Abstract
Mannich bases 2a-f derived from 3,4-dimethylphenol (1), formaldehyde and different amines are prepared and subjected to spectral (IR, 1H and 13C NMR) and elemental analyses. The inhibition of two human carbonic anhydrase (hCA, EC 4.2.1.1) isozymes I and II, with 1 and synthesized Mannich bases 2a-f and acetazolamide (AAZ) as a control compound was investigated in vitro by using the hydratase and esterase assays. In relation to hydratase and esterase activities of the half maximal inhibitory concentration (IC50) and the inhibition equilibrium constants (Ki)values were determined. Only two compounds (2a and 2e)exhibit weak hCA II inhibitory effects on esterase activity. IC50 and Ki values for 2a and 2e with respect to esterase activity of hCA II are0.88 × 103 and 6.3–7.6 µM and 0.44 × 103 and 19.0–96.4 µM,respectively. On the contrary, compounds 2b and 2d might be used as CA activators due to increasing esterase activity of hCA I and hCA II isozymes.
Introduction
Carbonic anhydrase (CA, EC 4.2.1.1) has a catalytic function of CO2 hydration. In the active site of CA, Zn(II) ion has a tetrahedral geometry and is coordinated by the three histidine imidazoles and a water moleculeCitation1. At least 16 CA isozymes were described up to now in mammals, the most active ones as catalysts for carbon dioxide hydration being CA II and CA IXCitation2–5. Several of these isozymes (hCA II and hCA IV) are present in human eyesCitation6–9 causing glaucoma, which is a group of diseases characterized by a gradual loss of the visual field due to an elevation in intraocular pressure (IOP), and being the second leading cause of blindness worldwideCitation9,Citation10. Since CA inhibitors have been shown to reduce IOP exclusively by lowering the aqueous humour flow and these compounds have been used for the treatment of glaucoma for yearsCitation11,Citation12. The rate-determining step for the CO2 hydration reaction catalyzed by CAs is the proton transfer reaction from the water bound to the Zn(II) ion to the reaction medium, with generation of the zinc hydroxide species of the enzymeCitation13–20.
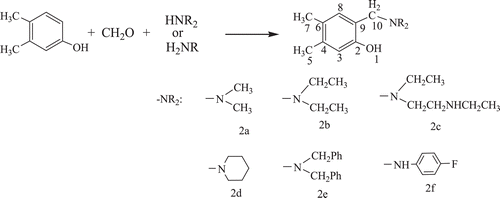
Several anions behave as inhibitors for the CO2 hydration reactionCitation21,Citation22 and coordinate directly to Zn(II) ion. A number of studies on the zinc model complexes as the active site of CA, which consist of amines and amino acid derivatives are the most investigated onesCitation23–31. The interaction of all 16 mammalian CA isozymes with several types of phenols, some of which are widely used as antioxidant food additives or as drugs has been also investigatedCitation32–35. Some antioxidant phenol derivatives have been showed effective hCA II inhibitory effects, in the same range as the clinically used sulfonamide acetazolamideCitation36. This has given impetus for the synthesis of Mannich bases from these compounds using Mannich reaction. Mannich reaction offers a convenient method for introduction of the basic aminoalkyl chain, which alters the biological profile and physicochemical characteristicsCitation37. Various drugs obtained from Mannich reaction have proved more effective and less toxic than their parent antibioticsCitation38. In the present study, previously synthesized 2aCitation39 and novel Mannich bases 2b-f have been prepared by using microwave assist reaction of 3,4-dimethylphenol (1) with formaldehyde and various amines () which contain both functional phenolic–OH group and -NHR or -NR2 groups. They have been characterized by spectral (IR, 1H and 13C NMR) and elemental analyses. Furthermore, we have purified human CA I and II (hCA I and hCA II) from erythrocytes and examined the in vitro inhibition effects of synthesized compounds together starting compound (1) and acetazolamide (AAZ) as a control compound.
Materials and methods
All reagents were of the highest grade, commercially available and used without further purification. 1H and 13C NMR spectra were recorded with DPX-300 MHz Bruker Avance NMR spectrometer (Bio spin Gmbh, Germany). Elemental analyses for C, H and N were performed on a Leco CHNS-932 instrument. IR spectra were recorded on a Bruker Optics, vertex 70 FT-IR spectrometer using ATR techniques. A domestic microwave oven manufactured by BEKO was used for the microwave-assisted reactions at highest power (1200 W) and 2450 MHz operating frequency.
The general experimental procedure for 2a-2f
To mixture of 3,4-dimethylphenol (1) (0.1 mol) and amine compound (0.1 mol), formaldehyde (0.1 mol) was added dropwise with stirring then irradiated by microwave. After cooling to room temperature the residue was washed with ethanol and dried in air.
2-((dimethylamino)methyl)-4,5-dimethylphenol (2a)
Reaction time: 15 min; (white solid, 90%); mp 70 ˚C; 1H-NMR (d6-DMSO, ppm):8.77 (s, 1H, H1), 6.77 (s, 1H, H3), 6.51 (s, 1H, H8), 3.46 (s, 2H, H10), 2.58 (s, 3H, H7), 2.48 (s, 3H, H5), 2.10 (s, 3H, CH3), 2.07 (s, 3H, CH3); 13C-NMR (d6-DMSO, ppm): 154.20 (C2), 138.64 (C4), 131.86 (C8), 128.32 (C6), 122.43 (C3), 119.81 (C9), 59.99 (C10), 47.21 (CH3), 18.96 (C5), 18.82 (C7); IR (cm−1): 3492 (OH), 3012 (C-H)ar., 2980, 2800 (C-H)aliph., 1600-1457 (Ar C=C), 820 (ring); Anal. Calcd for C11H17NO: C, 73.70; H, 9.56; N, 7.81. Found: C, 73.86; H, 9.15; N, 7.93.
2-((diethylamino)methyl)-4,5-dimethylphenol (2b)
Reaction time: 35 min; (brown oil, 89%); 1H-NMR (d6-DMSO, ppm):9.56 (s, 1H, H1), 6.65 (s, 1H, H3), 6.44 (s, 1H, H8), 3.50 (s, 2H, H10), 2.42 (q, J = 7.03 Hz 4H, CH2), 2.20 (s, 3H, H7), 2.10 (s, 3H, H5), 0.92 (t, J = 7.01 Hz 6H, CH3); 13C-NMR (d6-DMSO, ppm): 149,53 (C2), 136.72 (C4), 129.43 (C8), 125.50 (C6), 119.52 (C3), 116.67 (C9), 55.56 (C10), 45.15 (CH2), 19.02 (C5), 18.47 (C7), 10.97 (CH3); IR (cm−1): 3485 (OH), 3006 (C-H)ar., 2961, 2873 (C-H)aliph., 1600-1440 (Ar C=C), 750 (ring); Anal. Calcd for C13H21NO: C, 75.32; H, 10.21; N, 6.76. Found: C, 75.34; H, 10.26; N, 6.81.
2-((ethyl(2-(ethylamino)ethyl)amino)methyl)-4,5-dimethylphenol (2c)
Reaction time: 60 min; (white solid, 85%); mp 120˚C;1H-NMR (d6-DMSO, ppm):10.19 (s, 1H, H1), 6.80 (s, 1H, H3), 6.49 (s, 1H, H8), 3.57 (s, 2H, H10), 2.05 (s, 3H, H7), 2.09 (s, 3H, H5), 3.34 (s, 1H, NH), 2.58(t, J = 4.71 Hz 3H, CH3), 2.50 (q, J = 4.71 Hz 2H, CH2), 2.49 (t, J = 4.68 Hz 2H, CH2), 2.48 (m, 2H, CH2), 0.99-0.89 (m, 5H, C2H5); 13C-NMR (d6-DMSO, ppm): 151.32 (C2), 141.09 (C4), 131.11 (C8), 128.42 (C6), 119.99 (C3), 119.87 (C9), 57.79 (C10), 20.01 (C5), 19.99 (C7), 55.02 (NCH2CH2), 48.67 (NCH2CH3), 46.23 (NCH2CH2NH), 44.3 (NHCH2CH3), 15.68 (NHCH2CH3), 13.42 (NCH2CH3); IR (cm−1): 3490 (OH), 2977 (NH), 3011 (C-H)ar., 2968, 2831 (C-H)aliph., 1628-1458 (Ar C=C), 748 (ring); Anal. Calcd for C15H26NO: C, 71.95; H, 10.47; N, 11.19. Found: C, 71.94; H, 10.09; N, 11.45.
4,5-dimethyl-2-(morpholinomethyl)phenol (2d)
Reaction time: 30 min; (brown oil, 92%);1H-NMR (d6-DMSO, ppm):9.82 (s, 1H, H1), 6.82 (s, 1H, H3), 6.55 (s, 1H, H8), 3.58 (s, 2H, H10), 3.59 (t, J = 4.03 Hz 4H, OCH2), 2.40 (t, J = 4.03 Hz 4H, NCH2), 2.12 (s, 3H, H7), 2.11 (s, 3H, H5); 13C-NMR (d6-DMSO, ppm): 154.89 (C2), 136.29 (C4), 130.93 (C8), 126.33 (C6), 119.34 (C3), 117.80 (C9), 66.71 (OCH2), 59.26 (C10), 53.17 (NCH2), 19.63 (C5), 18.79 (C7). IR (cm−1); 3489 (OH), 3010 (C-H)ar., 2990, 2907 (C-H)aliph., 1635-1448 (Ar C=C), 750 (ring); Anal. Calcd for C13H19NO2: C, 70.56; H, 8.65; N, 6.33. Found: C, 70.62; H, 8.66; N, 6.37.
2-((dibenzylamino)methyl)-4,5-dimethylphenol (2e)
Reaction time: 35 min; (white solid, 96%); mp 96 ˚C; 1H-NMR (d6-DMSO, ppm):10.41 (s, 1H, H1), 7.28-7.87 (m, 10H, C6H5), 6.67 (s, 1H, H3), 6.66 (s, 1H, H8), 3.69 (s, 2H, H10), 3.62 (s, 4H, CH2), 2.20 (s, 3H, H7), 2.17 (s, 3H, H5); 13C-NMR (d6-DMSO, ppm): 153.62 (C2), 137.92 (C4), 137.46–126.98 (C6H5-CH), 132.94 (C8), 129.85 (C6), 119.85 (C3), 119.74 (C9), 63.56 (C6H5-C), 55.84 (C10), 19.20 (C5), 18.80 (C7); IR (cm−1): 3495 (OH), 3015 (C-H)ar., 2950, 2850 (C-H)aliph., 1560-1455 (Ar C=C), 736 (ring); Anal. Calcd for C23H25NO: C, 83.34; H, 7.60; N, 4.23. Found: C, 83.36; H, 7.41; N, 4.35.
2-((4-fluorophenylamino)methyl)-4,5-dimethylphenol (2f)
Reaction time: 20 min; (white solid, 82%); mp 145˚C;1H-NMR (d6-DMSO, ppm):9.13 (s, 1H, H1), 6.90 (s, 1H, H3), 6.92 (s, 1H, H8), 6.50 (m, 2H, C6H4), 6.40 (m, 2H, C6H4), 5.82 (s, 1H, NH), 4.06 (s, 2H, H10), 2.08 (s, 3H, H7), 2.04 (s, 3H, H5); 13C-NMR (d6-DMSO, ppm): 156.68 (C6H4-C), 151.02 (C2), 145.01 (C6H4-C), 138.52 (C4), 130.00 (C8), 129.01 (C6), 126.13 (C3), 120.01 (C9), 119.13 (C6H4-CH), 53.42 (C10), 116.52 (C6H4-CH), 21.01 (C5), 19.68 (C7); IR (cm−1): 3488 (OH), 3276 (NH), 3007 (C-H)ar., 2976, 2884 (C-H)aliph., 1502-1453 (Ar C=C), 763 (ring); Anal. Calcd for C15H16FNO: C, 73.45; H, 6.57; N, 5.71. Found: C, 73.36; H, 6.37; N, 5.85.
Purification of isoenzymes hCA-I and hCA-II from human erythrocytes
In order to purify hCA-I and hCA-II isoenzymes, first, human blood was centrifuged at 1500 rpm for 20 min, and after the removal of the plasma, the erythrocytes were washed with an isotonic solution (0.9% NaCl). After that, the erythrocytes were lysed with 1.5 volume of ice-cold water. The lysate was centrifuged at 20,000 rpm for 30 min to remove cell membranes and non-lysed cells. The pH of the supernatant was adjusted to 8.7 with tris and was then loaded onto an affinity column containing Sepharose-4B-L-tyrosine-p-aminobenzene sulfonamide as the binding group. After extensive washing with 25 mM tris–HCl/22 mM Na2SO4 (pH 8.7), the hCA-I and hCA-II isoenzymes were eluted with 1.0 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6)Citation40,Citation41. The amount of purified protein was estimated by the Bradford methodCitation42 and SDS–PAGE was carried out to determine whether the elute containing the enzymeCitation43.
Hydratase activity assay
Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and AndersonCitation44. CO2-hydratase activity as an enzyme unit (EU) was calculated by using the equation ((t0-tc)/tc) where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively. IC50 values (the concentration of inhibitor producing a 50% inhibition of CA activity) have been obtained as in vitro for the synthesized compounds 2a-f, 1 and AAZ as the control compound.
Esterase activity assay
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (CHEBIOS UV–VIS) according to the method described in the literatureCitation45,Citation46. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL of 0.05 M tris–SO4 buffer (pH 7.4), 1 mL of 3 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution. IC50 values have been obtained as in vitro for free the synthesized compounds 2a-f, 1 and AAZ as the control compound.
Determination of Ki values
The method for determination of Ki values is described elsewhereCitation47–51. In the first part of this study, IC50 values have been obtained as in vitro. IC50 of the inhibitors (the synthesized compounds 2a-f, 1 and AAZ as the control compound) were assayed by the hydrolysis of p-nitrophenylacetate on esterase activities of CA isoenzymes in the presence of various inhibitor concentrations. The absorbance was determined at 348 nm after 3 minCitation47. Regression analysis graphs were drawn by plotting inhibitor concentrations versus enzyme activity by using Microsoft Excel Program.
In the second part of the study, enzyme activity was measured in the presence of five different substrate concentrations at each of these inhibitor concentrations (30%, 50%, and 70%), and the data were linearized with Lineweaver–Burk plot in order to obtain Ki values.
Result and discussion
We herein report the syntheses of title compounds 2a-f by treatment of 3,4-dimethylphenol (1), with various amines in the presence of formaldehyde. The reactions were carried out under microwave (MW) irradiation and solvent free condition. The one-step Mannich reaction of 2a-f is given in .
1H-NMR and 13C-NMR spectra of 2a-f
The 1H and 13C-NMR spectra of compounds 2a-f were recorded in d6-DMSO solution at room temperature using TMS as internal standard. In the 1H NMR spectra the signals of the respective protons of the prepared compounds 2a-f were verified on the basis of their chemical shifts, multiplicities and coupling constants. The NMR spectra of 2a-f exhibited a broad singlet in the range 10.41-8.77 ppm which correspond to protons H1 (O-H)Citation40. The chemical shift of the aromatic protons of the benzene ring are observed in the range 6.90-6.65 ppm for H3 protons and 6.92-6.44 ppm for H8 protons. The 1H NMR spectra of 2a-f showed a singlet with 2 H intensity each, in the range 4.06-3.46 ppm assigned to the H10 (CH2) protons, confirming the Mannich condensation of 3,4-dimetylphenol, formaldehyde and aminesCitation52,Citation53. In the 1H NMR spectra of compounds 2a-f showed two singlets with 3 H intensity each, in the range 2.58-2.05 and 2.48-2.04 ppm for protons H7 and H5, respectively.
13C NMR spectra of 2a-f exhibited peaks in the range 154.89–149.53, 141.09-136.29, 132.94–129.43, 129.85–125.50, 126.13–119.34 and 120.01-116.67 ppm due to C2, C4, C8, C6, C3 and C9 carbon atoms, respectively. The carbon atoms (C10) for 2a-f were observed in the range 59.99-53.42 ppm. The signals in the range 21.01-18.96 and 19.99-18.47 are assigned to C5 and C7 carbon atoms, respectively.
FTIR measurements
The IR spectra of all compounds showed vibrational bands in the range 3495-3485 cm−1 for O-H group. The bands at 3276 and 2977 cm−1 are assigned to vibrations associated with the N-H moiety for compounds 2f and 2c, respectivelyCitation54. Aromatic C-H stretching vibrations for all compounds are observed in the range 3015-3006 cm−1. The bands in the range 2990-2950 and 2907-2800 cm−1 are attributable to aliphatic C-H stretching vibrations for compounds 2a-f. The IR spectra of all compounds showed vibrational bands in the range 1635-1440 cm−1 for C=C groupCitation55. The bands of ring are located in the range 820-736 cm−1.
In vitro inhibition studies
Inhibition effects on hCA I and hCA II isozymes of the synthesized compounds (2a-f) and acetazolamide (AAZ) as the control compound were studied by hydratase and esterase activity methods. The related Ki values were also determined for each compound in order to compare with inhibition effects of starting compound (1) and AAZ ().
Table 1. IC50 and Ki values forsynthesized compounds (2a-f), 1 and AAZ withhCA I and hCA II isozymes.
According to in vitro studies, there is no inhibition effects observed for 1 and synthesized compounds (2a-f) on hydratase activities of hCA I and hCA II isozymes, and for 2c and 2f on esterase activities of hCA I and hCA II isozymes. Starting compound 1 showed esterase activities on hCA I and hCA II. However, IC50 values for the esterase activities of 1 (1.03 × 103 µM for hCA I and 0.93 × 103 µM for hCA II, respectively) were greater than the IC50 values of AAZ (5.9 µM for hCA I and 4.3 µM for hCA II, respectively). In relation to esterase activities, the inhibition equilibrium constants (Ki) were also determined. Compound 1 exhibited Ki values as 8.5-79.6 µM for hCA I and 15.3-67.3 µM for hCA II, respectively indicating poorer inhibition effects compared to AAZ (5.0-6.1 µM for hCA I and 3.5-4.1 µM for hCA II, respectively).
Compounds 2a and 2e showed inhibitory effects on esterase activity of hCA II, nevertheless they did not showed any inhibitory effects of hCA I isozyme. The esterase IC50 and the Ki values for 2a and 2e (0.88 × 103 and 6.3–7.6 µM for hCA II, respectively and 0.44 × 103 and 19.0-96.4 µM for hCA II, respectively) are higher than the esterase IC50 and the Ki values of AAZ (4.3 and 3.5-4.1 µM for hCA II, respectively). Similar to 1, these compounds are also weaker inhibitors than AAZ. No inhibition effects were observed for compounds 2b and 2d on hCA I and hCA II isozymes but these compounds increased the esterase activities of these isozymes. Therefore, 2b and 2d compounds might be evaluated as carbonic anhydrase activators.
The compounds used in this study have both functional phenolic -OH group and -NHR or -NR2 groups together in a molecule which have not reinforced their CA (I and II) inhibitory effects. This might be due to acid-base reaction between them, although they are good CA inhibitors separatelyCitation23–28,Citation30,Citation32,Citation36.
Conclusion
An efficient, clean, economic, and one-pot procedure for the synthesis of Mannich bases (2a-f) has been used by the three-component coupling of aldehyde, amine, and 3,4-dimethylphenol (1) under microwave irradiation, solvent free conditions, short reaction time with excellent yields of the products. All of the synthesized compounds 2a-f have been completely characterized by IR, 1H and 13C NMR and elemental analyses and all results are consistent with the proposed structures (). Inhibition effects of synthesized compounds (2a-f)on hCA I and hCA II isozymes were studied by hydratase and esterase activity methods and then Ki values were determined. None of the synthesized compounds (2a-f) did not show any inhibition effect on hydratase activity of hCA I and hCA II. Only 2a and 2e showed weak inhibitory effects on esterase activity of hCA II. On the contrary, compounds 2b and 2d might be good CA activators due to increasing esterase activity of hCA I and hCA II isozymes.
Declaration of interest
This work was supported by grant (Grant No: 2008-1) from the Dumlupınar University Research Foundation and carried out in the Department of Chemistry of the Dumlupınar University.
References
- Lipscomb WN, Sträter N. Recent Advances in Zinc Enzymology. Chem Rev 1996;96:2375–2434.
- Gülçin İ, Beydemir Ş, Büyükokuroğlu ME. In vitro and in vivo effects of dantrolene on carbonic anhydrase enzyme activities. Biol Pharm Bull 2004;27:613–616
- Gülçin İ, Alici HA, Cesur M. Determination of in vitroantioxidant and radical scavenging activities of propofol. Chem Pharm Bull 2005;53:281–285
- Gülçin İ, Büyükokuroglu ME, Oktay M, Küfrevioglu Öİ. On the in vitro antioxidant properties of melatonin. J Pineal Res 2002;33:167–171.
- Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 2008;283:27799–27809.
- Di Fiore A, Scozzafava A, Winum JY, Montero JL, Pedone C, Supuran CT et al. Carbonic anhydrase inhibitors: binding of an antiglaucoma glycosyl-sulfanilamide derivative to human isoform II and its consequences for the drug design of enzyme inhibitors incorporating sugar moieties. Bioorg Med Chem Lett 2007;17:1726–1731.
- Innocenti A, Vullo D, Pastorek J, Scozzafava A, Pastorekova S, Nishimori I et al. Carbonic anhydrase inhibitors. Inhibition of transmembrane isozymes XII (cancer-associated) and XIV with anions. Bioorg Med Chem Lett 2007;17:1532–1537.
- Winum JY, Thiry A, Cheikh KE, Dogné JM, Montero JL, Vullo D et al. Carbonic anhydrase inhibitors. Inhibition of isoforms I, II, IV, VA, VII, IX, and XIV with sulfonamides incorporating fructopyranose-thioureido tails. Bioorg Med Chem Lett 2007;17:2685–2691.
- Scozzafava A, Banciu MD, Popescu A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of isozymes I, II and IV by sulfamide and sulfamic acid derivatives. J Enzym Inhib 2000;15:443–453.
- Schuman JS. Antiglaucoma medications: a review of safety and tolerability issues related to their use. Clin Ther 2000;22:167–208.
- Wilkinson BL, Bornaghi LF, Houston TA, Innocenti A, Vullo D, Supuran CT et al. Inhibition of membrane-associated carbonic anhydrase isozymes IX, XII and XIV with a library of glycoconjugate benzenesulfonamides. Bioorg Med Chem Lett 2007;17:987–992.
- Santos MA, Marques S, Vullo D, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of cytosolic/tumor-associated isoforms I, II, and IX with iminodiacetic carboxylates/hydroxamates also incorporating benzenesulfonamide moieties. Bioorg Med Chem Lett 2007;17:1538–1543.
- Ilies M, Scozzafava A, Supuran CT. Carbonic anhydrase activators. In: Carbonic Anhydrase – Its Inhibitors and Activators; Supuran CT, Scozzafava A, Conway J. Eds.Boca Raton; CRC Press:, 2004; pp 317–352;
- Temperini C, Scozzafava A, Supuran CT. Drug design studies of carbonic anhydrase activators. In Drug Design of Zinc-Enzyme Inhibitors – Functional, Structural, and Disease Applications; Supuran CT, Winum JY. Eds.; Wiley: Hoboken, 2009; pp 473–486.
- Briganti F, Mangani S, Orioli P, Scozzafava A, Vernaglione G, Supuran CT. Carbonic anhydrase activators: X-ray crystallographic and spectroscopic investigations for the interaction of isozymes I and II with histamine. Biochemistry 1997;36:10384–10392.
- Becker HM, Klier M, Schüler C, McKenna R, Deitmer JW. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc Natl Acad Sci USA 2011;108:3071–3076.
- Elder I, Tu C, Ming LJ, McKenna R, Silverman DN. Proton transfer from exogenous donors in catalysis by human carbonic anhydrase II. Arch Biochem Biophys 2005;437:106–114;
- Tripp BC, Ferry JG. A structure-function study of a proton transport pathway in the gamma-class carbonic anhydrase from Methanosarcina thermophila Biochemistry. 2000;39:9232–9240.
- Dave K, Ilies MA, Scozzafava A, Temperini C, Vullo D, Supuran CT. An inhibitor-like binding mode of a carbonic anhydrase activator within the active site of isoform II. Bioorg Med Chem Lett 2011;21:2764–2768
- Dave K, Scozzafava A, Vullo D, Supuran CT, Ilies MA. Pyridinium derivatives of histamine are potent activators of cytosolic carbonic anhydrase isoforms I, II and VII: solution and crystallographic studies. Org Biomol Chem 2011;9:2790–2800.
- Pocker Y, Deits TL. Effects of pH on anionic inhibition of carbonic anhydrase activities. J Am Chem Soc 1982; 104:2424–2434.
- Tibell L, Forsman C, Simonsson I, Lindskog S. Anion inhibition of CO2 hydration catalyzed by human carbonic anhydrase II. Mechanistic implications. Biochim Biophys Acta 1984;789:302–310.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Supuran CT, Scozzafava A. Activation of carbonic anhydrase isozymes. In: The Carbonic Anhydrases—New Horizons; Chegwidden WR, Carter N, Edwards Y. Eds.; Basel, Switzerland Birkhauser Verlag: 2000; pp 197–219.
- Temperini C, Scozzafava A, Puccetti L, Supuran CT. Carbonic anhydrase activators: X-ray crystal structure of the adduct of human isozyme II with L-histidine as a platform for the design of stronger activators. Bioorg Med Chem Lett 2005;15:5136–5141.
- Temperini C, Scozzafava A, Vullo D, Supuran CT. Carbonic anhydrase activators. Activation of isoforms I, II, IV, VA, VII, and XIV with L- and D-phenylalanine and crystallographic analysis of their adducts with isozyme II: stereospecific recognition within the active site of an enzyme and its consequences for the drug design. J Med Chem 2006;49:3019–3027.
- Temperini C, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase activators: L-Adrenaline plugs the active site entrance of isozyme II, activating better isoforms I, IV, VA, VII, and XIV. Bioorg Med Chem Lett 2007;17:628–635.
- Parkkila S, Vullo D, Puccetti L, Parkkila AK, Scozzafava A, Supuran CT. Carbonic anhydrase activators: activation of isozyme XIII with amino acids and amines. Bioorg Med Chem Lett 2006;16:3955–3959.
- Vullo D, Nishimori I, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase activators: an activation study of the human mitochondrial isoforms VA and VB with amino acids and amines. Bioorg Med Chem Lett 2007;17:1336–1340.
- Vullo D, Innocenti A, Nishimori I, Scozzafava A, Kaila K, Supuran CT. Carbonic anhydrase activators: activation of the human isoforms VII (cytosolic) and XIV (transmembrane) with amino acids and amines. Bioorg Med Chem Lett 2007;17:4107–4112.
- Nishimori I, Onishi S, Vullo D, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase activators: the first activation study of the human secretory isoform VI with amino acids and amines. Bioorg Med Chem 2007;15:5351–5357.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: interactions of phenols with the 12 catalytically active mammalian isoforms (CA I-XIV). Bioorg Med Chem Lett 2008;18:1583–1587.
- Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of mammalian isoforms I-XIV with a series of substituted phenols including paracetamol and salicylic acid. Bioorg Med Chem 2008;16:7424–7428
- Innocenti A, Hilvo M, Scozzafava A, Parkkila S, Supuran CT. Carbonic anhydrase inhibitors: Inhibition of the new membrane-associated isoform XV with phenols. Bioorg Med Chem Lett 2008;18:3593–3596
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–9105.
- Sentürk M, Gülçin I, Dastan A, Küfrevioglu OI, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of human erythrocyte isozymes I and II with a series of antioxidant phenols. Bioorg Med Chem 2009;17:3207–3211.
- Joshi S, Khosla N, Khare D, Sharda R. Synthesis and in vitro study of novel Mannich bases as antibacterial agents. Bioorg Med Chem Lett 2005;15:221–226.
- Joshi S, Khosla N, Tiwari P. In vitro study of some medicinally important Mannich bases derived from antitubercular agent. Bioorg Med Chem 2004;12:571–576.
- Bilgiç S, Bilgiç O, Büyükkıdan B, Gündüz M. Synthesis of chromans from the reaction of o-quinone methide precursor with substituted styrenes. J Chem Res 2007; 2007:76–79.
- Arslan B, Nalbantoğlu B, Demir N, Özdemir H, Küfrevioğlu Öİ. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Trop J Med Sci 1996;26:163.
- Rickli EE, Ghazanfar SA, Gibbons BH, Edsall JT. Carbonic anhydrases from human erythrocytes. Preparation and properties of two enzymes. J Biol Chem 1964;239:1065–1078.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli DK. Clevage of structural proteins during in assembly of the head of bacteriophage T4. Nature 1970;227:680.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–154.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Innocenti A, Scozzafava A, Parkkila S, Puccetti L, De Simone G, Supuran CT. Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg Med Chem Lett 2008;18:2267–2271.
- Landolfi C, Marchetti M, Ciocci G, Milanese C. Development and pharmacological characterization of a modified procedure for the measurement of carbonic anhydrase activity. J Pharmacol Toxicol Methods 1997;38:169–172.
- Bülbül M, Hisar O, Beydemir S, Ciftçi M, Küfrevioglu OI. The in vitro and in vivo inhibitory effects of some sulfonamide derivatives on rainbow trout (Oncorhynchus mykiss) erythrocyte carbonic anhydrase activity. J Enzyme Inhib Med Chem 2003;18:371–375.
- Ciftçi M, Bülbül M, Gül M, Gümüstekin K, Dane S, Süleyman H. Effects of nicotine and vitamin E on carbonic anhydrase activity in some rat tissues in vivo and in vitro. J Enzyme Inhib Med Chem 2005;20:103–108.
- Hisar O, Beydemir Ş, Bülbül M, Yanık T. Kinetic properties of carbonic anhydrase purified from gills of rainbow trout (Oncorhynchus mykiss). J Appl Anim Res 2006;30:185.
- Winum JY, Cecchi A, Montero JL, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with boron-containing sulfonamides, sulfamides, and sulfamates: toward agents for boron neutron capture therapy of hypoxic tumors. Bioorg Med Chem Lett 2005;15:3302–3306.
- Szady-Chelmieniecka A, Rozwadowski Z, Dziembowska T, Grech E, Wojciechowski G, Brzezinski B. Intramolecular hydrogen bonds in 3-diethylaminomethyl-5-R-salicylic aldehydes. J Mol Stru 2000;520:39.
- Chi KW, Ahn YS, Shim KT, Park TH, Ahn JS. One-pot synthesis of Mannich base using hyd aromatic rings and secondary amines. Bull Korean Chem Soc 1999;20:973.
- Palaniappan S, John A, Amarnath CA, Rao VJ. Mannich-type reaction in solvent free condition using reusable polyaniline catalyst Journal of Molecular Catalysis A: Chemical 2004;218: 47.
- Yenikaya C, Poyraz M, Sarı M, Demirci F, İlkimen H, Büyürkgüngör O. Synthesis, characterization and biological evaluation of a novel Cu(II) complex with the mixed ligands 2,6-pyridinedicarboxylic acid and 2-aminopyridine. Polyhedron 2009;28:3526.