Abstract
Heterocycles bearing nitrogen, sulphur and thiazole moieties constitute the core structure of a number of biologically interesting compounds. Benzothiazole, a group of xenobiotic compounds containing a benzene ring fused with a thiazole ring, are used worldwide for a variety of therapeutic applications. Benzothiazole and their heterocyclic derivatives represent an important class of compounds possessing a wide spectrum of biological activities. The myriad spectrum of medicinal properties associated with benzothiazole related drugs has encouraged the medicinal chemists to synthesize a large number of novel therapeutic agents. Several analogues containing benzothiazole ring system exhibit significant antitumour, antimicrobial, antidiabetic, anti-inflammatory, anticonvulsant, antiviral, antioxidant, antitubercular, antimalarial, antiasthmatic, anthelmintic, photosensitizing, diuretic, analgesic and other activities. This article is an attempt to present the research work reported in recent scientific literature on different pharmacological activities of benzothiazole compounds.
| Abbreviations | ||
| 11β-HSD1, | = | 11β-hydroxysteroid dehydrogenase type 1; |
| AD, | = | Alzheimer’s disease; |
| AEDs, | = | antiepileptic drugs; |
| AIDS, | = | acquired immune deficiency syndrome; |
| AMBAN, | = | 2-acetyl- 3-(6-methoxybenzothiazo)-2-yl-amino-acrylonitrile; |
| APB, | = | 6-amino-2-n-pentylthiobenzothiazole; |
| BCC, | = | basal cell carcinoma; |
| BHR, | = | bronchial hyper responsiveness; |
| BT, | = | benzothiazole; |
| CAs, | = | carbonic anhydrases; |
| CAIs, | = | carbonic anhydrases inhibitors ; |
| CYP, | = | cytochrome P450; |
| CC50, | = | 50% cytotoxic concentration; |
| CNS, | = | central nervous system; |
| COX, | = | cyclooxygenase; |
| CVS, | = | cardiovascular system; |
| EC50, | = | 50% effective concentration; |
| EFA, | = | efavirenz; |
| EZA, | = | ethoxzolamide; |
| FDA, | = | Food and Drug Administration; |
| GABA, | = | gamma amino butyric acid; |
| GI, | = | gastro intestinal; |
| GP, | = | guinea pig; |
| Hsp90, | = | heat shock protein 90; |
| HIV, | = | human immune deficiency virus; |
| HsNMT, | = | homo sapiens N-myristoyltransferase; |
| IC50, | = | 50% inhibitory concentration; |
| INH, | = | isoniazid; |
| ITC, | = | isothermal titration calorimetry; |
| JNK, | = | c-jun N-terminal kinase; |
| LO, | = | 5-lipooxygenase; |
| LTD4, | = | leukoetriene D4; |
| MAPK, | = | mitogenactivated protein kinase; |
| MIC, | = | minimum inhibitory concentration; |
| MTP, | = | microsomal triglyceride transfer protein; |
| NASIDS, | = | non steroidal anti-inflammatory; |
| NCEs, | = | new chemical entities; |
| NCI, | = | National Cancer Institute; |
| NMSC, | = | nonmelanoma skin cancer; |
| NNRTIs, | = | non-nucleoside reverse transcriptase inhibitors.; |
| OA, | = | ovalbumin; |
| PCA, | = | passive cutaneous anaphylaxis; |
| PDT, | = | photodynamic therapy; |
| PET, | = | positron emission tomography; |
| PfNMT, | = | N-myristoyltransferase of Plasmodium falciparum; |
| PZA, | = | pyridazinamide; |
| RIA, | = | radio immuno assay; |
| RIF, | = | rifampicin; |
| ROS, | = | reactive oxygen species; |
| RT, | = | reverse transcriptase; |
| SCE, | = | sister chromatid exchange; |
| ScMET, | = | subcutaneous metrazole; |
| TB, | = | tuberculosis; |
| TSA, | = | thermal shift assay; |
| WHO, | = | World Health Organisation |
Introduction
Chemistry of heterocycles lies at the heart of drug discoveryCitation1. Investigation of fortunate organic compounds for drug discovery has been a rapidly emerging theme in medicinal chemistry. Benzothiazole (BT) and its derivatives belong to an enormously important family of synthetic compounds of heterocyclic systemsCitation2. Benz-fused azoles are among the most imperative classes of molecules having a common heterocyclic scaffold in several biologically active and medicinally significant compounds. The attention of biologists was drawn to this series when the pharmacological profile of riluzole, 6‐trifluoromethoxy‐2‐benzothiazolamine, (: compound 3) a blocker of excitatory amino acid mediated neurotransmission, was discoveredCitation3–6. Since then, BT derivatives have been studied extensively and found to possess diverse chemical reactivity profile along with broad spectrum of biological actionsCitation7. These agents act as important pharmacophores exhibiting outstanding biological activities and hence play a vital role in field of medicinal chemistryCitation8. BT s are a family of heterocyclic compounds whose chemical skeleton is constituted from a benzene (1) ring fused with a thiazole (2) ring (: compound 4)Citation9.
Pharmacological activities
BT core is highly important scaffold for drug development, because it has demonstrated a wide spectrum of pharmacological activities. Important medicinal activities associated with this class of compounds as reported in current scientific literature are antitumour, LTD4 receptor antagonists, amyloid imaging agent in Alzheimer’s disease (AD)Citation10, antimicrobial, antidiabetic, muscarinic receptor agonistCitation11, antiparasiticCitation12 anti-inflammatory, anticonvulsant, immunomodulatory and neuroprotective, antiglutamate/antiparkinsonianCitation13 antitubercular and antiallergic activitiesCitation14. BT derivatives have been shown to be useful for treatment of various diseases including neurodegenerative disorders, local brain ischemia, Huntington’s disease and cancerCitation15. Important findings from literature on diverse biological activity profile of BTs are presented in the following sections.
Anticancer activity
Cancer is one of the most formidable afflictions in the worldCitation16. Despite immense advances in the field of basic and clinical research, cancer is currently the second leading cause of death after cardiovascular disorders claiming more than half a million deaths in USA alone in 2009. The major proportion of cancer caused deaths are those related to lung (30%), prostate (9%) and colorectum (9%) in men, and lung (26%), breast (15%), colorectum (9%) and ovarian (5%) cancers in women. Deaths from cancer worldwide are projected to continue rising with an estimated 12 million deaths in the year 2030Citation17. A major challenge to medicinal chemistry researchers is identification of novel structures that can be potentially useful in designing new, potent selective and less-toxic anticancer agentsCitation18. Despite of the fact that several important advances have been achieved over recent decades in the research and development of various anticancer drugs, current antitumor chemotherapy still suffers from two major limitations. First is the lack of selectivity of conventional chemotherapeutic agents for cancer tissues, bringing about unwanted side effects. Second is the acquisition of multiple-drug resistance by cancer cellsCitation19. Current scenario highlights the need for the discovery and development of new lead compounds of simple structure, exhibiting optimal in vivo antitumor potency and new mechanisms of action. The BT moiety with various substitutions shows antitumor activity. A series of potent and selective antitumour agents were developed. Substituted 2‐(4‐aminophenyl) BTs represent a potent, highly selective and novel mechanistic class of antitumor agents examined, in vitro, shows antitumor activity in ovarian, breast, lung, renal and colon carcinoma human cell linesCitation20–26. BT analogs exert their anticancer activities by acting on diverse molecular targets. Some important examples of such biotargets on which these derivatives interact are discussed in the following text.
Replication and mitosis inhibitors
Drugs of this category are mitotic inhibitors and bind to tubulin, a microtubular protein and prevent polymerization and assembly of microtubules. It causes disruption of mitotic spindles and interference in cytoskeletal functions. The chromosomes fail to move apart during mitosis leading to metaphase arrest. Tuylu et al. developed derivatives of 2-aryl-substituted (o-hydroxy-, m-bromo-, o-methoxy-, o-nitro-phenyl or 4-pyridyl)benzothiazoles (: compounds 5–9) and tested their mutagenicity using in vitro assays: in the Ames test with Salmonella typhimurium TA98 and TA100 strains and (ii) in the sister chromatid exchange in cultured human lymphocytes. Compounds 5, 7, 8 and 9 were found to cause significant increase in revertant colonies when compared with the solvent control whereas compound 8 showed most potent mutagenic activity against TA98 and less mutagenic activity for TA100Citation27.
Topoisomerase II inhibitor
Suk-June et al. introduced combinatorial method for synthesis of 2-(substituted-phenyl)benzothiazoles (: compounds 10–12) and reported their antitumour activity. The structure activity relationship studies indicated that the BT nucleus was essential for potent cytotoxicity, and the substitution at 3-position of the phenyl ring with alkyl or halogen groups increased the cytotoxicity of antitumour BTs. Among these analogs, compounds 10 and 11 were observed to possess the strongest inhibitory activity against topoisomerase II with the IC50 of 71.7 and 70.5 µM, respectively when compared with the antitumor agent etoposide with IC50 of 78.4 µM. Compounds 10 and 12 possessing amino substitution showed high topo ll activityCitation28.
Tyrosine kinase inhibitors
Protein kinases have fundamental role in signal transduction pathways. Moreover, aberrant kinase activity has also been observed in many diseases. In recent years, kinase inhibition has become a major area for therapeutic interventions and a variety of kinase inhibitor pharmacophores have been described in current literature.
A series of novel 2-phenyl-1,3-benzothiazoles (: compounds 13–24) were synthesized and evaluated for their anticancer activity against MCF-7 breast cancer cell line with the MTT assay by Bhuva and Kini. The compounds have been found to mimic the ATP-competitive binding of genistein and quercetin to tyrosine kinase. Moderate to good antibreast cancer activity was recorded against most of the compoundsCitation29.
Inhibitors of thioredoxin signaling system
Thioredoxin/thioredoxin reductase system has the potential to act as target for anticancer drug and a small molecule inhibitor of this system is in clinical development. Lion et al. evaluated in vitro antitumour properties of a new series of fluorinated benzothiazole-substituted-4-hydroxycyclohexa-2,5-dienones (: compounds 25–27). The new compounds were found to be of comparable activity as compared with the nonfluorinated precursors, in terms of antiproliferative activity in sensitive human cancer cell lines and inhibitory activity against the thioredoxin signaling system. Most potent antiproliferative activity was shown by the 5-fluoro analogue (25)Citation30.
Cytochrome P450 (CYP) inhibitors
Cytochrome P450 has a crucial role in mechanism of action of some antitumour agents. Hutchinson et al. observed that fluorinated 2-(4-aminophenyl)benzothiazoles were potently cytotoxic (GI50 < 1 nM) in vitro in sensitive human breast MCF-7 (ER+) and MDA-468 (ER-) cell lines but inactive (GI50 > 10 IM) against PC 3 prostate, non-malignant HBL 100 breast, and HCT 116 colon cells. Most potent derivative was 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole 28, which produced no exportable metabolites in the presence of sensitive MCF-7 cellsCitation31. Induction of cytochrome P450 CYP1A1, a crucial event in determining the antitumor specificity of this series of BTs, was not compromised. In the preceding year they reported synthesis of a series of water-soluble L-lysine- and L-alanyl-amide prodrugs of the lipophilic antitumour 2-(4-aminophenyl)benzothiazoles. The prodrugs exhibited the required pharmaceutical properties of good water solubility (in weak acid) and stability at ambient temperature and degradation to free base in vivo. The lysyl-amide of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (NSC 710305) 29 has been selected for Phase 1 clinical evaluationCitation32. Furthermore, they described the synthesis of a new series of antitumour 2-(4-aminophenyl)benzothiazole analogues substituted with cyano and alkynyl group at 3′ position (: compounds 30 and 31). Compound 30 was active against MCF-7 and MDA-468 with GI50 value 0.0057 and 0.0078 µM, respectively, and compound 31 depicted GI50 values 0.011 and 0.0088 µM against MCF-7 and MDA-468, respectivelyCitation33.
Heat shock protein 90 (Hsp90) inhibitors
Zhang et al. synthesized a series of benzo- and pyridino- thiazolothiopurines as potent heat shock protein 90 inhibitors. Structure activity relationship showed that in case of BT series, the best analogues contained a two-carbon linker substituted with the diethyl phosphate moiety. Compounds 32–34 () were most potent inhibitors of tumour growthCitation34.
Other anticancer BTs
Novel series of substituted 2-phenyl-benzothiazole and substituted 1,3-benzothiazole-2-yl-4-carbothiaote derivatives (: compounds 35–38) were synthesized by Devmurari et al. Reduction in tumour volume, packed cell volume, viable cell count and non-viable cell count by administration of BT derivatives was noticed when compared with EAC control mice. Compounds 35–38 showed very good anticancer activity; however, compound 38 demonstrated significant toxicityCitation35.
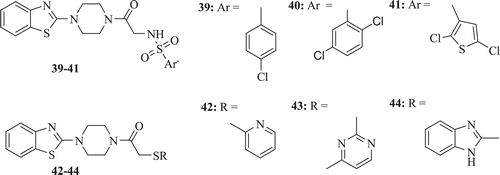
A series of BTs bearing piperazino-arylsulfonamides, arylthiol and sulphonamide moieties have been synthesized and evaluated, in vitro, for their antiproliferative activity against a large panel of human tumour derived cell lines by Al-Soud et al. Compounds 39–44 () were the most potent analogues in this series, showing activity against both the cell lines derived from haematological and solid tumours (CC50 range = 8–24 μM). Compound 40 was found to be selective and non cytotoxic to normal cellsCitation36.
Figure 9. Chemical structure of benzothiazole derivatives containing piperazino-arylsulfonamides, arylthiol and sulphonamides moieties.
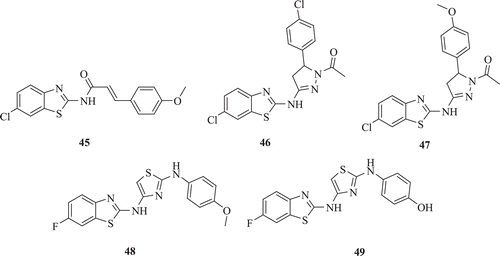
Amnerkar and Bhusari reported some new prop-2-eneamido, 1-acetyl-pyrazoline and thiazolyl substituted 2-aminobenzothiazoles (: compounds 45–49) for in vitro human tumour cell lines screening. On the basis of structure activity relationship, compound 45 was found to be active against renal cancer cell lines especially on RXF 393 with a growth percentage of 71.40Citation37.
Figure 10. Chemical structure of prop-2-eneamido, 1-acetyl-pyrazoline and thiazolyl substituted 2-aminobenzothiazoles.
Schnur et al. studied the antitumour property of N-(5-fluorobenzothiaol-2-yl)-2-guanidinothiazole-4-carboxamide in micro metastatic 3LL Lewis lung carcinoma in mice. Compound 50 () showed excellent therapeutic index relative to the cytotoxic anticancer drug adriamycinCitation38.
Leong et al. presented 2-(4-aminophenyl)benzothiazoles as potent and highly selective class of antitumour agents representing a mechanistic class distinct from clinically used chemotherapeutic agents. The result demonstrated that the generation of covalent adducts in sensitive cells between electrophilic reactive intermediates of 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203) (: compound 51) and DNA exacts lethal damage that precedes the exquisitely selective cell deathCitation39.
In vitro cytotoxic activity against HL‐60 and U‐937 cell lines and antimicrobial activity against bacterial and fungal strains of a series of BT derivatives (: compounds 52–59) were identified by Gupta et al. From the study, it was concluded that compounds 52, 53 and 55 exhibit significant activities against all bacterial and fungal strains. Compound 53 has better activity against Staphylococcus aureus, Proteus vulgaris, Staphylococcus epidermidis, Klebsiella pneumoniae and Candida albicans. Compound 56 has considerable activity against Bacillus subtilis and Escherichia coli. Except monomer 54 and 55 (methoxy and unsubstituted), all other compounds have significant activity against U‐937 lymphoma cell lines and compound 58 (methylene) exhibits better activity (1.8 μM) than the reference compound cisplatin (3.2 μM)Citation40.
Mortimer et al. evaluated a series of new 2-phenylbenzothiazoles for in vitro antitumor properties against human lung, breast and colon cancer cell lines. Among the synthesized compounds, 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (: compound 60) was found to possess exquisitely potent antiproliferative activity with GI50 <0.1 nM for MCF-7 and MDA-468Citation41.
Repicky et al. examined the antiproliferative- and apoptosis-inducing activities of 2-acetyl-3-(6-methoxybenzothiazo)-2-yl-amino-acrylonitrile (AMBAN) (: compound 61) towards human leukaemia HL60 and U937 cells. Results demonstrated that AMBAN inhibited the growth of HL60 and U937 cells with the IC50 of 30.37 µM (for 24 h), 19.39 µM (for 48 h and 72 h) and 11.31 µM (for 24 h), 3.55 µM (for 48 h), 2.52 µM (for 72 h), respectivelyCitation42.
A series of new amidino-nitro and amidino-amino substituted BTs (: compounds 62–63) was efficiently prepared by Racané et al. Diamidino substituted 2-phenylbenzothiazole 62 shows exceptionally prominent tumour cell-growth inhibitory activity and cytotoxicity whereas compound amino-amidine-2-phenylbenzothiazole 63 depicts inhibitory activity towards MCF-7 and H 460 cellsCitation43.
Some novel imidazobenzothiazole, imidazobenzoxazole, and imidazobenzoimidazole derivatives were investigated by Trapani et al. and their cytotoxic activity were determined at the National Cancer Institute in Derbyshire (UK) by testing against a panel of tumour cell lines. In vitro cytotoxic activity was reported by compounds 64–67 (). Compound 65 showed a score greater than the minimum values for xenograft testing together with a net cell kill in the hollow fiber assayCitation44.
A new series of 2-(4-aminophenyl)benzothiazoles (: compounds 68–72) was screened for antitumour activity by Dong-Fang et al. The molecules showed potent inhibitory activity in vitro in the nanomolar range against a panel of human breast cancer cell lines, but were found inactive (IC50 > 30 µM) against other cell typesCitation44,Citation45. They also synthesized and evaluated a series of potent and selective sulfamate salt derivatives of 2-(4-aminophenyl)benzothiazoles (: compounds 73–76) as potential prodrugs for parenteral administration. It was found that sulfamate salts were less active than their parent aminesCitation46.
Wells et al. synthesized a series of new antitumor agents, the BT-substituted quinol, ethers and esters (: compounds 77–85). The results showed that compound 77 possesses potent antitumour activity with IC50 of 0.24 µM against colon cell linesCitation47.
Vicini et al. evaluated three new series of benzo[d]isothiazole, BT and thiazole, Schiff’s bases against emergent and reemergent human and cattle infection diseases (AIDS, hepatitis B and C, TB, bovine viral diarrhoea) or against drug resistant cancers (leukemia, carcinoma, melanoma, multiple-drug resistant (MDR) tumours). All the benzo(d)isothiazole (: compounds 86–91) derivatives inhibited the growth of leukaemia cell lines. Benzo[d]isothiazole compounds showed a marked cytotoxicity against CD4+ lymphocytes (MT-4) with CC50 value between 4–9 µM that were used to support HIV-1 growthCitation48.
Yoshida et al. synthesized 2,6-dichloro-N-[2-(cyclopropanecarbonylamino)benzothiazole-6-yl]benzamides and tested for antitumour activity. Excellent in vivo inhibitory activity on tumour growth and excellent plasma concentration (t1/2 = 3.29 h) was shown by (: compound 92)Citation49.
Figure 21. Chemical structure of 2,6-dichloro-N-[2-(cyclopropanecarbonylamino) benzothiazole-6-yl]benzamides.
![Figure 21. Chemical structure of 2,6-dichloro-N-[2-(cyclopropanecarbonylamino) benzothiazole-6-yl]benzamides.](/cms/asset/ecc19e9c-3a6f-4f79-9353-c69c85cf291f/ienz_a_720572_f0021_b.gif)
Four classes of UK-1 analogues (: compound 93) were synthesized and their cytotoxicity testing against human A-549, BFTC-905, RD, MES-SA and HeLa carcinoma cell lines was determined by Shu-Ting et al.Citation50
Wan-Ping et al. provided evidence indicating that most of the 2-(4-aminophenyl)benzothiazoles (: compounds 94–103), under UVA light exposure, induced basal cell carcinoma (BCC) cell apoptosis. Due to these compounds having chromophoric structure and light absorption in the UVA range (320–400 nm), this in vitro study analyses the photosensitive effect of UVA-activated compounds in BCC cells. When cells were pre-treated with 2-(4-aminophenyl)benzothiazoles, UVA induces a markedly increased accumulation of sub-G1 phase and triggers apoptosis as revealed by the increased annexin V-FITC cells and the caspase-3 activation. Compounds treated with UVA induced a decrease in mitochondrial membrane potential (▵ψmt) and ATP via enhanced reactive oxygen species generation and promoted phosphorylation of extracellular signal-regulated kinase (ERK) and p38 MAPK expression. These results suggested that compounds treated with UVA elicit photosensitive effects in mitochondria processes, which involve ERK and p38 activation, and ultimately lead to BCC cell apoptosisCitation51.
Aiello et al. studied the antitumour properties of new fluorinated 2-aryl benzothiazoles, -benzoxazoles and -chromen-4-ones (: compounds 104–105) and compared with potent antitumour 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole. None of the compounds was able to recapitulate the antitumour potency of 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole. However, compounds 104 and 105 showed potent antitumour activity against MCF-7 and MDA-468 breast cancer cell lines with submicromolar GI50 value 0.57, 0.20 and 0.40, 0.21 µM, respectivelyCitation52.
Anti-infective activity profile
Historically, the use of anti-infective agents can be credited with saving more human lives than other area of medicinal therapy discovered to dateCitation53. Emergence of infectious diseases and multidrug resistance are the combined factors that remain important and challenging problems for the treatment of infectious diseasesCitation54. In addition, the treatment of infectious diseases is more complicated in immune-suppressed patients, such as those infected with the HIV, undergoing anticancer therapy or transplantsCitation55.
Antimicrobial activities
Microbes are causative agents for various types of diseases such as pneumonia, ameobiasis, typhoid, malaria, common cough and cold and also some severe diseases such as tuberculosis (TB), influenza, syphilis and AIDSCitation56. Infectious diseases caused by bacteria affect millions of people and are leading causes of death worldwideCitation57. During the past three decades, antimicrobial agents have been introduced at a rate exceeding our ability to integrate them into clinical practiceCitation58. Despite several advancements in the development of antimycotic agents, antifungal chemotherapy remains problematic in many cases and search for new antifungal agents continues to be an inevitable taskCitation59. Despite of numerous attempts to develop new antimicrobial agents, many problems remain to be solved for currently available antimicrobial drugsCitation60. BT still remains one of the most versatile classes of compounds against microbes, and therefore, are useful substructures for further molecular exploration.
Mistry and Desai synthesized heterocyclic 4-thiazolidinone benzothiazoles derivatives. The synthesized compounds were tested for their antibacterial activity against S. aureus and E. coli by measuring the inhibition area on agar plates (diffusimetric methods). Good activity against S. aureus and E. coli was shown by compounds 106, 110, 111 and 107, 112, 113, respectively. Compounds 108, 112, 113 and 109, 114, 115 depicted low activity against S. aureus and E. coli, respectively (: compounds 106–115)Citation61.
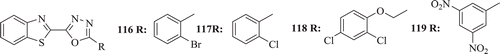
Rajeeva et al. evaluated some new 2-(5-substituted-1,3,4-oxadiazole-2-yl)-1,3- BT derivatives for in vitro antibacterial activity at a concentration of 100 µg/mL using DMSO as a control and ciprofloxacin as standard drug against Gram positive and Gram negative bacterial strain such as B. subtilis, Bacillus pummels, E. coli and Pseudomonas aeruginosa by disc diffusion method. The analogues (: compounds 116–119) were found to possess broad spectrum of antibacterial activityCitation62.
Figure 26. Chemical structure of 2-(5-substituted-1,3,4-oxadiazole-2-yl)-1,3-benzothiazole derivatives.
A series of 2-(benzo[d]thiazol-2-ylthio)-N-(2-oxoindolin-3-ylidene)acetohydrazide, 2-(benzo [d]thiazol-2-ylthio)-N-(2,4′-dioxospiro[indolin-3,2′-thiazolidin]-3′-yl)acetamide and 2′-((benzo[d]thiazol-2-ylthio)methyl)spiro[indoline-3,5′-thiazol1,3,4oxadiazol]-2-one compounds were developed and screened for their antibacterial activities by Kaur et al. Most potent antibacterial against K. pneumoniae, S. aureus and E. coli (zone of inhibition: 22, 22 and 20 mm, respectively) and anti-inflammatory compound of this series (: compounds 120–121) was compound 120 and most potent analgesic compound was 121Citation63.
A novel series of benzothiazolium derivatives (: compounds 122–123) substituted at position 5- and/or 6- on heterocyclic ring has been prepared and tested against Gram positive bacteria and yeast microorganisms by Sigmundova et al. Compounds 122 and 123 showed potent activity against B. subtilis and also 123 was active against Micrococcus luteusCitation64.
Gajdos et al. synthesized a series of new 2-styrylbenzothiazole-N-oxides (: compounds 124–127) and tested for their antimicrobial activities against Gram positive (S. aureus and B. subtilis) and Gram negative bacteria (E. coli and Pseudomonas aeruginosa) as well as a yeast (C. albicans) and a mould (Microsporum gypseum). However, weak activity was recorded when compared with previously prepared analogous benzothiazolium saltsCitation65.
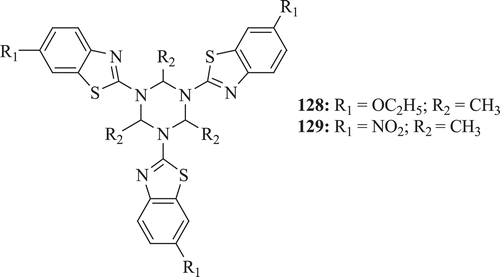
2,4,6-substituted-1,3,5-tris(benzothiazolyl)hexahydro-1,3,5-triazine derivatives (: compounds 128–129) were synthesized by Pareek et al. All compounds were evaluated for antibacterial, antifungal, acaricidal and antifeedant activities. Biological screening identified that very low activity was recorded against all bacterial strains with comparably higher antifungal activity. Results of acaricidal and antifeedent activities showed that compounds 128 and 129 possess good acaricidal and antifeedent activites, respectivelyCitation66.
Figure 30. Chemical structure of 2,4,6-substituted-1,3,5-tris(benzo-thiazolyl)hexahydro-1,3,5-triazine.
Barot et al. screened a series of substituted fluoro BT derivatives (: compounds 130–131) for their antibacterial, antioxidant activities and for inhibition of denaturation of protein. Excellent activity against S. aureus, B. subtilis was shown by compounds 130 and 131; moreover, compound 130 also demonstrated good antioxidant activityCitation11.
Novel BT-substituted thiazolidinone derivatives (: compounds 132–139) were evaluated for their inhibitory ability against Proteus mirabilis, S. aureus, Salmonella typhi and K. pnuemoniae by Nagarajan et al. Compounds 133, 134, 135 and 137 showed effective inhibition towards above mentioned four human pathogens as compared to 132, 136, 137 and 138Citation67.
Antimicrobial activity of new 2-alkenyl-6-aminobenzothiazoles (: compounds 140–141) was screened by Sidoova et al. Results demonstrated that compounds 140 and 141 exhibit better activity as compared with standard drug 6-amino-2-n-pentylthiobenzothiazole (APB)Citation68.
Malik et al. identified some new 2-amino-substituted-benzothiazole derivatives (: compounds 142–148) as antifungal agents. All the compounds exhibited moderate antifungal activity with minimum inhibitory concentration (MIC) range 0.63–0.31 µg/mLCitation69.
A novel series of Schiff bases of BT derivatives (: compounds 149–155) were screened for their antibacterial activity by Soni et al. The biological screening identified that antibacterial activity decreases with substitution at ortho and it increases with substitution at para position. Compounds 149, 154 and 155 showed maximum antibacterial activities and compounds 149, 150, 153 and 155 showed antifungal activities against both the C. albicans and Aspergillus niger organismsCitation70.
Latrofa et al. evaluated a series of N-cycloalkenyl-2-acylalkylidene-2,3-dihydro-1,3-benzothiazoles, N-cycloalkyl-2-acylalkylidene-2,3-dihydro-1,3-benzothiazoles and N-alkyl-2-acylalkylidene-2,3-dihydro-1,3-benzothiazoles for antifungal and antibacterial activity. Results revealed that N-cycloalkenyl compounds (: compounds 156–170) did not exhibit any significant antifungal activity, but antibacterial activity was found to increase with increase in the value of acyl chain (R1). Compounds 165 and 166 showed moderate antibacterial activities against Gram negative organismsCitation71.
Some new 1-aroyl-3-(substituted-2-benzothiazolyl)thioureas were evaluated for their antimicrobial activity by Saeed et al. All the compounds (: compounds 171–181) exhibited moderate antimicrobial activityCitation72.
Thiourea derivatives bearing BT moiety (: compounds 182–186) were reported with good antimicrobial activity by Saeed et al. Significant antimicrobial activity was showed by all the compoundsCitation73.
Bhusari et al. synthesized a series of sulphonamide derivatives having benzothiazole moiety (: compounds 187–189). All the test compounds have been assayed in vitro for antibacterial activity against B. subtilis and E. coli, and for antifungal activity against C. albicans. Antimycobacterial activity was tested against H37Rv strain of Mycobacterium tuberculosis. Moderate to good potency against different bacterial strains was observed by all the newly synthesized compounds. Among synthesized derivatives, compounds 187, 188 and 189 showed prominent antibacterial, antifungal and anti mycobacterium activity, respectivelyCitation74.
Franchini et al. synthesized 2-mercapto-1,3-benzothiazole (: compounds 190–199) derivatives with potent antimicrobial activity. The biological screening identified compound 194 as the most active one showing an interesting antibacterial activity with MIC values of 3.12 µg/mL against S. aureus and also compound 195 demonstrated wide antimicrobial activity against S. aureus and E. coli with MIC 1.25 and 25 µg/mL, respectivelyCitation75.
Bondock et al. described the synthesis of polyfunctionally substituted heterocycles (e.g. pyrazoles, isoxazole, pyrimidines, thiazolo[3,2-a]pyrimidine, tetrazolo[1,5-a]pyrimidine, pyrido[1,2-a]pyrimidine, 1,5-benzodiazepine, and pyrazolo[1,5-a]pyrimidine incorporating benzothiazole moiety by using enaminonitrile moiety and screened for their antimicrobial activity. Synthesized compounds (: compounds 200–204) showed moderate activity against the screened Gram positive bacteriaCitation76.
New and simple synthetic methods for the synthesis of ethyl-8-chloro-4-(4-substituted phenyl)-2[(N-ethoxyphthalimido)amino]-4H-pyrimidino[2,1-b1,3]benzothiazole-3-carboxylates 205–212 and 6-chloro-N-[3-{2-(4-substitutedphenyl)ethenyl}-1-N-ethoxyphthalimidoquinoxaline-2(1H)-ylidene]-1,3-benzothiazole-2-amines 208–211 was described by Sain et al. All the compounds (: compounds 205–212) exhibited good to moderate antimicrobial activityCitation77.
Baheti et al. synthesized and screened 15-iminobenzothiazolo[2,3-b]pyrimido[5,6-c]pyrimido[2,3-b]benzothiazole-14(H)-one derivatives (: compounds 213–222) for their antimicrobial activity and it was found that compound 220 showed 10, 10, 13, 10 mm zone of inhibition and similarly 221 demonstrated 10, 9, 10, 12 mm inhibition zone against S. aureus, B. substilis, E. coli and S. typhi, respectivelyCitation78.
Figure 43. Chemical structure of 15-iminobenzothiazolo[2,3-b]pyrimido[5,6-c]pyrimido[2,3-b]benzothiazole-14(H)-one derivatives.
![Figure 43. Chemical structure of 15-iminobenzothiazolo[2,3-b]pyrimido[5,6-c]pyrimido[2,3-b]benzothiazole-14(H)-one derivatives.](/cms/asset/cf5231b9-5469-4592-a3ef-2c12d7e4fe11/ienz_a_720572_f0043_b.gif)
Substituted pyrimido[2,1-b]benzothiazoles (: compounds 223–230) were evaluated against E. coli, S. aureus, Enterobacter for antibacterial activity and against C. albicans for antifungal activity at conc of 100 µg/disc by Gupta et al. Compounds 223, 227 and 226, 228 showed significant activity against E. coli, Enterobacter and S. aureus, respectively; moreover, potent antifungal activity against C. albicans was shown by compounds 224, 227, 228 and 230Citation79.
Vedavathi et al. evaluated fluorobenzothiazole incorporated with 1,3,4-thiadiazole derivatives for antimicrobial activities. Significant antibacterial and antifungal activity was demonstrated by all the compounds (: compounds 231–240)Citation80.
Kuchta et al. presented 6-amino-2-n-pentylthiobenzothiazole (APB) (: compound 241) as a promising new antifungal agent active against various Candida species in vitro, to inhibit the yeast mycelium conversion in C. albicans, and to be active in vivo, curing systemic candidosis in mice. Results showed that APB is an antifungal agent inhibiting ergosterol biosynthesis at the level of 4-demethylationCitation81.
New 2-methyl-3-[(substituted)-benzothiazole-2′-yl]4(3H)-quinazolinones (: compounds 242–244) have been synthesized and evaluated by Lakhan and Ral for antifungal activity. Compound 242 was relatively more active at the given dilutions against the fungi chosen in comparison with the commercial fungicideCitation82.
Polyfluorinated 2-benzylthiobenzothiazoles (: compounds 245–252) were evaluated for their fungicidal activity against Rhizoctonia solani, Botrytis cinereapers and Dothiorella gregaria at a dosage of 50 µg/mL by Huang and Guang-Fu. The outcomings of the study showed that compound 245 exhibited very significant activities against R. solani, B. cinereapers and D. gregariaCitation83.
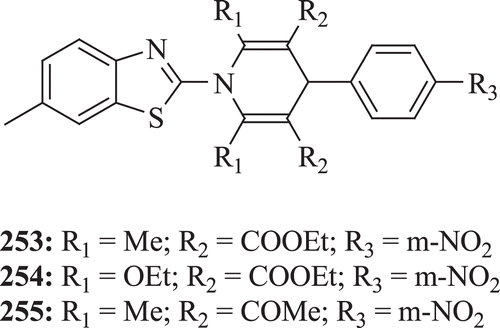
A series of N-(6-methylbenzothiazolyl)-2,3,5,6-tetrasubstituted-4-(aryl)-1,4-dihydropyridines were evaluated for antibacterial and antifungal activities by Mithlesh et al. Maximum antimicrobial activity was depicted by the compounds 253–255 ()Citation84.
Figure 49. Chemical structure of N-(6-methylbenzothiazolyl)-2,3,5,6-tetrasubstituted-4-(aryl)-1,4-dihydropyridines.
The antimicrobial activity of 3,5-diaryl-1-benzothiazolopyrazoline derivatives has been studied against various microbes by Sharma and Sharma. The screening data revealed that compounds (: compounds 256–
263) possess moderate activity against C. albicans, but compounds 256, 259, 261 and 262 show higher activity against Cladosporium cladosporoides and Curvularia lunataCitation85.
Argyropoulou et al. evaluated several thiazoles and BTs carrying a benzenesulfonamide moiety at position 2 of the heterocyclic nucleus as antimicrobial agents. All the derivatives (: compounds 264–268) demonstrated effective antibacterial properties against Gram positive bacteriaCitation86.
Alang et al. screened seven new derivatives of BTs (: compounds 269–280) for antibacterial activity. Compounds 271 and 275 were found to possess moderate activity against S.aureus and P. aeruginosa, while compounds 269 and 272 demonstrated similar sort of activity against E. coli. Compound 273 depicted excellent activity against P. aeruginosa, while compound 274 possessed prominent activity against S. epidermidis when tested at 1 mg/mL concentration taking ampicillin as a standard drugCitation87. They also evaluated a series of BTs for antifungal activity against C. albicans (MTCC 183), A. niger (MTCC 228), Candida tropicalis (MTCC 6192) and Fusarium oxysporium (MTCC 3656). Results showed that compound 276 possesses significant activity against C. albicans (MTCC 183), A. niger (MTCC 228) and F. oxysporium (MTCC 3656) while 278 shows comparable activity against C. albicans (MTCC 183), A. niger (MTCC 228) when tested at 1 mg/mL concentration taking clotrimazole as the standard drugCitation88.
Chaitanya et al. evaluated a series of BT derivatives (: compounds 281–296) for antimicrobial and anti-inflammatory activity. Compounds 281 and 291 depicted excellent antibacterial activity against E. coli and compounds 282–289 possess antifungal activity against A. niger. Moreover, compounds 289, 290, 292–296, possess potent antifungal activity against Aspergillus flavus and also compound 296 possesses good anti-inflammatory activity in carrageenan induced rat paw edema to the extent of 95.73% in comparison with the standard drug (82.56%).Citation89
Antiviral activity
In the 19th century, humans lost their fear of God and acquired a fear of microbes, whereas in the 20th century, the anonymously acclaimed fear of microbes has been superceded by the fear of human immunodeficiency virus (HIV)Citation90. HIV-I has been identified as the causative agent in the transmission and development of AIDSCitation91. AIDS is now a pandemic. In 2007, it was estimated by World Health Organisation (WHO) that 33.2 million people lived with the disease worldwide, and that AIDS killed an estimated 2.1 million people, including 330,000 childrenCitation92. In the past two and half decades, various compounds have been developed as medicinal candidates for the treatment of HIV infections aiming at one or several steps of the HIV-1 life cycle such as absorption, entry, fusion, un-coating, reverse transcription, integration, transcription and maturationCitation93. Reverse transcriptase (RT) is a key enzyme, which plays an important role in the replication of virus. Three non-nucleoside reverse transcriptase inhibitors namely nevirepine, delaviridine and efavirenz (EFV) have been approved by FDACitation94. However, prolonged treatment with antiretroviral drugs results in disease progression. These concerns have drawn a strong focus on research into new drugs targeting other aspects of HIV life cycle, including viral entry and integration into the host genome (integrase inhibitors)Citation95.
Akhtar et al. investigated a series of new BT derivatives (: compounds 297–298) for their antiviral activity. Results showed that none of the in vitro tested compounds was found to inhibit HIV-replication, at EC50 lower than the CC50, compared with the antiviral agent EFV, whereas biological screening for cytotoxicity observed that compounds 297 and 298 showed strong effect on leukaemia cell linesCitation96.
A series of 2,5,6-substituted benzoxazole, benzimidazole, BT and oxazole(4,5-b) pyridine derivatives were evaluated for antiviral activity against HIV-I reverse transcriptase enzyme by Akbay et al. Results demonstrated that compound 299 () posseses good antiviral activity (IC50 value 0.34 µmol/L)Citation97.
Nagarajan et al. synthesized inhibitors of HIV-1 protease with improved potency and antiviral activities by replacement of the urea moiety by benzothiazolesulfonamide moiety. Compounds 300–302 () were observed to be potent inhibitors of HIV-1 protease and also possess good oral bioavailabilityCitation98.
Antimalarial activity
Malaria is one of the most serious global health problems in subtropical and tropical zones of the world and travellers to these endemic areas. Global estimates of malaria show that at least 300 million people are afflicted, and 1–3 million people die annually from this disease. The disease is caused by four species of the malaria parasite of which Plasmodium falciparum is the most virulent and potentially deadly. Most serious issue is that malaria parasites develop resistance to clinically used chemotherapeutic agents such as chloroquine, mefloquine and pyrimethamine. Therefore, there is still a need for highly potent and low-priced antimalarialsCitation99,Citation100.
5-n-Undecyl and 5-n-pentadecyl-6-hydroxy-4,7-dioxobenzothiazole were tested for prophylactic antimalarial activity against Plasmodium gallinaceum in chicks by Friedman et al. and (: compound 303) was found to show prophylactic activity at 120 mg/kg in this sporozoite-induced malariaCitation101.
Burger and Sawhney evaluated a series of BT amino alcohols for antimalarial activity (: compounds 304–306). Results showed that several compounds possess weak activity against Plasmodium bergheiCitation102.
Bowyer et al. demonstrated the use of scintillation proximity assay for the identification of inhibitors showing activity against both recombinant N-myristoyltransferase of Plasmodium falciparum (PfNMT) and the cultured asexual stages of P. falciparum. Seven compounds showed >25% inhibitory activity against recombinant PfNMT. Data showed that three BT -containing compounds (307, 310 and 313) have IC50 values <50 μM for PfNMT. All the compounds showed some selectivity for PfNMT, with the exception of compound 308 () that depicted more inhibitory action to HsNMT as compared with PfNMT. Effect of these compounds on total parasitaemia, compared with controls containing 1% (v/v) DMSO only, shows that there was a significant reduction in parasitaemia at 100 µM to a level similar to the starting parasitaemia (approximately 1%). In the case of compounds 307 and 309 at 10 µM, there was an approximately 80% reduction in parasitaemia compared with the controlCitation103.
Antitubercular activity
TB is a contagious disease with high mortality worldwide and is currently the leading killer of the youth, women and AIDS patients worldwideCitation104. The statistics indicate that 3 million people worldwide die annually from complications of TB, and there are estimated 8 million new cases each year, 95% of which occur in developing countriesCitation105,Citation106. The actual development and clinical use of new therapeutics for TB have remained stagnant for years because of the complexity of the disease process, the treatment of which at present requires the administration of drug combinations over a period of 6 months and more importantly because of the alarming rise of drug-resistance. Treatment of mycobacterial infections, especially TB, has become an important problem due to the emergence of monodrug and multidrug-resistant strains of M. tuberculosis. It can be emphasized that there exists continuous demand for drugs of new structural classes with novel mechanisms of action other than isoniazid, rifampicin and pyridazinamide. Hence, discovery and development of novel, more active and less-toxic anti-TB agents is an imperative task for medicinal researchersCitation107.
Novel 3-nitro-2-(sub)-5,12-dihydro-5-oxobenzothiazolo[3,2-a]-1,8-naphthyridine-6-carboxylic acids were synthesized and evaluated for their antitubercular activities in vitro and in vivo against M. tuberculosis H37 Rv (MTB) and multidrug resistant M. tuberculosis (MDR-TB) by Dinakaran et al. Among the synthesized compounds, 2-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)-3-nitro-5,12-dihydro-5-oxobenzothiazolo[3,2-a]-1,8-naphthyridine-6-carboxylic acid (: compound 314) was found to be the most active compound in vitro with MIC of 0.19 and 0.04 µM against MTB and MTR-TB, respectivelyCitation108.
Figure 60. Chemical structure of 2-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)-3-nitro-5,12-dihydro-5-oxobenzothiazolo[3,2-a]-1,8-naphthyridine-6-carboxylic acid.
![Figure 60. Chemical structure of 2-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)-3-nitro-5,12-dihydro-5-oxobenzothiazolo[3,2-a]-1,8-naphthyridine-6-carboxylic acid.](/cms/asset/7d4dd9f9-d9ca-4824-a4cd-6829763162d4/ienz_a_720572_f0060_b.gif)
A new class of 2-methylbenzothiazole analogues (: 315–316) depicting appreciable anti-TB properties was identified by Huang et al. These agents were found to be active against replicating M. tuberculosis H37Rv. From the overall results, it was predicted that the most potent compounds 315 and 316 exhibited MICs of 1.4 and 1.9 μM, respectivelyCitation109.
Palmer et al. evaluated a series of benzothiazoline derivatives for antimicrobial activity against Gram positive and Gram negative bacteria, dermatophytes, Entamoeba histolytica and for in vitro and in vivo activity against M. tuberculosis. Results demonstrated that compound 317 () possesses the greatest activity against M. tuberculosisCitation110.
Anthelmintic activity
Helminth parasitism remains an underappreciated scourge of humans in most of the developing world. As many as 2 billion individuals harbour these parasites, with millions typically simultaneously infected with filariae, hookworms, whipworm, large roundworms and/or schistosomes, all of which often result in chronic, debilitating morbidityCitation111. Despite remarkable advances made in the chemotherapy of parasitic diseases, even today, successful treatment and eventual eradication of filariasis remains as one of the major public health problems of the tropics. Attempts to innovate new “structural leads” in the chemotherapy of helminthiasis have led to the discovery of a series of benzothizoles anthelmintics possessing potent activity against different helminth parasitesCitation112.
Anthelmentic activity of fluorobenzothiazole comprising sulfonamido pyrazole derivatives against earthworms, Perituma posthuma were demonstrated by Sreenivasa et al. Compounds 318–325 () showed significant activity as compared with the standard drug albendazoleCitation113.
Nadkarni et al. synthesized substituted phenyl imidazo-[2,1-b]benzothiazoles (: compounds 326–328) and tested their anthelmintic activity. Results showed that all the compounds exhibited potent anthelmintic activityCitation114.
Anti-inflammatory activity
Inflammatory diseases are widely prevalent throughout the world and inflammation remains a common as well as often poorly controlled disease, which can be life threatening in extreme form of allergy, autoimmune diseases and rejection of transplanted organs; and similarly, chronic inflammation has been found to mediate a wide variety of diseases including cardiovascular diseases, cancer, diabetes, arthritis, AD, pulmonary diseases and autoimmune diseases. Non steroidal anti-inflammatory drugs (NSAIDs) have been used for the treatment of ailments such as pain, fever and inflammation and also in chronic conditions such as arthritis, psoriasis and asthmaCitation115–120. NSAIDs principally act by two different mechanisms: a direct contact mechanism on the gastro intestinal (GI) mucosa through oral dose and a generalized systemic action appearing after intravenous dosingCitation121,Citation122. Their use is mainly restricted by their well known and serious adverse GI side effects such as gastroduodenal erosions, ulcerations and renal toxicities and therefore the discovery of new safer anti-inflammatory drugs represents a challenging goal for researchersCitation123,Citation124.
Paramashivappa et al. evaluated a series of 2-[[2-alkoxy-6-pentadecylphenyl)methyl]thio]-1H benzimidazoles/benzothiazoles and benzoxazoles from anacardic acid for cyclooxygenase-1 (COX-1) inhibition. Results depicted that compound 329 () was 470-fold selective towards COX-2 compared with COX-1Citation125.
Singh et al. synthesized a series of 2-substituted-9-chloro-8-fluoropyrimido [2,1-b]benzothiazole-3-cyano-4(H)-ones (: compounds 330–336) and screened their anti-inflammatory and analgesic activity by acetic acid induced writhing method in albino mice. Results showed that none of them could inhibit albumin denaturation significantly in comparison with standard drug diclofinac sodium, which exhibited 81% inhibition of albumin denaturationCitation126.
Figure 66. Chemical structure of 2-substituted-9-chloro-8-fluoropyrimido [2,1-b]benzothiazole-3-cyano-4(H)-ones.
![Figure 66. Chemical structure of 2-substituted-9-chloro-8-fluoropyrimido [2,1-b]benzothiazole-3-cyano-4(H)-ones.](/cms/asset/a7c1dfee-091e-4854-a789-f91e58953932/ienz_a_720572_f0066_b.gif)
Anti-inflammatory activity of 2-amino benzothiazole derivatives were reported by Venkatesh and Pandeya. Compounds 337–339 () were the most active compounds in comparison with standard drug diclofenac sodiumCitation127.
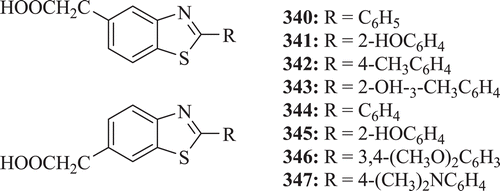
Wada et al. evaluated 2,5,6-trisubstituted benzothiazole compounds (: compounds 340–347) for their anti-inflammatory activity. It was found that presence of an acetic acid function was important for anti-inflammatory activity and also that 2-substituted 5-benzothiazoleacetic acid was better than 2-substituted 6-benzothiazoleacetic acid in anti-inflammatory activityCitation128.
Figure 68. Chemical structure of 2-substituted-5-benzothiazoleacetic acid and 6-benzothiazoleacetic-2-substituted acid.
Novel benzoheterocyclic[(methoxyphenyl)amino]oxoalkanoic acid esters have been screened as inhibitors of rat polymorphonuclear leukocytes 5-lipooxygenase (LO) in vitro and as inhibitors of leukoetriene D4 (LTD4) and ovalbumin induced bronchospasm in the guinea pig (GP) in vivo by Musser et al. Results revealed that compound 348 () was the most potent compound with IC50 of 0.36 µMCitation129.
Miscellaneous activities
Recent progress as anticonvulsant agents
Epilepsy is a syndrome of different cerebral disorders of the central nervous system (CNS) and is characterized by paroxysmal, excessive and hyper synchronous discharges of large number of neurons. The overall prevalence of the disease is 0.5–1.0% of the population and up to 50 million people are affected worldwide. Data show that only 28–30% of patients are poorly treated with currently available antiepileptic drugs and these drugs may also cause serious side effects including ataxia, nausea, mental dulling and hepatotoxicityCitation130–132.
Sharma et al. synthesized and evaluated a series of isatin schiff’s bases (: compounds 349–357) for their anticonvulsant activity; moreover, their toxicity screening was also carried out. Among the synthesized compounds, 350–352 showed potent anticonvulsant activity and compound 350 showed 100% protection at 300-mg dose in MES test and 60% promising protection in subcutaneous metrazole test upto 0.5 h; however, this compound has also shown 87.5% nontoxic effectCitation133.
In order to develop suitable anticonvulsant agents, Rana et al. synthesized a series of 1,3-benzothiazol-2-yl-benzamides. Among the hits discovered, compounds 358–360 () emerged as anticonvulsants with no neurotoxicityCitation134.
Anticonvulsant property of 1,3-benzothiazol-2-yl-semicarbazones were evaluated by Siddiqui et al. Results showed that compounds 361–364 () possessed 100% protection at both 0.5 h- and 4 h-time intervals except compound 361 whose percentage protection decreased to 83.3% at the 4 h indicating rapid onset but shorter duration of action against maximal electroshock (MES) testCitation135.
Recent advances in antidiabetic activity
Diabetes mellitus is one of the major crippling diseases in the world, leading to huge economic loses. This metabolic disorder is a heterogeneous group of diseases characterized by derangement in carbohydrate, protein and/ or fat metabolism caused by defective production of insulin. Current estimates show that at least 150 million people worldwide have diabetes, of which two-thirds are residing in developing countries. The total number of people with diabetes is predicted to rise to about 300 million by the year 2025, with one-third of affected individuals living in India and China aloneCitation136–138.
Pattan et al. synthesized a new series of 2-amino[5′-(4-sulphonylbenzylidine)2,4-thiazolidinedione]-7-chloro-6-fluorobenzothiazoles and screened for their antidiabetic activity on albino mice. All the compounds 365–370 () showed significant antidiabetic activity, but compounds 365–367 showed maximum antidiabetic activityCitation139.
Figure 73. Chemical structure of series of 2-amino[5'-(4-sulphonylbenzylidine)2,4-thiazolidinedione]-7-chloro-6-fluorobenzothiazoles.
![Figure 73. Chemical structure of series of 2-amino[5'-(4-sulphonylbenzylidine)2,4-thiazolidinedione]-7-chloro-6-fluorobenzothiazoles.](/cms/asset/30139537-ed9f-4017-bf69-dab87aa99f2e/ienz_a_720572_f0073_b.gif)
A new series of 2-thioether-benzothiazoles (: compounds 371–375) has been synthesized and evaluated for c-jun N-terminal kinase inhibition by De et al. Results depicted that compounds 371 and 375 show similar activity, whereas compounds 373–374 were inactive due to stearic hindranceCitation140.
Zandt et al. discovered 3-[(4,5,7-trifluorobenzothiazol-2-yl)methyl]indole-N-acetic acid (Lidorestat) (: compound 376) as highly potent and selective inhibitors of aldose reductase with an IC50 of 5 nM for treatment of chronic diabetic complicationsCitation141.
Su et al. evaluated BT derivatives as selective inhibitors of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) possessing a considerable potential in treatments for metabolic diseases, such as diabetes mellitus type 2 or obesity. Results demonstrated that compounds 377 and 378 () show inhibition for 11β-HSD1 greater than 80% at 10 µMCitation142.
Diuretic activity
N-{(substituted)-1,3-benzothiazol-2-yl}-1,1-biphenyl-4-carboxamide derivatives (: compound 379) were screened by Shaharyar and Ansari for their in vivo diuretic activity. Urinary output of this synthesized compound was highly significant 16.08 ± 0.650 (p < 0.01), i.e. increased by >300% with respect to control urinary outputCitation143.
Developments in cardiovascular activity profile
Yoshino et al. evaluated a series of 4-(benzothiazol-2-yl)benzylphosphonic acid dialkyl ester derivatives (: compounds 380–385) for coronary vasodilatory activity by Langendorff’s method in the isolated guinea pig heart. Results revealed that the potency of diethyl derivative 380 was superior to that of reference compounds papaverine hydrochloride or diltiazem hydrochlorideCitation144.
Vu et al. evaluated a series of triamide derivatives bearing a BT core as potent microsomal triglyceride transfer protein inhibitors (: compound 386). Results demonstrated that compound 386 lowered the plasma triglycerides, glucose and insulin levels at doses as low as 3 mg/kg and also had a low systemic exposureCitation145.
Antioxidant agent
Cressier et al. reported the synthesis and antioxidant property of new compounds derived from benzothiazoles and thiadiazoles. It was noticed that only thiols, thiosulfonic acids and phosphorothioates exhibit evident antioxidant activity and 2-mercapto-6-methylbenzothiazole (: compound 387) show an efficient radioprotective effect at LD99.9/30Days-IRRCitation146.
Karali et al. evaluated antioxidant property of 3H-spiro[1,3-benzothiazole-2,30-indol]-20(10H)-ones derivatives (: compounds 388–395). Compounds 388, 389 and 391 were observed to be the most active antioxidant agentsCitation147.
Antiasthmatic activity
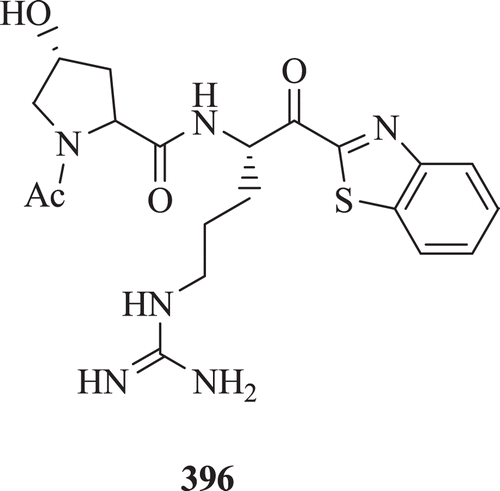
Benothiazole ketone as a potent, reversible, low molecular weight tryptase inhibitors were studied by Costanzo et al. Results showed that transition-state mimetics possessing a heterocyclic activated ketone group possess potent tryptase inhibitory activity with a Ki value of 10 nM (: compound 396)Citation148.
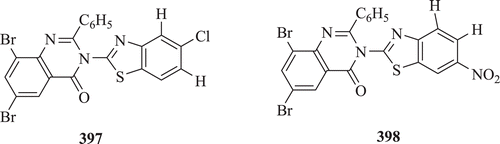
Laddha et al. evaluated the phosphodiesterase inhibitory properties of two series of 6,8-disubstituted-2-phenyl-3-(substituted benzothiazole-2-yl)-4[3H]-quinazolinone. Compounds 6,8-dibromo-2-phenyl-3-(5-chloro benzothiazole-2-yl)-4[3H]-quinazolinone (: compound 397) with IC50 of 1438 ± 85 µM and 6,8-dibromo-2-phenyl-3-(6-nitro benzothiazole-2-yl)-4[3H]-quinazolinone 398 with IC50 of 1520 ± 48 µM were found to be more potent than standard drug theophyllineCitation149.
Antiallergic activity
Wade et al. synthesized 2- or 3-carboxy-4H-pyrimido [2,1-b]-benzazol-4-ones (: compounds 399–401). These acidic compounds were tested in the rat passive cutaneous anaphylaxis assay as potential antiallergic agents. Incorporation of an acidic functionality [carboxylic acid, N-(1H-tetrazole-5-yl)carboxamide, or tetrazole] at either the 2- or 3- position of the 4H-pyrimado[2,1-b]benzazole-4-one ring system gives good antiallergenic propertyCitation150.
Role in AD
Alzheimer’s disease is a group of progressive neurodegenerative disorder pathologically characterized by the deposition of β-amyloid (Aβ) peptide into amyloid plaques in the extracellular brain parenchyma and by intraneuronal neurofibrillary tangles caused by the abnormal phosphorylation of the tau proteinCitation151.
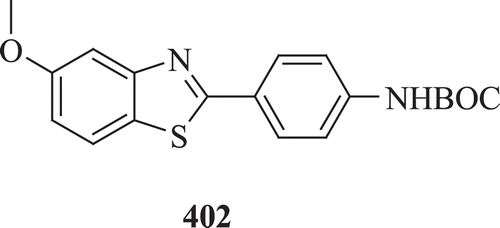
A novel and convergent palladium catalyzed 2-arylbenzothiazoles was synthesized (: compound 402) by Majo et al. The key step in the synthesis was a Suzuki biaryl coupling of 2-bromobenzothiazole with aryl boronic acids to provide a variety of 2-arylbenzothiazole derivatives in good yield. The synthetic utility of this methodology was demonstrated by the synthesis of 2-(4-aminophenyl)-6-methoxybenzothiazole, a positron emission tomography proves precursor for the in vivo imaging of ADCitation152.
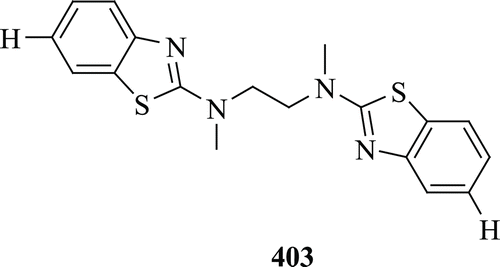
Byeon et al. evaluated some new ferulic acid and BT dimers for their specific binding affinities to Aβ fibrils. Compound 403 () has shown excellent binding affinity to Aβ fibrilsCitation153.
Anticarbonic anhydrase activity
In humans, 16 different isozymes have been described, several of these isozymes are considered as drug targets. They constitute interesting targets for the design of pharmacological agents useful in the treatment or prevention of a variety of disorders such as glaucoma, acid-base disequilibria, epilepsy and various other neuromuscular diseases such as altitude sickness, edema and obesityCitation154. Carbonic anhydrases (CAs) are zinc-containing metalloenzymes and plays crucial role in various physiological processes including carbon dioxide and hydrocarbon transport, acid homeostasis, biosynthetic reactions and various pathological processes, especially tumor progression. Therefore, CAs are interesting targets for pharmaceutical researchCitation155. Carbonic anhydrase inhibitors (CAIs) were used as diuretics and antiglaucoma drugs and also have the potential to be used as novel antiobesity, anticancer and anti-infective drugsCitation156–166. Ethoxzolamide (EZA), carbonic anhydrase inhibitor, was the first approved drug with BT scaffold. Baranauskiene and Matulis described the intrinsic binding parameters (Gibbs free energy, enthalpy, entropy and heat capacity) for several inhibitors, including EZA, trifluoromethanesulfonamide and acetazolamide, binding to recombinant human CA XIII isozyme by using isothermal titration calorimetry and thermal shift assay (TSA) methods. Intrinsic enthalpy of EZA binding is −42.1 kJ/mol, intrinsic Gibbs free energy −52.8 and the intrinsic entropy of binding is 35.9 J/(mol × K)Citation167.
EZA, an almost forgotten inhibitor of the metalloenzyme carbonic anhydrase, was already the lead molecule to obtain the second generation inhibitors, dorzolamide and brinzolamide, clinically used antiglaucoma agents with topical action, as well as various other investigational agents. Di Fiore et al. discussed the inhibition data of EZA with the 12 catalytically active mammalian isozymes (CA I-CA XIV) and the X-ray crystal structure with the cytosolic, ubiquitous isoform CA II. The structural analysis suggested that the introduction of bulky substituents on the bicyclic ring system of CAIs may represent a powerful strategy to obtain compounds with diverse inhibition profiles and selectivity for the various mammalian CAs. These data are presumably useful for the design of novel CA inhibitors incorporating such bicyclic rings, targeting various CA isozymesCitation168.
Marketed drugs
Some important and clinically used drugs having BT ring in their structures are presented in the .
Table 1. Some successful benzothiazole based clinically available drugs.
Conclusion
The aforementioned literature reveals that BT is a versatile heterocyclic scaffold having high potential for the development of new chemical entities for treatment of infectious diseases, cancer, CNS and cardiovascular system disorders. The broad spectrum antibacterial and antifungal activity of BT derivatives could lead to a new series of antimicrobials. The BT derivatives have demonstrated promising anticancer and anti-inflammatory activities. Potent antitumour activity demonstrated by 2-(4-aminophenyl)benzothiazole derivatives have reinforced their perspective role in these therapies. Thus, BT scaffold is not only synthetically important but also possesses a diverse range of promising pharmacological activities. The biological profiles of these new BT derivatives would represent a fruitful matrix for further development of better medicinal agents.
Declaration of interest
The authors report no conflicts of interest.
References
- Chatrabhuji PM, Nimavat KS, Vyas KB, Undavia NK. Synthesis and antimicrobial activity of some 2 -aryl-3-[(4-methyl cinnamoyl amino)-4-oxo-thiazolidines with synthesis and antimicrobial activity of some 2(4-hydroxyphenyl)-3-[(4-methyl cinnamoyl amino)-4-oxo-thiazolidines. RJPBCS 2010;1:451–455.
- Londhe BS, Pratap UR, Mali JR, Mane RA. Synthesis of 2-Arylbenzothiazoles catalyzed by biomimetic catalyst β-cyclodextrin. Bull Korean Chem Soc 2010;31:2329–2332.
- Evindar G, Batey RA. Parallel synthesis of a library of benzoxazoles and benzothiazoles using ligand-accelerated copper-catalyzed cyclizations of ortho-halobenzanilides. J Org Chem 2006;71:1802–1808.
- Tang J, Wu LL, Huang X. Convenient solid-phase synthesis of benzothiazole derivatives. Chin Chem Lett 2003;14:885–886.
- Malik JK, Manvi FV, Nanjwade BK, Singh S, Purohit P. Review of the 2-amino substituted benzothiazoles: Different methods of the synthesis. Der Pharmacia Lett 2010;2:347–359.
- Prabhu PP, Pande S, Shastry CS. Synthesis and biological evaluation of schiff’s bases of some new benzothiazole derivatives as antimicrobial agents. IJCRGG 2011;3:185–191.
- Priyanka, Sharma NK, Jha KK. Benzothiazole: The molecule of diverse biological activities. Int J Curr Pharm Res 2010;2:1–6.
- Muttu CT, Bhanushali MD, Hipparagi SM, Tikare VP, Karigar A. Microwave assisted synthesis and evaluation of some fluoro chloro 2-N-(substituted Schiff’s bases)aminobenzothiazoles derivatives for their antiinfllamatory activity. IJRAP 2010;1:522–528.
- Yadav PS, Devprakash, Senthilkumar GP. Benzothiazole: Different methods of synthesis and diverse biological activities. Int J Pharm Sci Drug Res 2011;3:1–7.
- Rey V, Soria-Castro SM, Arguello JE, Penenory AB. Photochemical cyclization of thioformanilides by chloranil: An approach to 2-substituted benzothiazoles. Tetrahedron Lett 2009;50:4720–4723.
- Barot HK, Mallika G, Sutariya BB, Shukla J, Nargund LVG. Synthesis of nitrogen mustards of fluoro-benzothiazoles of pharmacological interest. RJPBS 2010;1:124–129.
- Guo HY, Li JC, Shang YL. A simple and efficient synthesis of 2-substituted benzothiazoles catalyzed by H2O2/HCl. Chin Chem Lett 2009;20:1408–1410.
- Jaseer EA, Prasad DJC, Dandapat A, Sekar G. An efficient copper(II)-catalyzed synthesis of benzothiazoles through intramolecular coupling-cyclization of N-(2-chlorophenyl)benzothioamides. Tetrahedron Lett 2010;51:5009–5012.
- Sathe BS, Jaychandran E, Jagtap VA, Sreenivasa GM. Antitubercular activity of fluoro benzothiazole comprising potent thaizolidinone. Int J Pharma Bio Sci 2010;1:495–500.
- Choi MM, Kim EA, Hahn HG, Nam KD, Yang SJ, Choi SY et al. Protective effect of benzothiazole derivative KHG21834 on amyloid beta-induced neurotoxicity in PC12 cells and cortical and mesencephalic neurons. Toxicology 2007;239:156–166.
- Campos J, Núñez C, Díaz JJ, Sánchez RM, Gallo MA, Espinosa A. Anticancer bisquaternary heterocyclic compounds: A ras-ional design. Farmaco 2003;58:221–229.
- Xie M, Ujjinamatada RK, Sadowska M, Lapidus RG, Edelman MJ, Hosmane RS. A novel, broad-spectrum anticancer compound containing the imidazo[4,5-e][1,3]diazepine ring system. Bioorg Med Chem Lett 2010;20:4386–4389.
- Bhaskar VH, Mohite PB. Synthesis characterization and evaluation of anticancer activity of some tetrazole derivatives. J Optoelectron Biomed Mater 2010;2:249–259.
- Menta E, Palumbo M. Novel antineoplastic agents. Exp Opin Ther Patents 1997;7:1401–1426.
- Dubey R, Shrivastava PK, Basniwal PK, Bhattacharya S, Moorthy NS. 2-(4-aminophenyl) benzothiazole: A potent and selective pharmacophore with novel mechanistic action towards various tumour cell lines. Mini Rev Med Chem 2006;6:633–637.
- Shi DF, Bradshaw TD, Wrigley S, McCall CJ, Lelieveld P, Fichtner I et al. Antitumor benzothiazoles. 3. Synthesis of 2-(4-aminophenyl)benzothiazoles and evaluation of their activities against breast cancer cell lines in vitro and in vivo. J Med Chem 1996;39:3375–3384.
- Bradshaw TD, Chua MS, Orr S, Matthews CS, Stevens MF. Mechanisms of acquired resistance to 2-(4-aminophenyl)benzothiazole (CJM 126, NSC 34445). Br J Cancer 2000;83:270–277.
- Bradshaw TD, Shi DF, Schultz RJ, Paull KD, Kelland L, Wilson A et al. Influence of 2-(4-aminophenyl)benzothiazoles on growth of human ovarian carcinoma cells in vitro and in vivo. Br J Cancer 1998;78:421–429.
- Bradshaw TD, Wrigley S, Shi DF, Schultz RJ, Paull KD, Stevens MF. 2-(4-Aminophenyl)benzothiazoles: Novel agents with selective profiles of in vitro anti-tumour activity. Br J Cancer 1998;77:745–752.
- Bradshaw TD, Chua MS, Browne HL, Trapani V, Sausville EA, Stevens MF. In vitro evaluation of amino acid prodrugs of novel antitumour 2-(4-amino-3-methylphenyl)benzothiazoles. Br J Cancer 2002;86:1348–1354.
- Bradshaw TD, Bibby MC, Double JA, Fichtner I, Cooper PA, Alley MC et al. Preclinical evaluation of amino acid prodrugs of novel antitumor 2-(4-amino-3-methylphenyl)benzothiazoles. Mol Cancer Ther 2002;1:239–246.
- Tuylu BA, Zeytinoglu HS, Isikdag I. Synthesis and mutagenicity of 2-aryl-substitute (o-hydroxy- m-bromo- o-methoxy- o-nitro-phenyl or 4-pyridyl) benzothiazole derivatives on Salmonella typhimurium and human lymphocytes exposed in vitro. Biologia 2007;62:626–632.
- Choi SJ, Park HJ, Lee SK, Kim SW, Han G, Choo HY. Solid phase combinatorial synthesis of benzothiazoles and evaluation of topoisomerase II inhibitory activity. Bioorg Med Chem 2006;14:1229–1235.
- Bhuva HA, Kini SG. Synthesis, anticancer activity and docking of some substituted benzothiazoles as tyrosine kinase inhibitors. J Mol Graph Model 2010;29:32–37.
- Lion CJ, Matthews CS, Wells G, Bradshaw TD, Stevens MF, Westwell AD. Antitumour properties of fluorinated benzothiazole-substituted hydroxycyclohexa-2,5-dienones (‘quinols’). Bioorg Med Chem Lett 2006;16:5005–5008.
- Hutchinson I, Chua MS, Browne HL, Trapani V, Bradshaw TD, Westwell AD et al. Antitumor benzothiazoles. 14. Synthesis and in vitro biological properties of fluorinated 2-(4-aminophenyl)benzothiazoles. J Med Chem 2001;44:1446–1455.
- Hutchinson I, Jennings SA, Vishnuvajjala BR, Westwell AD, Stevens MF. Antitumor benzothiazoles. 16. Synthesis and pharmaceutical properties of antitumor 2-(4-aminophenyl)benzothiazole amino acid prodrugs. J Med Chem 2002;45:744–747.
- Hutchinson I, Bradshaw TD, Matthews CS, Stevens MF, Westwell AD. Antitumour benzothiazoles. Part 20: 3′-cyano and 3′-alkynyl-substituted 2-(4′-aminophenyl)benzothiazoles as new potent and selective analogues. Bioorg Med Chem Lett 2003;13:471–474.
- Zhang L, Fan J, Vu K, Hong K, Le Brazidec JY, Shi J et al. 7′-substituted benzothiazolothio- and pyridinothiazolothio-purines as potent heat shock protein 90 inhibitors. J Med Chem 2006;49:5352–5362.
- Devmurari VP, Pandey S, Goyani MB, Nandanwar RR, Jivani NP, Perumal P. Synthesis and anticancer activity of some novel 2-substituted benzothiazole derivatives. Int J Chem Tech Res 2010;2:681–689.
- Al-Soud YA, Al-Sadoni HH, Saeed B, Jaber IH, Beni-Khalid MO, Al-Masoudi NA et al. Synthesis and in vitro antiproliferative activity of new benzothiazole derivatives. ARKIVOC 2008;15:225–238.
- Amnerkar ND, Bhusari KP. Preliminary anticancer activity of some prop-2-eneamido thiazole and 1-acetyl-pyrazolin derivatives of aminobenzothiazoles. Dig J Nano Bio 2010;5:177–184.
- Schnur RC, Fliri AF, Kajiji S, Pollack VA. N-(5-fluorobenzothiazol-2-yl)-2-guanidinothiazole-4-carboxamide. A novel, systemically active antitumor agent effective against 3LL Lewis lung carcinoma. J Med Chem 1991;34:914–918.
- Leong CO, Gaskell M, Martin EA, Heydon RT, Farmer PB, Bibby MC et al. Antitumour 2-(4-aminophenyl)benzothiazoles generate DNA adducts in sensitive tumour cells in vitro and in vivo. Br J Cancer 2003;88:470–477.
- Gupta SD, Moorthy NSHN, Sanyal U. Synthesis cytotoxic evaluation in silico pharmacokinetic and QSAR study of some benzothiazole derivatives. Int J Phar Pharm Sci 2010;2:57–60.
- Mortimer CG, Wells G, Crochard JP, Stone EL, Bradshaw TD, Stevens MF et al. Antitumor benzothiazoles. 26.(1) 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J Med Chem 2006;49:179–185.
- Repicky A, Jantova S, Cipak L. Apoptosis induced by 2-acetyl-3-(6-methoxybenzothiazo)-2-yl-amino-acrylonitrile in human leukemia cells involves ROS-mitochondrial mediated death signaling and activation of p38 MAPK. Cancer Lett 2009;277:55–63.
- Racané L, Kralj M, Suman L, Stojkovic R, Tralic-Kulenovic V, Karminski-Zamola G. Novel amidino substituted 2-phenylbenzothiazoles: Synthesis, antitumor evaluation in vitro and acute toxicity testing in vivo. Bioorg Med Chem 2010;18:1038–1044.
- Trapani G, Franco M, Latrofa A, Reho A, Liso G. Synthesis, in vitro and in vivo cytotoxicity, and prediction of the intestinal absorption of substituted 2-ethoxycarbonyl-imidazo[2,1-b]benzothiazoles. Eur J Pharm Sci 2001;14:209–216.
- Dong-Fang S, Bradshaw TD, Wrigley S, Mccall CJ, Lelieveld P, Fichtner I et al. Antitumor benzothiazoles 31 Synthesis of 2-(4-aminophenyl)benzothiazoles and evaluation of their activities against breast cancer cell lines in vitro and in vivo. J Med Chem 1996;39:3375–3384.
- Shi DF, Bradshaw TD, Chua MS, Westwell AD, Stevens MF. Antitumour benzothiazoles. Part 15: The synthesis and physico-chemical properties of 2-(4-aminophenyl)benzothiazole sulfamate salt derivatives. Bioorg Med Chem Lett 2001;11:1093–1095.
- Wells G, Bradshaw TD, Diana P, Seaton A, Shi DF, Westwell AD et al. Antitumour benzothiazoles. Part 10: The synthesis and antitumour activity of benzothiazole substituted quinol derivatives. Bioorg Med Chem Lett 2000;10:513–515.
- Vicini P, Geronikaki A, Incerti M, Busonera B, Poni G, Cabras CA et al. Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg Med Chem 2003;11:4785–4789.
- Yoshida M, Hayakawa I, Hayashi N, Agatsuma T, Oda Y, Tanzawa F et al. Synthesis and biological evaluation of benzothiazole derivatives as potent antitumor agents. Bioorg Med Chem Lett 2005;15:3328–3332.
- Shu-Ting H, I-Jen H, Chen C. Synthesis and anticancer evaluation of bis(benzimidazoles) bis(benzoxazoles) and benzothiazoles. Bioorg Med Chem 2006;14:6106–6119.
- Wan-Ping H, Yin-Kai C, Chao-Cheng L, Hsin-Su Y, Yi-Min T, Shu-Mei H et al. Synthesis and biological evaluation of 2-(4-aminophenyl)benzothiazole derivatives as photosensitizing agents. Bioorg Med Chem 2010;18:6197–6207.
- Aiello S, Wells G, Stone EL, Kadri H, Bazzi R, Bell DR et al. Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (PMX 610, NSC 721648). J Med Chem 2008;51:5135–5139.
- Sharma PC, Jain A, Jain S. Fluoroquinolone antibacterials: A review on chemistry, microbiology and therapeutic prospects. Acta Pol Pharm 2009;66:587–604.
- Sharma PC, Alawadhi N, Sharma A, Pahwa R, Rajak H. Fluoroquinolones in urinary tract infections: A study on perception analysis of prescribers. Der Chemica Sinica 2010;1:84–90.
- Chawla R, Sahoo U, Arora A, Sharma PC, Radhakrishnan V. Microwave assisted synthesis of some novel 2-pyrazoline derivatives as possible antimicrobial agents. Acta Pol Pharm 2010;67:55–61.
- Mishra R, Tomar I. Pyrimidine: The molecule of diverse biological and medicinal importance. Int J Pharm Sci Res 2011;2:758–771.
- Gouveia FL, de Oliveira RM, de Oliveira TB, da Silva IM, do Nascimento SC, de Sena KX et al. Synthesis, antimicrobial and cytotoxic activities of some 5-arylidene-4-thioxo-thiazolidine-2-ones. Eur J Med Chem 2009;44:2038–2043.
- Sharma PC, Jain A, Jain S, Pahwa R, Yar MS. Ciprofloxacin: Review on developments in synthetic, analytical, and medicinal aspects. J Enzyme Inhib Med Chem 2010;25:577–589.
- Bellman R. Clinical pharmacokinetics of systemically administered antimycotics. Curr Clin Pharmacol 2007;2:37–58.
- Sharma PC, Jain S. Synthesis and in vitro antibacterial activity of some novel N-nicotinyl-1-ethyl-6-flouro-1,4-dihydro-7-piperazine-1-yl-4-oxoquinoline-3-carboxylates. Acta Pol Pharm Drug Res 2008;65:551–586.
- Mistry KM, Desai KR. Synthesis of novel heterocyclic 4-thiazolidinone derivatives and their antibacterial activity. E J Chem 2004;1:189–193.
- Rajeeva B, Srinivasulu N, Shantakumar SM. Synthesis and antimicrobial activity of some new 2-substitued benzothiazole derivatives. E J Chem 2009;6:775–779.
- Kaur H, Kumar S, Singh I, Saxena KK, Kumar A. Synthesis, characterization and biological activity of substituted benzothiazole derivatives. Dig J Nanomater Bios 2010;5:67–76.
- Sigmundova I, Zahradnik P, Magdolen P, Bujdakova H. Synthesis and study of new antimicrobial benzothiazoles substituted on heterocyclic ring. ARKIVOC 2008;8:183–192.
- Gajdos P, Magdolen P, Zahradník P, Foltínová P. New conjugated benzothiazole-N-oxides: Synthesis and biological activity. Molecules 2009;14:5382–5388.
- Pareek PK, Mithlesh, Kriplani P, Ravikant, Ojha KG. Rapid synthesis and biological activities of some new benzothiazol-2-ylhexahydro-s-triazine derivatives. Phosphorus Sulfur and Silicon 2010;185:1338–1345.
- Nagarajan AS, Kamalraj S, Muthumary J, Reddy BSR. Synthesis of biologically active benzothiazole substituted thiazolidinone derivatives via cyclization of unsymmetrical imine. Indian J Chem 2009;48B:1577–1582.
- Sidoova E, Loos D, Bujdakova H, Kallova J. New anticandidous 2-alkylthio-6-aminobenzothiazoles. Molecules 1997;2:36–42.
- Malik JK, Manvi FV, Nanjwade BK, Singh S. Synthesis and screening of some new 2-amino substituted benzothiazole derivatives for antifungal activity. Drug Invention Today 2009;1:32–34.
- Soni B, Ranawat MS, Sharma R, Bhandari A, Sharma S. Synthesis and evaluation of some new benzothiazole derivatives as potential antimicrobial agents. Eur J Med Chem 2010;45:2938–2942.
- Latrofa A, Franco M, Lopedota A, Rosato A, Carone D, Vitali C. Structural modifications and antimicrobial activity of N-cycloalkenyl-2-acylalkylidene-2,3-dihydro-1,3-benzothiazoles. Farmaco 2005;60:291–297.
- Saeed A, Rafique H, Hameed A, Rasheed S. Synthesis and antibacterial activity of some new 1-aroyl-3-(substituted-2-benzothiazolyl)thioureas. Pharm Chem J 2008;42:191–195.
- Saeed S, Rashid N, Jones PG, Ali M, Hussain R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur J Med Chem 2010;45:1323–1331.
- Bhusari KP, Amnerkar ND, Khedekar PB, Kale MK, Bhole RP.Synthesis and in vitro antimicrobial activity of some new 4-amino-n-(13-benzothiazol-2-yl)benzenesulphonamide derivatives. Asian J Res Chem 2008;1:23–28.
- Franchini C, Muraglia M, Corbo F, Florio MA, Di Mola A, Rosato A et al. Synthesis and biological evaluation of 2-mercapto-1,3-benzothiazole derivatives with potential antimicrobial activity. Arch Pharm (Weinheim) 2009;342:605–613.
- Bondock S, Fadaly W, Metwally MA. Enaminonitrile in heterocyclic synthesis: Synthesis and antimicrobial evaluation of some new pyrazole, isoxazole and pyrimidine derivatives incorporating a benzothiazole moiety. Eur J Med Chem 2009;44:4813–4818.
- Sain DK, Thandhaney B, Joshi A, Hussain N, Talesara GL. Synthesis, characterization and biological evaluation of some heterocyclic compounds containing ethoxyphthalimide moiety via key intermediate 6-chloro 1,3-benzothiazole 2-amine. Indian J Chem 2010;49B:818–825.
- Baheti KG, Jadhav JS, Suryavanshi AT, Kuberkar SV. Novel synthesis and antibacterial activity of 1,5-iminobenzothiazolo[2,3-b]pyrimido[5,6-c]pyrimido[2,3-b]benzothiazole-14(H)-one and its 310 disubstituted derivatives. Indian J Chem 2005;44B:834–837.
- Gupta S, Ajmera N, Gautam N, Sharma R, Gautam DC. Novel synthesis and biological activity of pyrimido[2,3-b]benzothiazoles. Indian J Chem 2009;48B:853–857.
- Vedavathi M, Somashekar B, Sreenivasa GM, Jayachandran E. Synthesis, characterization and antimicrobial activity of fluorobenzothiazole incorporated with 1,3,4-thiadiazole. J Pharm Sci Res 2010;2:53–63.
- Kuchta T, Léka C, Farkas P, Bujdáková H, Belajová E, Russell NJ. Inhibition of sterol 4-demethylation in Candida albicans by 6-amino-2-n-pentylthiobenzothiazole, a novel mechanism of action for an antifungal agent. Antimicrob Agents Chemother 1995;39:1538–1541.
- Lakhan R, Ral BJ. Synthesis of some new 3-(2-benzothiazolyl)-4(3H)-quinazoliones as antifungal agents. J Chem Eng Data 1986;31:501–502.
- Huang W, Yang GF. Microwave-assisted, one-pot syntheses and fungicidal activity of polyfluorinated 2-benzylthiobenzothiazoles. Bioorg Med Chem 2006;14:8280–8285.
- Mithlesh, Pareek PK, Kant R, Shukla SK, Ojha KG. Rapid synthesis and biological evaluation of 1,4 dihydropyridine derivatives containing a benzothiazolyl moiety. Cent Eur J Chem 2010;8:163–173.
- Sharma V, Sharma KV. Synthesis and biological activity of some 3,5-diaryl-1-benzothiazolopyrazoline derivatives: Reaction of chalcone with 2-hydrazinobenzothiazoles. E J Chem 2009;6:348–356.
- Argyropoulou I, Geronikaki A, Vicini P, Zani F. Synthesis and biological evaluation of sulfonamide thiazole and benzothiazole derivatives as antimicrobial agents. ARKIVOC 2009;6:89–102.
- Alang G, Kaur R, Kaur G. Synthesis and antibacterial activity of some new benzothiazole derivatives. Acta Pharm Sci 2010;52:213–218.
- Alang G, Kaur R, Singh A, Budhlakoti P, Singh A, Sanwal R. Synthesis, characterization and antifungal activity of certain(e)-1-(1-(substitutedphenyl)ethylidene)-2-(6-methylbenzo[d]thiazol-2-yl) hydrazine analogues. IJPBA 2010;1:56–61.
- Chaitanya MS, Nagendrappa G, Vaidya VP. Synthesis, biological and pharmacological activities of 2-methyl-4H-pyrimido[2,1-b][1,3]benzothiazoles. J Chem Pharm Res 2010;2:206–213.
- Sharma PC, Sharma OP, Vasudeva N, Mishra DN, Singh SK. Anti-HIV substances of natural origin-An updated account. Natural Product Radiance 2006;5:70–77.
- Kakkar S, Mohanraj K, Sharma PC. Simple and rapid RP-HPLC method to determine the purity of the antiretroviral drug Lamivudine. Lat Am J Pharm 2010;29:1075–1081.
- Kharb R, Shahar Yar M, Sharma PC. Recent advances and future perspectives of triazole analogs as promising antiviral agents. Mini Rev Med Chem 2011;11:84–96.
- Yi-Sheng X, Cheng-Chu Z, Zi-Guo J, Li-Ming H, Ru-gang Z. Design, synthesis and anti-HIV integrase evaluation of 4-oxo- 4h-quinolizine-3-carboxylic acid derivatives. Molecules 2009;14:868–883.
- De CE. Non-nucleoside reverse transcriptase inhibitors (NNRTIs): Past, present and future. Chem Biodiversity 2004;1 44.
- Ellis D, Kuhen KL, Anaclerio B, Wu B, Wolff K, Yin H et al. Design, synthesis, and biological evaluations of novel quinolones as HIV-1 non-nucleoside reverse transcriptase inhibitors. Bioorg Med Chem Lett 2006;16:4246–4251.
- Akhtar T, Hameed S, Al-Masoudi NA, Loddo R, La Colla P. In vitro antitumor and antiviral activities of new benzothiazole and 1,3,4-oxadiazole-2-thione derivatives. Acta Pharm 2008;58:135–149.
- Akbay A, Oren I, Temiz-Arpaci O, Aki-Sener E, Yalcin I. Synthesis and HIV-1 reverse transcriptase inhibitor activity of some 2,5,6-substituted benzoxazole benzimidazole benzothiazole and oxazolo(4,5-b)pridine derivatives. Arzneim-forsch/Drug Res 2003;4:266–271.
- Nagarajan SR, De Crescenzo GA, Getman DP, Lu HF, Sikorski JA, Walker JL et al. Discovery of novel benzothiazolesulfonamides as potent inhibitors of HIV-1 protease. Bioorg Med Chem 2003;11:4769–4777.
- Pudhom K, Kasai K, Terauchi H, Inoue H, Kaiser M, Brun R et al. Synthesis of three classes of rhodacyanine dyes and evaluation of their in vitro and in vivo antimalarial activity. Bioorg Med Chem 2006;14:8550–8563.
- Seebacher W, Brun R, Weis R. New 4-aminobicyclo[2.2.2]octane derivatives and their activities against Plasmodium falciparum and Trypanosoma b. rhodesiense. Eur J Pharm Sci 2004;21:225–233.
- Friedman MD, Stotter PL, Porter TH, Folkers K. Synthesis of alkyl-4,7-dioxobenzothiazoles with prophylactic antimalarial activity. J Med Chem 1973;16:1314–1316.
- Burger A, Sawhney SN. Antimalarials 111 Benzothiazole amino alcohols. Antimalarials 11:270–272.
- Bowyer PW, Gunaratne RS, Grainger M, Withers-Martinez C, Wickramsinghe SR, Tate EW et al. Molecules incorporating a benzothiazole core scaffold inhibit the N-myristoyltransferase of Plasmodium falciparum. Biochem J 2007;408:173–180.
- Kharb R, Yar MS, Sharma PC. New insights into chemistry and anti-infective potential of triazole scaffold. Curr Med Chem 2011;18:3265–3297.
- Karali N, Gürsoy A, Kandemirli F, Shvets N, Kaynak FB, Ozbey S et al. Synthesis and structure-antituberculosis activity relationship of 1H-indole-2,3-dione derivatives. Bioorg Med Chem 2007;15:5888–5904.
- Aridoss G, Amirthaganesan S, Kim MS, Kim JT, Jeong YT. Synthesis, spectral and biological evaluation of some new thiazolidinones and thiazoles based on t-3-alkyl-r-2,c-6-diarylpiperidin-4-ones. Eur J Med Chem 2009;44:4199–4210.
- Kolavi G, Hegde V, Khazi I, Gadad P. Synthesis and evaluation of antitubercular activity of imidazo[2,1-b][1,3,4]thiadiazole derivatives. Bioorg Med Chem 2006;14:3069–3080.
- Dinakaran M, Senthilkumar P, Yogeeswari P, Sriram D. Antitubercular activities of novel benzothiazolo naphthyridone carboxylic acid derivatives endowed with high activity toward multi-drug resistant tuberculosis. Biomed Pharmacother 2009;63:11–18.
- Huang Q, Mao J, Wan B, Wang Y, Brun R, Franzblau SG et al. Searching for new cures for tuberculosis: Design, synthesis, and biological evaluation of 2-methylbenzothiazoles. J Med Chem 2009;52:6757–6767.
- Palmer PJ, Trigg RB, Warrington JV. Benzothiazolines as antituberculous agents. J Med Chem 1971;14:248–251.
- Geary TG, Woo K, McCarthy JS, Mackenzie CD, Horton J, Prichard RK et al. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int J Parasitol 2010;40:1–13.
- Abuzar S, Sharma S, Fatma N, Gupta S, Murthy PK, Katiyar JC et al. Studies in potential filaricides. 18. Synthesis of 2,2′-disubstituted 5,5′-dibenzimidazolyl ketones and related compounds as potential anthelmintics. J Med Chem 1986;29:1296–1299.
- Sreenivasa GM, Jayachandran E, Shivakumar B, Jayaraj KK, Kumar Vijay MMJ. Synthesis of bioactive molecule fluoro benzothiazole comprising potent heterocyclic moieties for anthelmentic activity. Arch Pharm Sci Res 2009;1:150–157.
- Nadkarni AB, Vijayalaxmi KR, Khadse GB.Synthesis and anthelmintic activity of substituted phenyl imidazo [2,1-b]benzothiazoles. Indian J Heterocycl Chem 2000;9:309–310.
- Kumar V, Sharma A, Sharma PC. Synthesis of some novel 2,5-disubstituted thiazolidinones from a long chain fatty acid as possible anti-inflammatory, analgesic and hydrogen peroxide scavenging agents. J Enzyme Inhib Med Chem 2011;26:198–203.
- Sharma PC, Kaur G, Pahwa R, Sharma A, Rajak H. Quinazolinone analogs as potential therapeutic agents. Curr Med Chem 2011;18:4786–4812.
- Verma A, Saraf SK. 4-thiazolidinone–a biologically active scaffold. Eur J Med Chem 2008;43:897–905.
- Sondhi SM, Singhal N, Johar M, Reddy BS, Lown JW. Heterocyclic compounds as inflammation inhibitors. Curr Med Chem 2002;9:1045–1074.
- Geronikaki AA, Lagunin AA, Hadjipavlou-Litina DI, Eleftheriou PT, Filimonov DA, Poroikov VV et al. Computer-aided discovery of anti-inflammatory thiazolidinones with dual cyclooxygenase/lipoxygenase inhibition. J Med Chem 2008;51:1601–1609.
- Giria RS, Thakerb HM, Giordanoc T, Williamsc J, Rogersc D, Sudersanamb V et al. Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazoline-4-one derivatives as inhibitors of NF-κB and AP-1 mediated transcription activation and as potential antiinflammatory agents. Eur J Med Chem 2009;44:2184–2189.
- Sharma PC, Yadav S, Pahwa R, Kaushik D, Jain S. Synthesis and evaluation of novel prodrugs of naproxen. Med Chem Res 2011;20:648–655.
- Papadopoulou C, Geronikaki A, Hadjipavlou-Litina D. Synthesis and biological evaluation of new thiazolyl/benzothiazolyl-amides, derivatives of 4-phenyl-piperazine. Farmaco 2005;60:969–973.
- Aakashdeep, Jain S, Sharma PC. Synthesis and antiinflammatory activity of some novel biphenyl-4-carboxylic acid 5-(arylidene)-2-(aryl)-4-oxothiazoldin-3-yl amides. Acta Pol Pharm Drug Res 2010;67:63–67.
- Gaba M, Singh D, Singh S, Sharma V, Gaba P. Synthesis and pharmacological evaluation of novel 5-substituted-1-(phenylsulfonyl)-2-methylbenzimidazole derivatives as anti-inflammatory and analgesic agents. Eur J Med Chem 2010;45:2245–2249.
- Paramashivappa R, Phani Kumar P, Subba Rao PV, Srinivasa Rao A. Design, synthesis and biological evaluation of benzimidazole/benzothiazole and benzoxazole derivatives as cyclooxygenase inhibitors. Bioorg Med Chem Lett 2003;13:657–660.
- Singh HP, Sharma CS, Gautam CP.Synthesis and pharmacological screening of some novel 2-arylhydrazino and 2-aryloxy-pyrimido [2,1-b] benzothiazole derivatives. American-Eurasian J Scientific Res 2009;4:222–228.
- Venkatesh P, Pandeya SN. Synthesis, characterisation and antiinflammatory activity of some 2-amino benzothiazole derivatives. Int J Chem Tech Res 2009;1:1354–1358.
- Wada J, Suzuki T, Iwasaki M, Miyamatsu H, Ueno S, Shimizu M. A new nonsteroidal antiinflammatory agent. 2-Substituted 5- or 6-benzothiazoleacetic acids and their derivatives. J Med Chem 1973;16:930–934.
- Musser JH, Kubrak DM, Chang J, DiZio SM, Hite M, Hand JM et al. Leukotriene D4 antagonists and 5-lipoxygenase inhibitors. Synthesis of benzoheterocyclic [(methoxyphenyl)amino]oxoalkanoic acid esters. J Med Chem 1987;30:400–405.
- Cheng-Xi W, Wu D, Zhi-Gang S, Kyu-Yun C, Zhe-Shan Q. Synthesis of 6-(3-substituted-4H-1,2,4-triazol-4-yl)-2-phenylbenzo[d]oxazoles as potential anticonvulsant agents. Med Chem Res 2010;19:925–935.
- Amin KM, Rahman DE, Al-Eryani YA. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg Med Chem 2008;16:5377–5388.
- Amnerkar ND, Bhusari KP. Synthesis, anticonvulsant activity and 3D-QSAR study of some prop-2-eneamido and 1-acetyl-pyrazolin derivatives of aminobenzothiazole. Eur J Med Chem 2010;45:149–159.
- Sharma PP, Pandeya SN, Roy RK, Verma AK, Gupta S. Synthesis and anticonvulsant activity of some novel isatin schiff’s bases. Int J Chem Tech Res 2009;1:758–763.
- Rana A, Siddiqui N, Khan SA, Ehtaishamul Haque S, Bhat MA. N-{[(6-substituted-1,3-benzothiazole-2-yl)amino]carbonothioyl}-2/4-substituted benzamides: Synthesis and pharmacological evaluation. Eur J Med Chem 2008;43:1114–1122.
- Siddiqui N, Rana A, Khan SA, Bhat MA, Haque SE. Synthesis of benzothiazole semicarbazones as novel anticonvulsants–the role of hydrophobic domain. Bioorg Med Chem Lett 2007;17:4178–4182.
- Mishra MK, Ray D, Barik BB. Microcapsules and transdermal patch: A comparative approach for improved delivery of antidiabetic drug. AAPS PharmSciTech 2009;10:928–934.
- Tripathi BK, Srivastava AK. Diabetes mellitus: Complications and therapeutics. Med Sci Monit 2006;12:RA130–RA147.
- Mohan V, Madan Z, Jha R, Deepa R, Pradeepa R. Diabetes- social and economic perspectives in the new millennium. Int J Diab Dev Countries 2004;24:29–35.
- Pattan SR, Suresh Pujar VD, Reedy VVK, Rasal VP, Koti BC. Synthesis and antidiabetic activity of 2-amino[5′(4-sulphonylbenzylidine)-2,4-thiazolidinedione]-7-chloro-6-fluorobenzothiazole. Indian J Chem 2005;44B:2404–2408.
- De SK, Chen LH, Stebbins JL, Machleidt T, Riel-Mehan M, Dahl R et al. Discovery of 2-(5-nitrothiazol-2-ylthio)benzo[d]thiazoles as novel c-Jun N-terminal kinase inhibitors. Bioorg Med Chem 2009;17:2712–2717.
- Van Zandt MC, Jones ML, Gunn DE, Geraci LS, Jones JH, Sawicki DR et al. Discovery of 3-[(4,5,7-trifluorobenzothiazol-2-yl)methyl]indole-N-acetic acid (lidorestat) and congeners as highly potent and selective inhibitors of aldose reductase for treatment of chronic diabetic complications. J Med Chem 2005;48:3141–3152.
- Su X, Vicker N, Ganeshapillai D, Smith A, Purohit A, Reed MJ et al. Benzothiazole derivatives as novel inhibitors of human 11beta-hydroxysteroid dehydrogenase type 1. Mol Cell Endocrinol 2006;248:214–217.
- Yar MS, Ansari ZH. Synthesis and in vivo diuretic activity of biphenyl benzothiazole-2-carboxamide derivatives. Acta Pol Pharm Drug Res 2009;66:387–392.
- Yoshino K, Kohno T, Uno T, Morita T, Tsukamoto G. Organic phosphorus compounds. 1. 4-(Benzothiazol-2-yl)benzylphosphonate as potent calcium antagonistic vasodilator. J Med Chem 1986;29:820–825.
- Vu CB, Milne JC, Carney DP, Song J, Choy W, Lambert PD et al. Discovery of benzothiazole derivatives as efficacious and enterocyte-specific MTP inhibitors. Bioorg Med Chem Lett 2009;19:1416–1420.
- Cressier D, Prouillac C, Hernandez P, Amourette C, Diserbo M, Lion C et al. Synthesis, antioxidant properties and radioprotective effects of new benzothiazoles and thiadiazoles. Bioorg Med Chem 2009;17:5275–5284.
- Karali N, Güzel O, Ozsoy N, Ozbey S, Salman A. Synthesis of new spiroindolinones incorporating a benzothiazole moiety as antioxidant agents. Eur J Med Chem 2010;45:1068–1077.
- Costanzo MJ, Yabut SC, Almond HR Jr, Andrade-Gordon P, Corcoran TW, De Garavilla L et al. Potent, small-molecule inhibitors of human mast cell tryptase. Antiasthmatic action of a dipeptide-based transition-state analogue containing a benzothiazole ketone. J Med Chem 2003;46:3865–3876.
- Laddha SS, Wadodkar SG, Meghal SK. cAMP-dependent phosphodiesterase inhibition and SAR studies on novel 6,8-disubstituted 2-phenyl-3-(substituted benzothiazole-2-yl)-4[3H]-quinazolinone. Med Chem Res 2009;18:268–276.
- Wade JJ, Toso CB, Matson CJ, Stelzer VL. Synthesis and antiallergic activity of some acidic derivatives of 4H-pyrimido[2,1-b]benzazol-4-ones. J Med Chem 1983;26:608–611.
- Chitra V, Kumar PK. Neuroprotective studies of rubia cordifolia linn on β-amyloid induced cognitive dysfunction in mice. Int J Pharm Tech Res 2009;1:1000–1009.
- Majo VJ, Prabhakaran J, Mann JJ, Kumar JSD. An efficient palladium catalyzed synthesis of 2-arylbenzothiazoles. Tetrahedron Lett 2003;44:8535–8537.
- Byeon SR, Jin YJ, Lim SJ, Lee JH, Yoo KH, Shin KJ et al. Ferulic acid and benzothiazole dimer derivatives with high binding affinity to beta-amyloid fibrils. Bioorg Med Chem Lett 2007;17:4022–4025.
- Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Supuran CT, Fiore AD, Alterio V, Monti SM, Simone GD. Recent advances in structural studies of the carbonic anhydrase family: The crystal structure of human CA IX and CA XIII. Curr Pharm Des 2010;16:3346–3254.
- Supuran CT. Carbonic anhydrases as drug targets–an overview. Curr Top Med Chem 2007;7:825–833.
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–189.
- Supuran CT. Carbonic anhydrase inhibition with natural products: Novel chemotypes and inhibition mechanisms. Mol Divers 2011;15:305–316.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. Metal binding functions in the design of carbonic anhydrase inhibitors. Curr Top Med Chem 2007;7:835–848.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. Design of zinc binding functions for carbonic anhydrase inhibitors. Curr Pharm Des 2008;14:615–621.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. New zinc binding motifs in the design of selective carbonic anhydrase inhibitors. Mini Rev Med Chem 2006;6:921–936.
- Winum JY, Scozzafava A, Montero JL, Supuran CT. Sulfamates and their therapeutic potential. Med Res Rev 2005;25:186–228.
- Scozzafava A, Mastrolorenzo A, Supuran CT. Carbonic anhydrase inhibitors and activators and their use in therapy. Expert Opin Ther Patents 2006;16:1627–1664.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: Current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Di Fiore A, Scozzafava A, Winum JY, Montero JL, Pedone C, Supuran CT et al. Carbonic anhydrase inhibitors: Binding of an antiglaucoma glycosyl-sulfanilamide derivative to human isoform II and its consequences for the drug design of enzyme inhibitors incorporating sugar moieties. Bioorg Med Chem Lett 2007;17:1726–1731.
- Baranauskiene L, Matulis D. Intrinsic thermodynamics of ethoxzolamide inhibitor binding to human carbonic anhydrase XIII. BMC Biophys 2012;5:12.
- Di Fiore A, Pedone C, Antel J, Waldeck H, Witte A, Wurl M et al. Carbonic anhydrase inhibitors: The X-ray crystal structure of ethoxzolamide complexed to human isoform II reveals the importance of thr200 and gln92 for obtaining tight-binding inhibitors. Bioorg Med Chem Lett 2008;18:2669–2674.
- Scheetz ME II, Carlson DG, Schinitsky MR. Frentizole, a novel immunosuppressive, and azathioprine: Their comparative effects on host resistance to Pseudomonas aeruginosa, Candida albicans, herpes simplex virus, and influenza (Ann Arbor) virus. Infect Immun 1977;15:145–148.
- Shi-Wei C, Ze-Rong L, Xiang-Yuan L. Prediction of antifungal activity by support vector machine approach. J Mol Struc Theochem 2005;731:73–81.
- Arakawa R, Ito H, Takano A, Okumura M, Takahashi H, Takano H et al. Dopamine D2 receptor occupancy by perospirone: A positron emission tomography study in patients with schizophrenia and healthy subjects. Psychopharmacology (Berl) 2010;209:285–290.
- Fichtner I, Monks A, Hose C, Stevens MF, Bradshaw TD. The experimental antitumor agents Phortress and doxorubicin are equiactive against human-derived breast carcinoma xenograft models. Breast Cancer Res Treat 2004;87:97–107.
- Vinodhini C, Sravani MVND, Bhanuprakash M, Devi MS, Imran M, Osman MOA et al. Method development and validation of pramipexole dihydrochloride monohydrate in tablet dosage form by uv and visible spectrophotometric methods. Int J Res Phar Biomed Sci 2011;2:680–686.
- Jain A, Sharma R, Gahalain N. Anxiety disorder: An overview. Int J Pharmacy Life Sci 2010;1:396–409.
- Satyanarayana B, Saravanan M, Kumari KS, Lokamaheshwari DP, Sridhar Ravishankar R, Nageswari A et al. Synthesis and spectral characterization of related compounds of riluzole an amyotrophic lateral sclerosis drug substance. ARKIVOC 2008;14:109–114.
- Voropai ES, Samtsov MP, Kaplevskii KN, Maskevich AA, Stepuro VI, Povarova OI et al. Spectral properties of thioflavin-t and its complexes with amyloid fibrils. J Appl Spectros 2003;70:868–874.
- Tanaka R, Kawamura T, Hirayama N. Structure of tiaramide. Anal Sci 2007;23:105–106.
- Ding Q, Huang XG, Wu J. Facile synthesis of benzothiazoles via cascade reactions of 2-iodoanilines, acid chlorides and Lawesson’s reagent. J Comb Chem 2009;11:1047–1049.

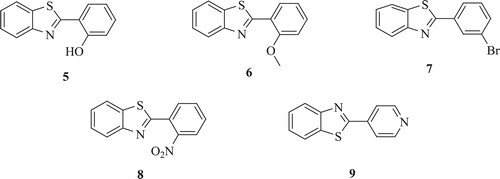
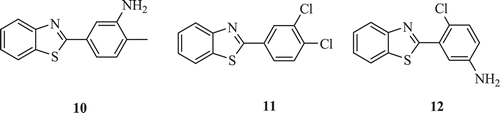
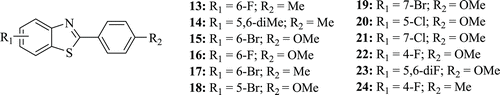
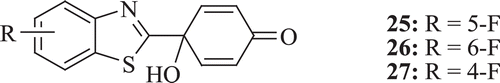
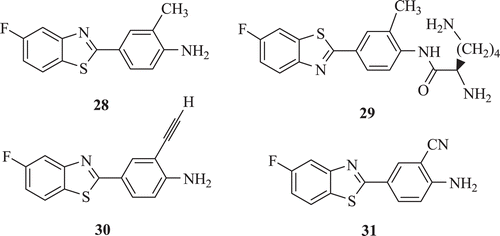
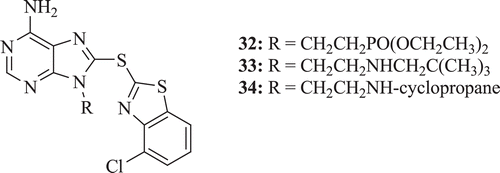
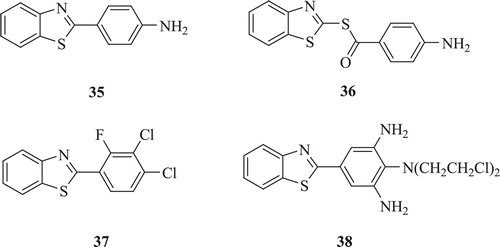
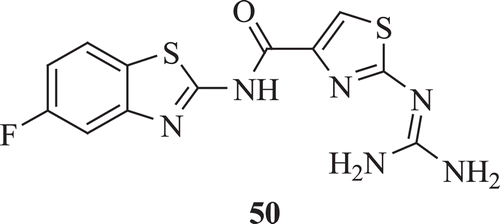
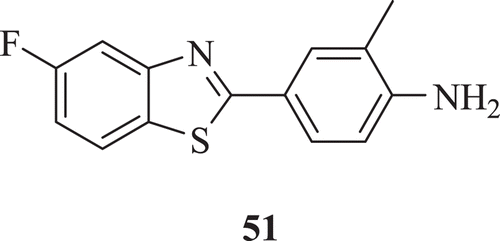
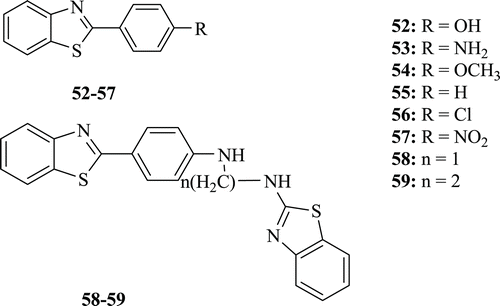
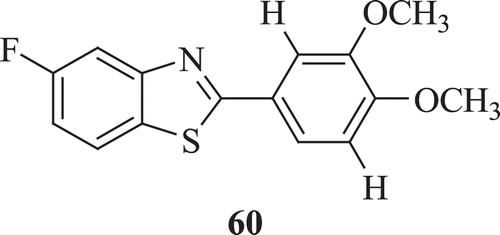
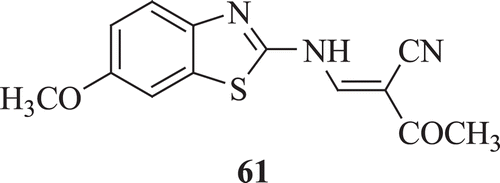
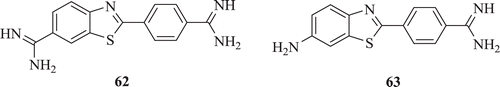
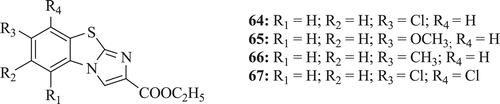
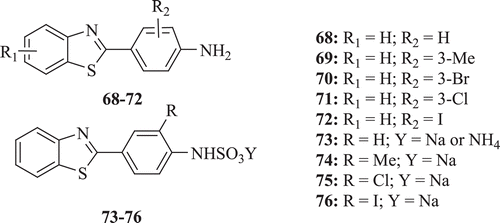
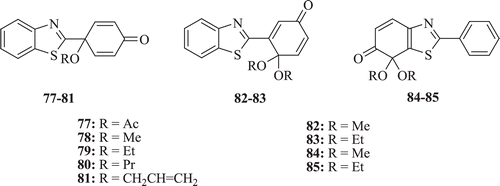
![Figure 20. Chemical Structures of benzo[d]isothiazole, benzothiazole and thiazole Schiff's bases.](/cms/asset/4006e367-08c4-45f2-bcc7-0e529f504d08/ienz_a_720572_f0020_b.gif)
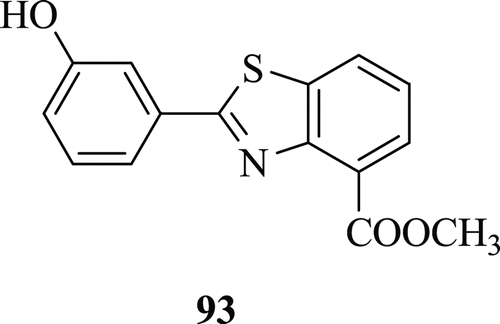
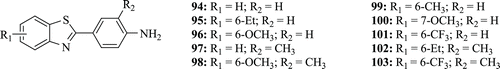
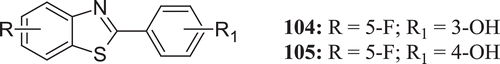
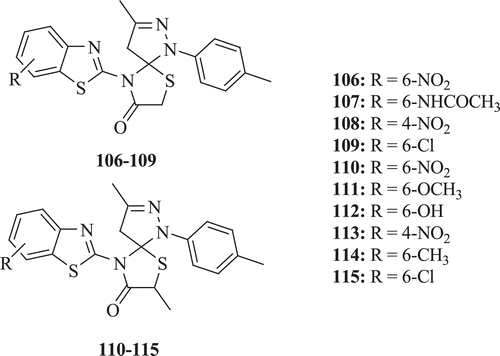
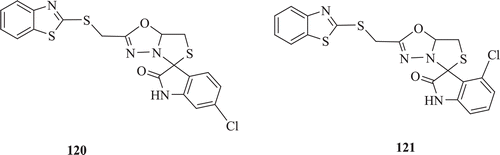
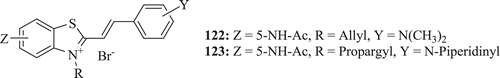
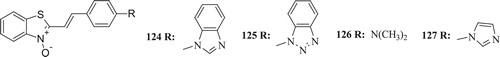
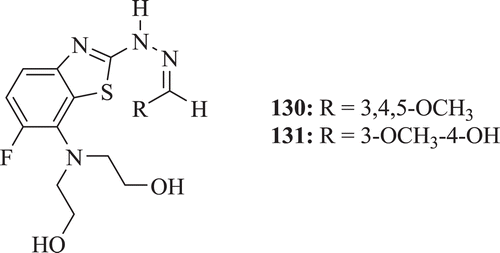
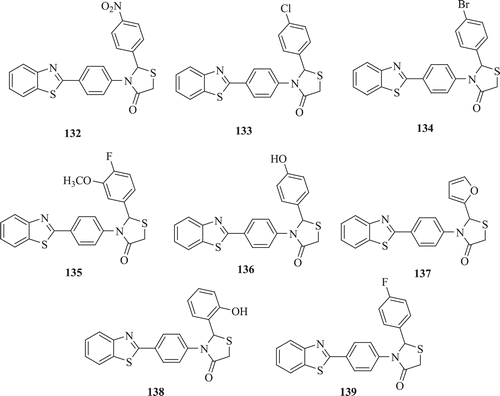
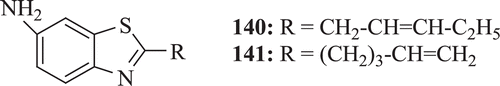
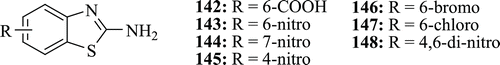
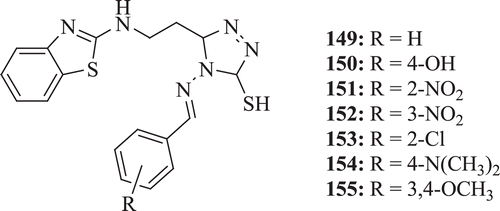
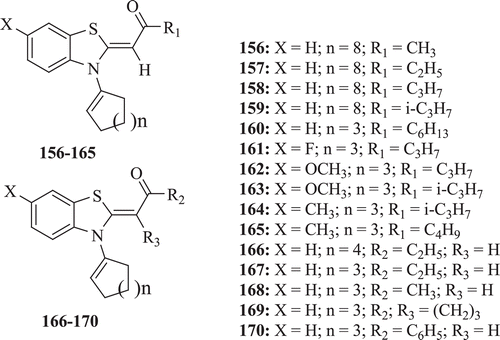
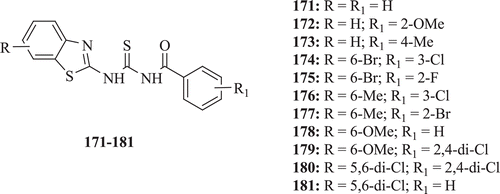
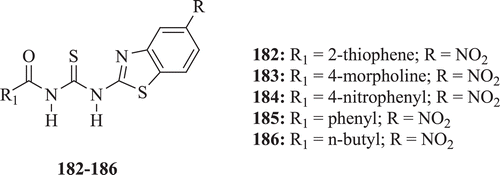
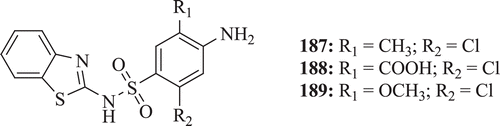
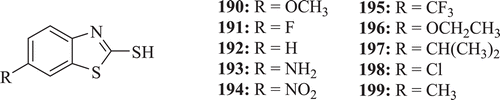
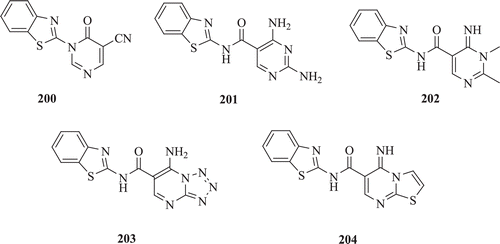
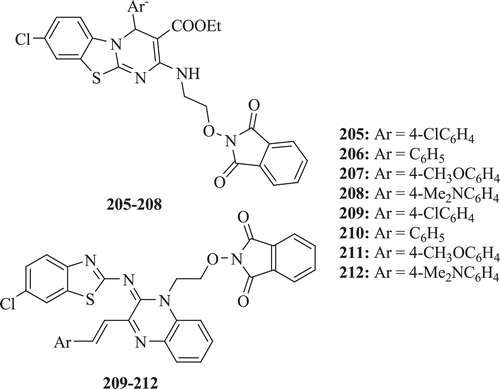
![Figure 44. Chemical structure of substituted pyrimido[2,1-b]benzothiazoles.](/cms/asset/71ed4513-2f0c-4bc6-add1-ca1fec478b73/ienz_a_720572_f0044_b.gif)
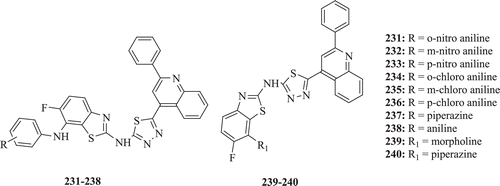
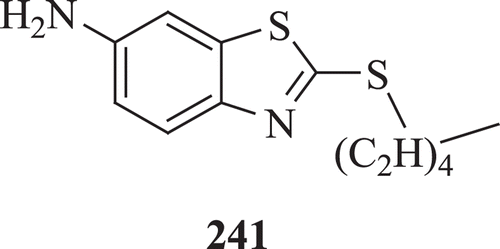
![Figure 47. Chemical structure of 2-methyl-3-[(substituted)benzothiazole-2′-yl]4(3H)-quinazolinones.](/cms/asset/7d447d52-3b69-4ffd-a858-a833c7585f98/ienz_a_720572_f0047_b.gif)
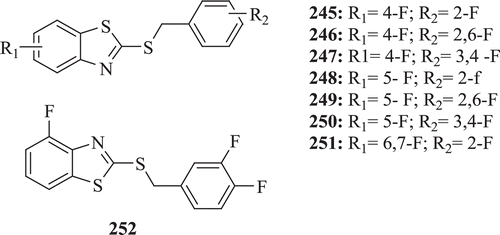
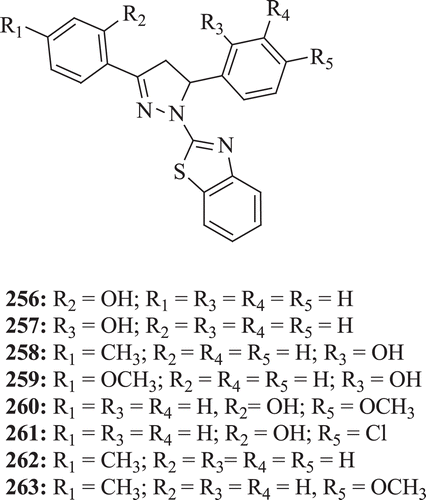
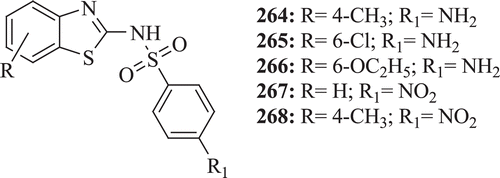
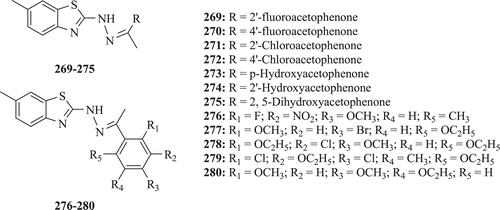
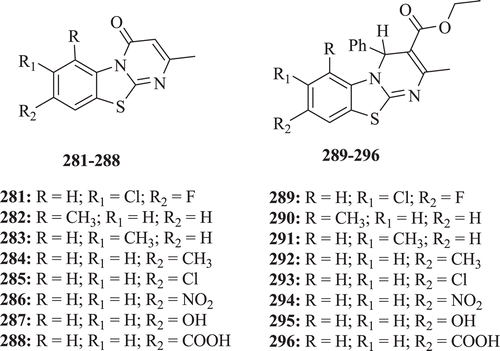
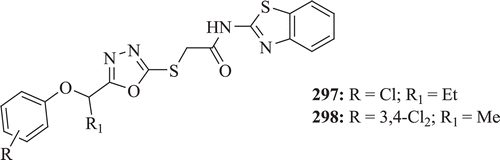
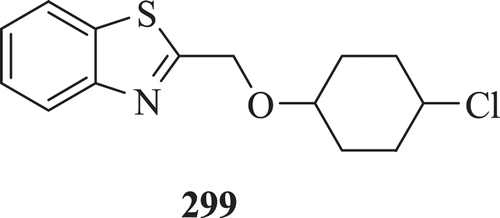
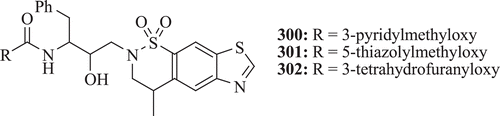
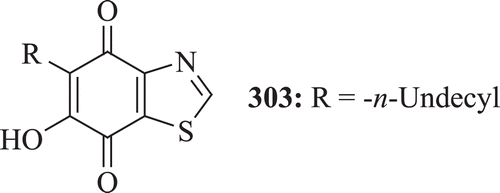
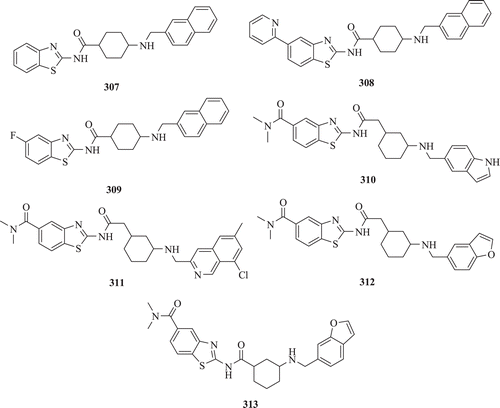
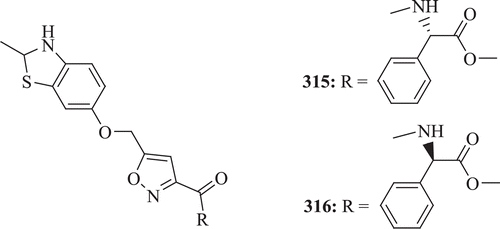
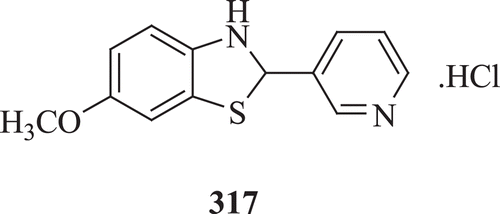
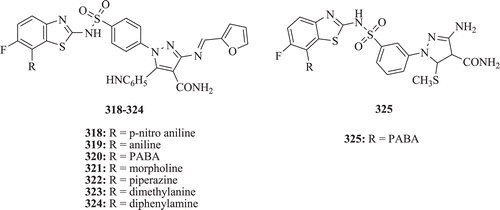
![Figure 64. Chemical structure of phenyl imidazo-[2,1-b]benzothiazole derivatives.](/cms/asset/2a28e297-46b7-4b4a-b7aa-d170bb1d1abf/ienz_a_720572_f0064_b.gif)
![Figure 65. Chemical structure of 2-[[2-alkoxy-6-pentadecylphenyl)methyl]thio]-1H-benzothiazole.](/cms/asset/0cc24070-70cd-457a-8ae2-9f505e3d2e98/ienz_a_720572_f0065_b.gif)
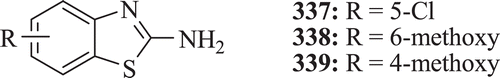
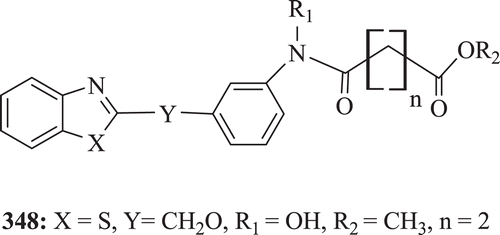
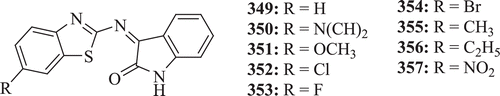
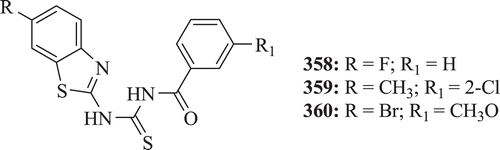
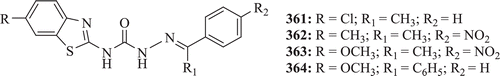
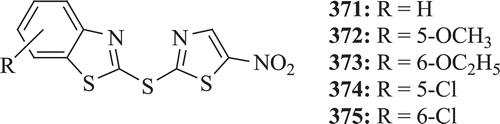
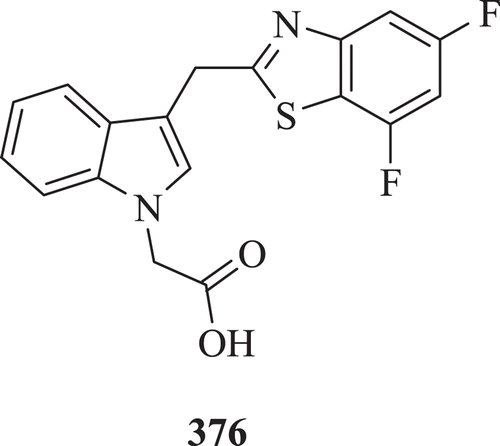

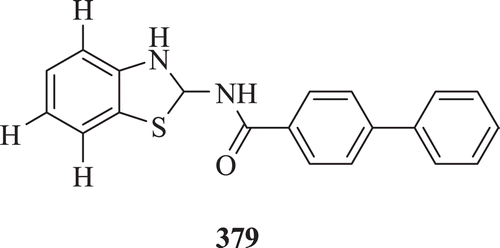
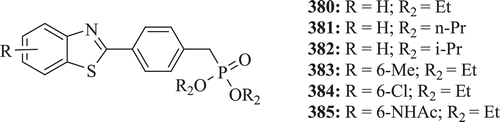
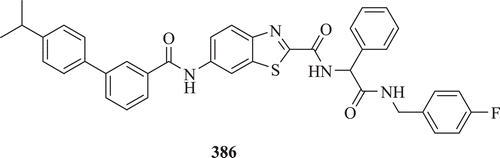
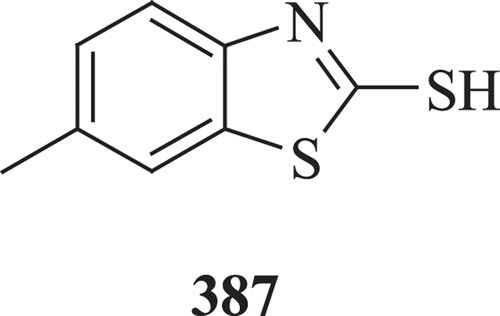
![Figure 81. Chemical structure of 3H-spiro[1,3-benzothiazole-2,30-indol]-20(10H)-ones derivatives.](/cms/asset/e89302ea-c98e-4dda-b411-64723d8d5be9/ienz_a_720572_f0081_b.gif)