Abstract
Three novel metal complexes of N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-4-benzoyl-1-(3-nitrophenyl)-5-phenyl-1H-pyrazole-3-carboxamide which possess strong carbonic anhydrase (CA) inhibitory properties have been synthesised. The structure of these compounds has been investigated by elemental analysis, FT-IR, LC/MS, UV–vis spectrophotometric method and magnetic susceptibility. Human carbonic anhydrase isoenzymes hCA-I and hCA-II were purified from erythrocyte cells by the affinity chromatography. The inhibitory effects of newly synthesized complexes and acetazolamide (AAZ) as a control compound on hydratase and esterase activities of these isoenzymes have been studied in vitro by comparing IC50 and Ki values and it has been found that the newly synthesised complexes behave as very powerful inhibitors against hCA-I and hCA-II than parent ligand (1) and than AAZ.
Introduction
Carbonic anhydrase (CA, EC 4.2.1.1) is a zinc metalloenzyme that catalyzes the reversible reactions of CO2 and water: CO2 + H2O → H+ + HCO3−. The treatment of glaucoma with inhibitors of the metalloenzyme carbonic anhydrase (CA II and CA IV) is very effective in reducing elevated intraocular pressure (IOP) due to CO2, characteristic of this diseaseCitation1–5. The 16 different isoenzymes of carbonic anhydrase (CA, EC 4.2.1.1) are known in humansCitation6–10. Sulfonamides inhibiting the zinc enzyme carbonic anhydrase are widely used pharmacological agents for the treatment of glaucomaCitation11–16. 1,3,4-Thiadiazole-2-sulfonamide derivativesCitation17–21 played a critical role in the development of the antiglaucoma drugs with carbonic anhydrase (CA) inhibitory actionCitation22,Citation23. Neutral sulfonamides are expected to be poor ligands because of the withdrawal of the electron density from the nitrogen atom onto the electronegative oxygen atoms. However, if the sulfonamide N atom bears a dissociable hydrogen atom, this same electron-withdrawing effect increases its acidity and, in the deprotonated form, sulfonamide anions are effective σ-donor ligands. Metal complexes of sulfonamides containing a large number of main group or transition metal ions, were shown to possess very strong CA inhibitory propertiesCitation24–29. Coordination metal compounds of heterocyclic sulfonamides are 10–100 times more active as CA inhibitors than the free sulfonamidesCitation24–29.
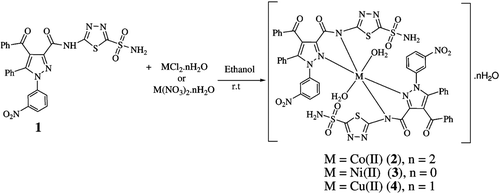
The ligand 1 used in this study has already been developed in our laboratories as CA inhibitorCitation30 and we only prepared its metal complexes 2, 3 and 4 by the reaction of 1 with Co(II), Ni(II) and Cu(II) (). These complexes can be seen as candidates for lowering intraocular pressure (IOP). The newly synthesised complexes 2-4 were characterised by spectroscopic methods in order to assign their structures, and were assayed as CA inhibitors against hCA-I and hCA-II isoenzymes.
Materials and methods
Metal salts (CoCl2.6H2O, Ni(NO3)2.6H2O and CuCl2.2H2O) and the other reagents were the highest grade commercially available and used without further purification. Compound 1 was synthesized by literature methodsCitation30. Elemental analyses for C, H, N and S were performed on a Leco CHNS-932 instrument. Mass spectra data were determined by Varian Mat III 80 eV. IR spectra were recorded on a Bruker Optics, vertex 70 FT-IR spectrometer using ATR techniques. The UV-vis spectra were carried out with a SHIMADZU UV-2550 spectrometer in the range 900–200 nm. Magnetic susceptibility measurements at room temperature were taken using a Sherwood Scientific Magway MSB MK1 model magnetic balance by the Gouy method using Hg[Co(SCN)4] as calibrant.
General procedure for syntheses of complexes 2-4
A solution of metal salt (7.54 × 10−5 mol) in 10 mL ethanol was added with continuous stirring to 10 mL of an ethanolic solution containing N-[5-(aminosulfonyl)-1,3,4-thiadiazol-2-yl]-4-benzoyl-1-(3-nitrophenyl)-5-phenyl-1H-pyrazole-3-carboxamide (1) (1.5 × 10−4 mol). The pH of the reaction mixture was adjusted by addition of 0.1 M aqueous NaOH solution in the range between 6.0 and 7.0. The reaction mixture was stirred for 3 h at room temperature to give a solid of complexes 2-4.
Synthesis of [CoL2(H2O)2].2H2O (2)
Pink solid, 40%, m.p. decompose; IR (cm−1): 3500-3200 (-OH), 3385 and 3265 (-NH2), 3094 (ar-CH), 1662 (benzoyl C=O), 1602 (amide C=O), 1535, 1492 (C=C and C=N), 1349 and 1173 (-SO2), 595 (Co-O) and 515 (Co-N); UV-vis, [λ (nm), ϵmax (M−1 cm−1)]: 267 (8690), 282 (18530), 330 (25720) (π-π*), 587 (220), 666 (240), 787(290) (d-d); MS(CI) m/z: 1207.0 [Co(C25H17N7O6S2)2]+, 1157.08 [(C25H17N7O6S2)2]+, 575.9 [C25H17N7O6S2]+; Anal. Calcd for C50H40CoN14O16S4: C, 46.91; H, 3.15; N, 15.32; S, 10.02. Found: C, 46.93; H, 3.09; N, 15.07; S, 10.35.
Synthesis of [NiL2(H2O)2] (3)
Blue solid, 65%, m.p. decompose; IR (cm−1): 3500-3200 (-OH), 3222(-NH2), 3068 (ar-CH), 1667 (benzoyl C=O), 1600 (amide C=O), 1530, 1501 (C=C and C=N), 1348 and 1173 (-SO2), 593 (Ni-O) and 512 (Ni-N); UV-vis, [λ (nm), ϵmax (M−1 cm−1)]: 275 (10890), 282 (18370), 329 (24450) (π-π*), 414 (440), 564 (400), 797(270) (d-d); MS(CI) m/z: 1206.0 [Ni(C25H17N7O6S2)2]+, 1157.08 [(C25H17N7O6S2)2]+, 1151.0 [(C25H17N7O6S2)2]+, 576.0 [(C25H17N7O6S2)]+; Anal. Calcd for C50H36NiN14O14S4:C, 48.28; H, 2.92; N,1 5.76; S, 10.31. Found: C, 47.64; H, 2.52; N, 15.43; S, 10.24.
Synthesis of [CuL2(H2O)2].H2O (4)
Green solid, 70%, m.p. 300°C; IR (cm−1): 3500-3200 (-OH), 3191(-NH2), 3066 (ar-CH), 1668 (benzoyl C=O), 1616 (amide C=O), 1530, 1500 (C=C and C=N), 1348 and 1169 (-SO2), 591 (Cu-O) and 487 (Cu-N); UV-vis, [λ (nm), ϵmax (M−1 cm−1)]: 273 (4530), 282 (9500), 312 (8540) (π-π*), 699 (180) (d-d); MS(CI) m/z: 1210.8 [Cu(C25H17N7O6S2)2]+, 1150.9 [(C25H17N7O6S2)2]+, 576.0 [(C25H17N7O6S2)]+; Anal. Calcd for C50H38CuN14O15S4: C, 47.41; H, 3.02; N, 15.48; S, 10.13. Found: C, 47.69; H, 2.96; N, 15.05; S, 10.98.
Purification of isoenzymes hCA-I and hCA-II from human erythrocytes
In order to purify hCA-I and hCA-II isoenzymes, first, human blood was centrifuged at 1500 rpm for 20 min, and after the removal of the plasma, the erythrocytes were washed with an isotonic solution (0.9 % NaCl). After that, the erythrocytes were lysed with 1.5 volume of ice-cold water. The lysate was centrifuged at 20,000 rpm for 30 min to remove cell membranes and non-lysed cells. The pH of the supernatant was adjusted to 8.7 with Tris and was then loaded onto an affinity column containing Sepharose-4B-L-tyrosine-p-aminobenzene sulfonamide as the binding group. After extensive washing with 25 mM Tris–HCl/22 mM Na2SO4 (pH 8.7), the hCA-I and hCA-II isoenzymes were eluted with 1.0 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6)Citation31,Citation32. The amount of purified protein was estimated by the Bradford methodCitation33 and SDS–PAGE was carried out to determine whether the elute containing the enzymeCitation34. SDS polyacrylamide gel electrophoresis was performed after the purification of the enzyme (Supplementary Figure 1).
Hydratase activity assay
Carbonic anhydrase activity was assayed by following the hydration of CO2 according to the method described by Wilbur and AndersonCitation35. CO2-hydratase activity as an enzyme unit (EU) was calculated by using the equation ((t0-tc)/tc) where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively. IC50 values (the concentration of inhibitor producing a 50% inhibition of CA activity) have been obtained as in vitro for the newly synthesized compounds 2-4 and AAZ as the control compound.
Esterase activity assay
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25°C using a spectrophotometer (CHEBIOS UV–VIS) according to the method described in the literatureCitation36,Citation37. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL of 0.05 M Tris–SO4 buffer (pH 7.4), 1 mL of 3 mM 4-nitrophenylacetate, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution. IC50 values have been obtained as in vitro for free the novel synthesized compounds 2-4 and AAZ as the control compound.
Determination of Ki values
The method for determination of Ki values is described elsewhereCitation38–42. In the first part of this study, IC50 values have been obtained as in vitro. IC50 of the inhibitors (the synthesized complexes (2-4) and AAZ as the control compound) were assayed by the hydrolysis of p-nitrophenylacetate on esterase activities of CA isoenzymes in the presence of various inhibitor concentrations. The absorbance was determined at 348 nm after 3 minCitation38. Regression analysis graphs were drawn by plotting inhibitor concentrations versus enzyme activity by using Microsoft Excel Program.
In the second part of the study, enzyme activity was measured in the presence of five different substrate concentrations at each of these inhibitor concentrations (30%, 50%, and 70%), and the data were linearized with Lineweaver-Burk plot in order to obtain Ki values.
Results and discussion
We herein report the syntheses of the complexes 2-4 by treatment of 1, with metal salts in ethanol ().
FT-IR measurments
The modifications of the characteristic IR bands of 1Citation30 are indicative of the deprotonation and/or coordination of the ligand to the metal ions.
The bands in the region 3500–3200 cm−1 are associated with the stretching vibrations of the O-H bond in the complexes 2-4. Two stretching frequencies were observed for the asymmetric (3385 cm−1) and symmetric (3265 cm−1) vibrations of the sulfonamido -NH2 moiety for compound 2. This band was observed at 3222 cm−1 for 3 and 3191 cm−1 for 4. The bands corresponding to the amide ν(C=O) vibration of the pyrazole carboxamide (1651 cm−1) of 1Citation30 is observed as a single band shifted to lower frequencies (about 1616–1600 cm−1) in the complexes (2-4). This fact could be attributed to the modifications that take place on the deprotonation of the nitrogen atom of carboxamido groupCitation43. The changes found in the bands assigned to the ν(C=C) and ν(C=N) vibrations are at 1535–1530 and 1501–1492 cm−1 for complexes 2-4 and as these vibrations appear at 1588 and 1494 cm−1 for 1Citation30. These data are also attributed to the coordination occurring from N atom of pyrazole ring of 1Citation30. M-O and M-N vibrations are observed in the range 595–591 and 515–487 cm−1 for complexes 2-4.
UV/vis spectrum and magnetic susceptibility
The electronic spectra of compounds 2-4 were recorded in DMSO solutions at a 1 × 10−3 M concentration at room temperature. The electronic spectra of the complexes confirmed their geometry. The electronic spectrum of 2 exhibits three intense absorption bands at 267, 282 and 330 nm attributed to π-π* transitions. These bands are observed at 275, 282 and 329 nm for compound 3 and 273, 282 and 312 nm for compound 4 similarly to other sulfonamide derivatives containing 1,3,4-thiadiazole-2-sulfonamide ringCitation44. In addition, the electronic spectra of complexes (2 and 3) show three weak intensity bands at 587, 666 and 787 nm for complex 2 and the bands at 414, 564 and 797 nm for complex 3 and a band at 699 nm for compound 4 which may tentatively be assigned to octahedral geometry of metal(II) ions with d7, d8, d9, respectivelyCitation45.
Magnetic susceptibility measurements were carried out on powdered samples at room temperature. The effective magnetic moments, 4.91 B.M. for 2, 3.64 B.M. for 3 and 1.89 B.M. for 4 are consistent with d7, d8, d9 octahedral metal (II) complexes, respectivelyCitation46,Citation47.
Mass spectra of 2-4
The mass spectra give additional structural information about the chemical structure of the studied complexes 2-4. None of the mass spectra of complexes shows a molecular ion (M+) peak. The peaks at m/z 1207.0, 1157.08 and 575.9 for 2, m/z 1206.0, 1151.0 and 576.0 for 3 and m/z 1210.8, 1150.9 and 576.0 for 4 are due to the fragments, [ML2]+ and [L2]+ and [L]+, respectively which suggest the monomeric nature of the complexes. These data confirm the proposed formulas of the complexes ().
In vitro inhibition studies
Inhibition effects on hCA-I and hCA-II isoenzymes of the newly synthesized compounds (2-4) and acetazolamide (AAZ) as a control compound were studied by hydratase and esterase activity methods and Ki values were determined for each compound and compared to inhibition effect of free ligand (1)Citation30 ().
Table 1. IC50 and Ki values of AAZ and 1 and newly synthesized compounds (2-4) for hCA I and hCA II isoenzymes.
According to the in vitro studies, the IC50 values of hydratase activities of newly synthesized compounds 2, 3 and 4 (0.49, 0.075 and 0.19 µM for hCA-I and 0.013, 0.0625 and 2.2 µM for hCA-II, respectively) are generally lower than the IC50 values of 1 (1.2 and 0.4 µM for hCA-I and hCA-II, respectively)Citation30 and of AAZ (3.3 and 2.4 µM for hCA-I and II, respectively). The IC50 values of esterase activities of compounds 2-4 are in the same trend with hydratase activities of 2-4 (0.25, 0.125 and 0.280 µM for hCA-I and 0.1, 0.170 and 0.260 for hCA-II, respectively). The IC50 values of esterase activities for free ligand (1)Citation30 and AAZ, have been obtained as 1.4 and 4.6 µM for hCA-I and 3.0 and 3.9 µM for hCA-II, respectively.
In relation to the esterase activities, the inhibition equilibrium constants (Ki) were also determined. The pyrazole carboxamide group of 1 might form a hydrogen bond with the histidine residue in the active site of CA to inhibit the isozymes (Ki = 1.40 and 3.0 µM for hCA-I and II), resulting in less movement of the carbonic acid toward CO2 production as suggested in a studyCitation48. The coordination compounds (2-4) show remarkable inhibition on hCA-I and II (Ki: 0.28, 0.081 and 0.316 µM and 0.145, 0.175 and 0.325 µM, respectively), having a higher inhibition as compared to 1, as well as to the control compound AAZ (2.8 and 2.1 µM for hCA I and II), which is probably due to the further effect of the metal(II) ions on the histidine residues in the active site of carbonic anhydraseCitation49,Citation50. Especially compound 2 has shown remarkable inhibition against hCA-II and compound 3 has shown remarkable inhibition against hCA-I isoenzymes. The order of the metal ions for the inhibition of hCA-I for hydratase activity is Ni>Cu>Co, and of hCA-I for esterase activity is Ni>Co>Cu, and of hCA-II for hydratase activity is Co>Ni>Cu, and of hCA-II for esterase activity is also Co>Ni>Cu.
Conclusions
We propose here three novel metal complexes 2-4 of heterocyclic sulfonamide 1; we found that these compounds proved to be stronger CA inhibitors than ligand 1 and AAZ against hCA-I and hCA-II isoforms. Electronic spectra, magnetic measurements and the other analyses show good agreement with the proposed structures of newly synthesized complexes 2-4.
Declaration of interest
This work was supported by The Scientific and Research Council of Turkey (TÜBİTAK) with Grant No. TBAG-104T406.
Supplementary Material
Download PDF (126.6 KB)References
- Supuran CT, Scozzafava A, Conway J, Eds. (2004). Carbonic anhydrase—Its Inhibitors and activators. Boca Raton: CRC Press, 1–363.
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–777.
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Marquis RE, Whitson JT. Management of glaucoma: focus on pharmacological therapy. Drugs Aging 2005;22:1–21.
- Yenikaya C, Sari M, Bülbül M, Ilkimen H, Celik H, Büyükgüngör O. Synthesis, characterization and antiglaucoma activity of a novel proton transfer compound and a mixed-ligand Zn(II) complex. Bioorg Med Chem 2010;18:930–938.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. New zinc binding motifs in the design of selective carbonic anhydrase inhibitors. Mini Rev Med Chem 2006;6:921–936.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. Sulfamates and their therapeutic potential. Med Res Rev 2005;25:186–228.
- Yenikaya C, Sarı M, Ilkimen H, Bülbül M, Büyükgüngör O. Synthesis, structural and antiglaucoma activity studies of a novel amino salicylato salt and its Cu(II) complex. Polyhedron 2011;30:535–541.
- Maren TH. Carbonic anhydrase: General prospectives and advances in glaucoma research. Drug Dev Res 1987;10:255–276.
- Scozzafava A, Menabuoni L, Mincione F, Briganti F, Mincione G, Supuran CT. Carbonic anhydrase inhibitors. Synthesis of water-soluble, topically effective, intraocular pressure-lowering aromatic/heterocyclic sulfonamides containing cationic or anionic moieties: is the tail more important than the ring? J Med Chem 1999;42:2641–2650.
- Borras J, Scozzafava A, Menabuoni L, Mincione F, Briganti F, Mincione G et al. Carbonic anhydrase inhibitors: synthesis of water-soluble, topically effective intraocular pressure lowering aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl moieties: is the tail more important than the ring? Bioorg Med Chem 1999;7:2397–2406.
- Scozzafava A, Briganti F, Mincione G, Menabuoni L, Mincione F, Supuran CT. Carbonic anhydrase inhibitors: synthesis of water-soluble, aminoacyl/dipeptidyl sulfonamides possessing long-lasting intraocular pressure-lowering properties via the topical route. J Med Chem 1999;42:3690–3700.
- Supuran CT, Scozzafava A, Menabuoni L, Mincione F, Briganti F, Mincione G. Carbonic anhydrase inhibitors. Part 71. Synthesis and ocular pharmacology of a new class of water-soluble, topically effective intraocular pressure lowering sulfonamides incorporating picolinoyl moieties. Eur J Pharm Sci 1999;8:317–328.
- Supuran CT, Mincione F, Scozzafava A, Briganti F, Mincione G, Ilies MA. Carbonic anhydrase inhibitors, part 52: Metal complexes of heterocyclic sulfonamides: A new class of strong topical intraocular pressurelowering agents in rabbits. Eur J Med Chem 1998;33:247–254.
- Roblin RO, Clapp JW. The preparation of heterocyclic sulfonamides. J Am Chem Soc 1950;72:4890–4892.
- Miller WH, Dessert AM, Roblin RO. Heterocyclic sulfonamides as carbonic anhydrase inhibitors. J Am Chem Soc 1950;72:4893–4896.
- Vaughan JR, Eichler JA, Anderson GW. Heterocyclic sulfonamides as carbonic anhydrase inhibitors. 2-Acylamido- and 2-sulfonamido-1,3,4-thiadiazole-5-sulfonamides. J Org Chem 1956;21:700–701.
- Young RW, Wood KH, Vaughan JR, Anderson GW. 1,3,4-Thiadiazole- and Thiadiazolinesulfonamides as carbonic anhydrase inhibitors. Synthesis and structural studies. J Am Chem Soc 1956;78:4649–4654.
- Supuran CT. Carbonic anhydrase inhibitors. (1994). In: Puscas I, ed. Carbonic Anhydrase and Modulation of Physiologic and Pathologic Processes in the Organism. Timisoara: Helicon, 29–111.
- Mincione F, Menabuoni L, Briganti F, Mincione G, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: inhibition of isozymes I, II and IV with N-hydroxysulfonamides–a novel class of intraocular pressure lowering agents. J Enzym Inhib 1998;13:267–284.
- Sugrue MF. The preclinical pharmacology of dorzolamide hydrochloride, a topical carbonic anhydrase inhibitor. J Ocul Pharmacol Ther 1996;12:363–376.
- Sumalan SL, Casanova J, Alzuet G, Borrás J, Castiñeiras A, Supuran CT. Synthesis and characterization of metal(II)-8-quinolinsulfonamidato (sa-) complexes (M = Co, Ni, Cu, and Zn). Crystal structure of [Zn(sa)2(NH3)]NH3 complex. Carbonic anhydrase inhibitory properties. J Inorg Biochem 1996;62:31–39.
- Supuran CT. Thienothiopyransulfonamides as complexing agents for the preparation of dual carbonic anhydrase inhibitors. Met Based Drugs 1995;2:327–330.
- Borras V, Cristea T, Supuran CT. Complexes with biologically active ligands. Part 5. Zn(ll) and Cd(ll) coordination compounds of hydrazine and heterocyclic sulfonamides as inhibitors of the zinc enzyme carbonic anhydrase. Main Group Met Chem 1996;19:339–410.
- Mincione G, Scozzafava A, Supuran CT. Carbonic Anhydrase Inhibitors. Part 46 Inhibition of Carbonic Anhydrase Isozymes I, II and IV With Trifluoromethylsulfonamide Derivatives and Their Zinc(II) and Copper(II) Complexes. Met Based Drugs 1997;4:27–34.
- Borràs J, Casanova J, Cristea T, Gheorghe A, Scozzafava A, Supuran CT et al. Complexes With Biologically Active Ligands. Part 6 Ni(II) Coordination Compounds of Hydrazine and Heterocyclic Sulfonamides as Inhibitors of the Zinc Enzyme Carbonic Anhydrase. Met Based Drugs 1996;3:143–148.
- Scozzafava A, Supuran CT. Complexes With Biologically Active Ligands. Part 10 Inhibition of Carbonic Anhydrase Isozymes I and II With Metal Complexes of Imidazo[2,1-b ]-1,3,4-Thiadiazole-2-Sulfonamide. Met Based Drugs 1997;4:19–26.
- Bülbül M, Kasimogullari R, Küfrevioglu OI. Amide derivatives with pyrazole carboxylic acids of 5-amino-1,3,4-thiadiazole 2-sulfonamide as new carbonic anhydrase inhibitors: synthesis and investigation of inhibitory effects. J Enzyme Inhib Med Chem 2008;23:895–900.
- Arslan O, Nalbantoğlu B, Demir N, Özdemir H, Küfrevioğlu Öİ. A new method for the purification of carbonic anhydrase isozymes by affinity chromatography. Trop J Med Sci 1996;26:163–166.
- Rickli EE, Ghazanfar SA, Gibbons BH, Edsall JT. Carbonic anhydrases from human erythrocytes. Preparation and properties of two enzymes. J Biol Chem 1964;239:1065–1078.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Wilbur KM, Anderson NG. Electrometric and colorimetric determination of carbonic anhydrase. J Biol Chem 1948;176:147–154.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Innocenti A, Scozzafava A, Parkkila S, Puccetti L, De Simone G, Supuran CT. Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg Med Chem Lett 2008;18:2267–2271.
- Landolfi C, Marchetti M, Ciocci G, Milanese C. Development and pharmacological characterization of a modified procedure for the measurement of carbonic anhydrase activity. J Pharmacol Toxicol Methods 1997;38:169–172.
- Bülbül M, Hisar O, Beydemir S, Ciftçi M, Küfrevioglu OI. The in vitro and in vivo inhibitory effects of some sulfonamide derivatives on rainbow trout (Oncorhynchus mykiss) erythrocyte carbonic anhydrase activity. J Enzyme Inhib Med Chem 2003;18:371–375.
- Ciftçi M, Bülbül M, Gül M, Gümüstekin K, Dane S, Süleyman H. Effects of nicotine and vitamin E on carbonic anhydrase activity in some rat tissues in vivo and in vitro. J Enzyme Inhib Med Chem 2005;20:103–108.
- Hisar O, Beydemir Ş, Bülbül M, Yanık T. Kinetic properties of carbonic anhydrase purified from gills of rainbow trout (Oncorhynchus mykiss). J Appl Anim Res 2006;30:185–188.
- Winum JY, Cecchi A, Montero JL, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors. Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with boron-containing sulfonamides, sulfamides, and sulfamates: toward agents for boron neutron capture therapy of hypoxic tumors. Bioorg Med Chem Lett 2005;15:3302–3306.
- Pedregosa JC, Casanova J, Alzuet G, Borrás J, García-Granda S, Diaz MR, Rodríguez AG. Metal com-plexes of 5-tertbutyloxycarbonylamido-1,3,4-thiadiazole 2-sulfonamide (B-H2ats), a carbonic anhydrase inhibitor. Synthesis and characterization of the copper(II) complex. Crystal structures of B-H2ats and the [Cu(B-ats)(NH3)2]2 dimer complex. Inorganica Chimica Acta 1995;123:117–124.
- Kirillova MV, Da Silva MFCG, Kirillov AM, Da Silva JJRF, Pombeiro AJL. 3D hydrogen bonded heteronuclear Co-II, Ni-II, Cu-II and Zn-II aqua complexes derived from dipicolinic acid. Inorg Chim Acta 2007;360:506–512.
- Barros-García FJ, Bernalte-García A, Luna-Giles F, Maldonado-Rogado MA, Viňuelas-Zahínos E. Synthesis, characterization and crystal structures of a bidentate indazole–thiazine derivative ligand and its cobalt (II) and cadmium (II) metal complexes. Polyhedron 2006; 25:52–60.
- Ajibade PA, Kolawole GA, O’Brien P, Helliwell M, Raftery J. Cobalt(II) complexes of the antibiotic sulfadiazine, the X-ray single crystal structure of [Co(C10H9N4O2S)2(CH3OH)2]. Inorganica Chimica Acta 2006;359:3111–3116.
- Chandra S, Kumar R. Synthesis electrochemistry and spectral studies on cobalt(II) and manganese (II) complexes with 12-,14-,15-, and 18-membered N4, N2O2, N2O2S, N6 donor macrocyclic ligands. Synth React Inorg Met-Org Nano-Met Chem 2005;35:161–170.
- Bayram E, Senturk M, Kufrevioglu OI, Supuran CT. In vitro inhibition of salicylic acid derivatives on human cytosolic carbonic anhydrase isozymes I and II. Bioorg Med Chem 2008;16:9101–9105.
- Khadikar PV, Joshi S, Kashkhedikar SG, Heda BD. Metal-complexes of 5-sulphosalicylic acid and their antimicrobial activity. Indian J Pharm Sci 1984;46:209–211.
- Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther 1997;74:1–20.