and 3g was determined to be 800 ± 42, 822 ± 41, 799 ± 26, 811 ± 33 and 786 ± 23 mg/kg, respectively.
There were no mortality or symptoms similar to those obtained after the tested compounds treatment observed in the DMSO treated control animals.
Discussion
Triton WR-1339 has been widely used as a model to produce acute hyperlipidemia in animals by inhibiting the enzyme lipoprotein lipaseCitation19,Citation20. In this model, the peak plasma triglyceride level was reached at 12 h and the plasma total cholesterol level was reached after 24 hCitation7,Citation21–24. In the course of this study, the same model gave a similar pattern of lipid profile changes either 12 or 24 h after Triton WR-1339 administration ( and ).
The results of the current study demonstrated the potential hypolipidemic effect of 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-8-yl)-1H-indole-2-carboxamide derivatives. Compounds 3c, 3d, 3e, 3f and 3g significantly reduced serum TG and increased serum HDL-C after 12 and 24 h of Triton administration.
Yamamoto and his colleagues reported that the large decrease in plasma HDL-C levels due to Triton WR-1339 injection results mostly from a progressive displacement of the apo A-1 protein from the HDL surface without loss of lipid. Meanwhile the large increase in plasma TG levels due to Triton administration results mostly from an increase of very low-density lipoprotein (VLDL) secretion by the liver accompanied by strong reduction of VLDL and LDL catabolismCitation25,Citation26.
Accordingly, given that the proportion of triglyceride in VLDL is many times higher than cholesterol, it is not surprising that the hypolipidemic activity of compounds 3c–3g was significantly higher for triglycerides than for cholesterol. This result suggests that our compounds are able to restore, at least partially, catabolism of B-lipoproteins as hypothesized by many works with other lipid-lowering agentsCitation27,Citation28.
Promisingly, compounds 3c–3g at a dose of 15 mg/kg body weight 12 and 24 h after Triton injection had the same potential in reducing TG levels and in increasing HDL-C levels compared to bezafibrate at a dose of 100 mg/kg body weight, which in this study has been used as standard reference hypolipidemic drug.
Furthermore, total cholesterol levels were not significantly changed which agrees with the mechanism of action of fibrates in that their total cholesterol-lowering activity is not strongly marked, but the triglycerides decreasing effect of them is very impressive especially by stimulation of the gene expression of lipoprotein lipaseCitation29.
The LD50 study showed that 5-fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-8-yl)-1H-indole-2-carboxamide derivatives exhibit no toxicity and are well tolerated by experimental animals.
The pharmacological effect of compounds 3c–3g confirmed the essentiality of the presence of the three structural components (aromatic heterocyclic ring capable of hydrogen bond formation, carboxamide linkage and a lipophilic area) for the lipid lowering activityCitation12–15.
Our compounds have shown these structural features through the presence of the 5-fluoroindole (H-bond donar) linked by a carboxamide linkage to the anthraquinone moiety (lipophilic component).
Compound 3g was found to have better pharmacological activity than compound 3c. This finding can be explained by the fact that compound 3g has a more extended structure due to the presence of longer carboxamide linkage than compound 3c, which in turn enhance its fitting with the target.
The results indicated that compound 3e was found to be more potent than compound 3d and 3f. When compared with compounds 3d and 3f, compound 3e had polar and acidic functional group on the anthraquinone moiety with no lipophilic substituents as found in compound 3d and 3f. This may lead to a conclusion that the presence of hydrophilic and/or acidic substituents explains the improved pharmacological activity of compound 3e.
However, future work is needed to investigate which structural component (hydrophilic or the acidic functional group) specifically has attributed more to improve the pharmacological activity.
Conclusion
5-Fluoro-N-(9,10-dihydro-9,10-dioxoanthracen-8-yl)-1H-indole-2-carboxamide derivatives; compounds 3c–3g were shown to improve lipid abnormalities such as hypertriglyceridemia and hypercholesterolemia, and then elevated HDL levels in Triton induced rats, suggesting them as possible useful candidates in the treatment of patients with lipid abnormalities. These findings are compatible with our previous published data, which confirm that compounds possess indole-2-carboxamide nucleus have lipid lowering effectCitation8–15,Citation30. The results are highly promising but more studies are necessary to figure out the exact mechanism of action of these novel compounds as antihyperlipidemic agents.
Acknowledgement
The authors wish to express their sincere appreciation to Al-Zaytoonah Private University of Jordan for financial support and to Ghadeer Albadawi for technical support.
Declaration of interest
All authors declare that the work of the current article has not been published else where, either completely, in part, or in any other form and that the manuscript has not been submitted to another journal.
References
- Sunil C, Ignacimuthu S, Kumarappan C. Hypolipidemic activity of Symplocos cochinchinensis S. Moore leaves in hyperlipidemic rats. J Nat Med 2012;66:32–38.
- Yokozawa T, Ishida A, Cho EJ, Nakagawa T. The effects of Coptidis Rhizoma extract on a hypercholesterolemic animal model. Phytomedicine 2003;10:17–22.
- Xiaoli Y, Kai H, Xiaokang Z, Baoshun Z, Xin C, Jun Y, Xuegang L. Synthesis and antihyperlipidemic efficiency of berberine-based HMG-CoA reductase inhibitor. Med Chem Res 2011, DOI: 10.1007/s00044-011–9651-z.
- Koh KK, Quon MJ, Rosenson RS, Chung WJ, Han SH. Vascular and metabolic effects of treatment of combined hyperlipidemia: focus on statins and fibrates. Int J Cardiol 2008;124:149–159.
- Al-Hiari Y, Shattat G, Al-Qirim T, El-Huneidi W, Sheikha GA, Hikmat S. Antihyperlipidemic properties of novel N-(benzoylphenyl)-5-substituted-1H-indole-2-carboxamides in Triton WR-1339-induced hyperlipidemic rats. Molecules 2011;16:8292–8304.
- Hayashi H, Niinobe S, Matsumoto Y, Suga T. Effects of Triton WR-1339 on lipoprotein lipolytic activity and lipid content of rat liver lysosomes. J Biochem 1981;89:573–579.
- Friedman M, Byers SO. Mechanism underlying hypercholesteremia induced by triton WR-1339. Am J Physiol 1957;190:439–445.
- Bosies E, Heerdt R, Kuknle F, Schmidt H, Stach H. Hypoglycemically and hypolipidemically active derivatives of phenylalkane carboxylic acids. US Patent 150207, 1980.
- Dasseux H, Oniciu D. Ketone compounds and compositions for cholesterol management and related uses. US Patent 325529, 2002.
- Kopin S, Carey M, Wang D. Methods of altering intestinal motility and absorption of hydrophobic compounds through the use of agonists and/or antagonists of the cholecystokinin–1 receptor. US Patent 224869,2005.
- Sher M, Ellsworth A. Triglyceride and triglyceride–like prodrugs of glycogen phosphorylase inhibiting compounds. US Patent 7098235,2003.
- Shahwan M, Shattat G, Al-Qirim T, Sheikha GA, Al-Hiari Y, El-Huneidi W et al. Synthesis and pharmacological evaluation of novel substituted and unsubstituted N-(benzoylphenyl)-1H-indole-2-carboxamides as potent antihypertriglyceridemic agents. Z Naturforsch, C, J Biosci 2010;65:309–316.
- Shattat G, Al-Qirim R, Al-Hiari Y, Sheikha GA, Al-Qirim T, El-Huneidi W et al. Synthesis and anti-hyperlipidemic evaluation of N-(benzoylphenyl)-5-fluoro-1H-indole-2-carboxamide derivatives in Triton WR-1339-induced hyperlipidemic rats. Molecules 2010;15:5840–5849.
- Al-Qirim T, Shahwan M, Shattat G, Al-Hiari Y, Abu-Sheikha G, Zaidi S. Pharmacological evaluation of novel indole-2-carboxamides as potent lipid-lowering agents in Triton-WR-1339-induced hyperlipidemic rats. Z Naturforsch, C, J Biosci 2009;64:619–625.
- Shattat G, Al-Qirim T, Sweidan K, Shahwan M, El-Huneidi W, Al-Hiari Y. The hypolipidemic activity of novel benzofuran-2-carboxamide derivatives in Triton WR-1339-induced hyperlipidemic rats: a comparison with bezafibrate. J Enzyme Inhib Med Chem 2010;25:751–755.
- Nakajima T, Tanaka N, Kanbe H, Hara A, Kamijo Y, Zhang X et al. Bezafibrate at clinically relevant doses decreases serum/liver triglycerides via down-regulation of sterol regulatory element-binding protein-1c in mice: a novel peroxisome proliferator-activated receptor alpha-independent mechanism. Mol Pharmacol 2009;75:782–792.
- Mori Y, Tokutate Y, Oana F, Matsuzawa A, Akahane S, Tajima N. Bezafibrate-induced changes over time in the expression of uncoupling protein (UCP) mRNA in the tissues: a study in spontaneously type 2 diabetic rats with visceral obesity. J Atheroscler Thromb 2004;11:224–231.
- Litchfield JT Jr., Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 1949;96:99–113.
- Schurr PE, Schultz JR, Parkinson TM. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids 1972;7:68–74.
- Kellner A, Correll JW, Ladd AT. The influence of intravenously administered surface-active agents on the development of experimental atherosclerosis in rabbits. J Exp Med 1951;93:385–398.
- Ohmori K, Yamada H, Yasuda A, Yamamoto A, Matsuura N, Kiniwa M. Effects of a novel anti-hyperlipidemic agent, S-2E, on blood lipid levels in rats with fructose-induced hypertriglyceridemia. Pharmacology 2004;72:240–246.
- Harnafi H, Bouanani Nel H, Aziz M, Serghini Caid H, Ghalim N, Amrani S. The hypolipidaemic activity of aqueous Erica multiflora flowers extract in Triton WR-1339 induced hyperlipidaemic rats: a comparison with fenofibrate. J Ethnopharmacol 2007;109:156–160.
- Lauk L, Galati EM, Forestieri AM, Kirjavainen S, Trovato A. Mucuma pruriens infusion lowers cholesterol and total lipid plasma levels in the rats.Phytother Res 1989;3:263–264.
- Khanna AK, Chander R, Singh C, Srivastava AK, Kapoor NK. Hypolipidemic activity of Achyranthus aspera Linn in normal and triton induced hyperlipemic rats. Indian J Exp Biol 1992;30:128–130.
- Yamamoto K, Byrne R, Edelstein C, Shen B, Scanu AM. In vitro effect of Triton WR-1339 on canine plasma high density lipoproteins. J Lipid Res 1984;25:770–779.
- Otway S, Robinson DS. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol (Lond) 1967;190:321–332.
- Campillo JE, Torres MD, Dominguez E, Romero A, P´erez C. Ficus carica leaf administration reduces hypertrygliceridaemia in streptozotocin diabetic rats. Diabetologia 1994;37:A213.
- Pérez C, Canal JR, Campillo JE, Romero A, Torres MD. Hypotriglyceridaemic activity of Ficus carica leaves in experimental hypertriglyceridaemic rats. Phytother Res 1999;13:188–191.
- Staels B, Dallongeville J, Auwerx J, Schoonjans K, Leitersdorf E, Fruchart JC. Mechanism of action of fibrates on lipid and lipoprotein metabolism. Circulation 1998;98:2088–2093.
- Al-Hiari Y, Shattat G, Al-Qirim T, El-Huneidi W, Abu Sheikha G,Hikmat S. Antihyperlipidemic properties of novel N-(benzoylphenyl)-5-substituted-1H-indole-2-carboxamides in triton WR-1339-induced hyperlipidemic rats. Molecules 2011;16:8292–8304.