Abstract
Numerous compounds have been prepared in order to improve the pharmacological profile of insulinotropic activities. In the present paper, we report the synthesis and the in vitro insulin releasing activity of the 6-methyl-chromonyl-2,4-thiazolidinediones (IIIa–c, IVa–c, Va–c). Compounds IIIb, IIIc, IVa–c, Va and Vc (at lower concentration; 0.001 mg/mL) were able to increase insulin release in the presence of 5.6 mmol/L glucose. In this series, the most potent compound is IVa having methyl group at N3 position of TZD ring.
Introduction
Diabetes mellitus (DM) is increasing in prevalence worldwide, a condition characterized by both insulin resistance (poor tissue insulin sensitivity) and impaired insulin secretion from the pancreatic β cellsCitation1. Thiazolidinediones (TZDs) or glitazones, ligands of the peroxisome–proliferator-activated receptor-γ, which are a class of antihyperglycemic agents that decrease insulin resistance and improve insulin action, thereby improving glycemic control and potentially preserving β-cell functionCitation2.
There are two widely used commercial products derived from TZD, rosiglitazone (Avandia), and pioglitazone (Actos). The known risk profile for TZD includes weight gain, fluid retention, and, in some patients, a worsening of congestive heart failureCitation3. On 23 September 2010, the FDA announced their decision to require a Risk Evaluation and Mitigation Strategy for rosiglitazone, which will limit its availabilityCitation4,Citation5. In USA, rosiglitazone was removed from the retail pharmacy stores by 18 November in 2011. In this case, pioglitazone is the only TZD-based drug available in the USA and European marketsCitation5.
The traditional Chinese medicines or natural products are commonly used in diets for the control or/and treatment of diabetes and its complications. Some traditional Chinese medicines having anti-diabetic activity appear to be effective for both the control of blood glucose and the modification of the course of diabetic complications without side-effects. Some flavonoids have been isolated from traditional Chinese medicines for anti-diabetes. Most of them showed a mechanism to improve the function of β cells of pancreatic isletCitation6.
Flavonoids-related chromone are well known naturally occurring heterocyclic compounds with oxygen as a heteroatom. They are present mainly in seeds, fruit skin, peel, bark or flowers and exert a wide range of biological activities. In addition, it was reported that the health benefits of flavonoids are usually linked to two properties: antidiabetic and antioxidant activityCitation7.
These promising properties led to numerous chemical works focusing on the synthesis and the structural modifications of flavones-chromones.
With prevalence of type 2 diabetes and limited number of insulin sensitizers, there is a huge demand for a new drug in safety. For these reasons, significant efforts are ongoing to develop novel TZDs, which retain their insulin-sensitizing activity and are devoid of activities that cause adverse effects.
In the last few years, we are trying to connect TZDs with some natural ring systems which are already exist in our diet and safe. Previously, we reported the synthesis and insulin releasing activity of flavonyl-TZDsCitation8–12, chromonyl-TZDsCitation13–15 and thiazolyl-TZDsCitation16–18. A significant insulinotropic effect was seen with several of these TZD compounds.
In this study, considering these knowledge; in our screening program to search for antidiabetic compounds, the 2,4-TZD, imidazolidine-2,4-dione and 2-thioxo-imidazolidine-4-one containing chromone ring were synthesized. Thus, it was hoped that this would help to evaluate the difference at the biological activities. Their insulin releasing activities in INS-1 cells were evaluated.
Experimental
Chemistry
Melting points were measured on an electrothermal 9100 type apparatus (Electrothermal Engineering, Essex, UK) and uncorrected. All instrumental analyses were performed in Central Lab. of Pharmacy Faculty of Ankara University. 1H NMR spectra were measured with a VARIAN Mercury 400 FT-NMR spectrometer (Palo Alto, CA, USA) in CDCl3 and DMSO-d6. All chemical shifts were reported as δ (ppm) values. Elementary analyses were determined on a Leco CHNS 932 analyzer (Leco, St. Joseph, MI, USA) and satisfactory results ±0.4% of calculated values (C, H, N) were obtained. For the chromatographic analysis Merck Silica Gel 60 (230–400 mesh, ASTM, American Society for Testing and Materials) was used. The chemical reagents used in synthesis were purchased from E. Merck (Darmstadt, Germany) and Aldrich (Milwaukee, MI, USA). 2,4-TZD (I)Citation19 was synthesized according to the literature.
Crystals suitable for X-ray analysis were obtained by recrystallization of compounds Va, Vb and Vc from ethylacetate: n-hexane (4:1) The data collection was performed on a CAD-4 diffractometer employing graphite-monochromated CuKα radiation (λ = 1.54184 Å). Three standard reflections were measured every two hours. The structure was solved by direct methods. The refinement was made with anisotropic temperature factors for all non-hydrogen atoms. The hydrogen atoms were generated geometrically. An empirical Ψ scan absorption correction was applied. Crystallographic data (excluding structure factors) for compounds Va–c have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no CCDC 864612, CCDC 864613, CCDC 864614, respectively. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (Fax: +44-1223-336033; e-mail:depositcdc.cam.ac.uk).
General synthesis of compounds IIIa–c
A mixture of 3-formyl chromone (I) (0.001 mol) and thiazolidine-2,4-dione/imidazolidine-2,4-dione/2-thioxo-imidazolidine-4-one (IIa–c) (0.001 mol) was refluxed in the presence of 1 mL glacial acetic acid and sodium acetate (0.001 mol) for 5 h. The crude product was crystallized from DMF.
(Z)-5-(6-Methyl-4-oxo-4H-chromen-3-yl methylene)-thiazolidine-2,4-dione) (IIIa)
Yield: 87.4%, mp: 257°C (Ref. Citation20 mp: 257°C)
(Z)-5-(6-Methyl-4-oxo-4H-chromen-3-yl methylene)-imidazolidine-2,4-dione) (IIIb)
Yield: 41.8 %, mp: 332°C, 1H NMR (DMSO-d6): δ = 2.44 (s, 3H, –CH3), 6.33 (s, 1H, =CH), 7.60 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.66 (dd, 1H, J7,8 = 8.80 Hz, J7,5 = 2.00 Hz, 7-H), 7.90 (s, 1H, 5-H), 8.71 (s, 1H, 2-H), 10.31 (s, 1H, NH), 11.28 (s, 1H, NH), MS (ESI+) m/z (rel intensity):312 (M + CH3CN, 100%), 217.9 (M + 1, 72%), Anal. for C13H8N2O4·0.2H2O: Calc. C: 61.40; H: 3.80; N: 10.23%. Found C: 61.47; H: 4.00; N: 10.22%.
(Z)-5-(6-Methyl-4-oxo-4H-chromen-3-yl methylene)-2-thioxoimidazolidine-4-one) (IIIc)
Yield: 88.8%, mp: 337°C, 1H NMR (DMSO-d6): δ = 2.45 (s, 3H, –CH3), 6.37 (s, 1H, =CH), 7.64 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.70 (dd, 1H, J7,8 = 8.40 Hz, J7,5 = 1.60 Hz, 7-H), 7.95 (s, 1H, 5-H), 8.89 (s, 1H, 2-H), 11.79 (s, 1H, NH), 12.39 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 287.5 (M + 1, 100), Anal. for C14H10N203S: Calc. C: 58.73; H: 3.52; N: 9.78; S: 11.20%. Found C: 59.10; H: 3.73; N: 9.81; S: 11.02%.
General synthesis of compounds IVa–c
Compounds IIIa–c (0.0004 mol) and anhydrous sodium carbonate (0.0004 mol) were dissolved in 3 mL DMF. Methyl iodide (0.0008 mol) was added to this mixture and stirred at 40°C for 3 h. The reaction mixture was poured on to ice. The residue was filtered off. The filtrate was purified by column chromatography using silica gel 60 (230–400 mesh ASTM) as the adsorbent and petroleum ether: chloroform (1:1) as the eluent.
(Z)-3-Methyl-5-(6-methyl-4-oxo-4H-chromen-3-yl methylene)-thiazolidine-2,4-dione) (IVa)
Yield: 71.5%, mp: 220°C, 1H NMR (CDCl3): δ = 2.48 (s, 3H, –CH3), 3.24 (s, 3H, –CH3), 7.82 (s, 1H, =CH), 7.41 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.55 (dd, 1H, J7,8 = 8.80 Hz, J7,5 = 2.40 Hz, 7-H), 8.05 (d, 1H, J5,7 = 1.20 Hz, 5-H), 8.17 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 302.8 (M + 1, 100), Anal. for C15H11NO4S: Calc. C: 59.79; H: 3.68; N: 4.65; S: 10.64%. Found C: 59.78; H: 3.91; N: 4.70; S: 10.39%.
(Z)-1,3-Dimethyl-5-(6-methyl-4-oxo-4H-chromen-3-yl methylene)-imidazolidine-2,4-dione) (IVb)
Yield: 64.2%, mp: 144°C, 1H NMR (CDCl3): δ = 2.48 (s, 3H, –CH3), 3.10 (s, 3H, –CH3), 3.14 (s, 3H, –CH3), 6.58 (s, 1H, =CH), 7.40 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.54 (dd, 1H, J7,8 = 8.40 Hz, J7,5 = 2.00 Hz, 7-H), 7.91 (d, 1H, J5,7 = 1.20 Hz, 5-H), 8.05 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 299.7 (M + 1, 100), Anal. for C16H14N2O4: Calc. C: 64.42; H: 4.73; N: 9.39%. Found C: 64.12; H: 4.92; N: 9.36%.
(Z)-3-Methyl-5-(6-methyl-4-oxo-4H-chromen-3-yl methylene)-2-methylsulfanyl-3,5-dihydro-imidazol-4-one) (IVc)
Yield: 47.7%, mp: 270°C, 1H NMR (CDCl3): δ = 2.47 (s, 3H, –CH3), 2.71 (s, 3H, –CH3), 3.17 (s, 3H, –CH3), 7.38 (d, 1H, J8,7 = 8.80 Hz, 8-H), 7.42 (s, 1H, =CH), 7.51 (dd, 1H, J7,8 = 8.40 Hz, J7,5 = 2.40 Hz, 7-H), 8.08 (d, 1H, J5,7 = 1.20 Hz, 5-H), 9.65 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 315.6 (M + 1, 100), Anal. for C16H14N203S: Calc. C: 61.13; H: 4.49; N: 8.91; S: 10.20%. Found C: 61.52; H: 4.65; N: 8.95; S: 10.11%.
General synthesis of compounds Va–c
Compounds IIIa–c (0.0008 mol) and anhydrous sodium carbonate (0.0016 mol) were dissolved in 5 mL DMF. Ethyl iodide (0.0032 mol) was added to this mixture and stirred at 40°C for 3 h. The reaction mixture was poured on to ice. The residue was filtered off. The filtrate was purified by column chromatography using silica gel 60 (230–400 mesh ASTM) as the adsorbent and petroleum ether: chloroform (1:1) as the eluent.
(Z)-3-Ethyl-5-(6-methyl-4-oxo-4H-chromen-3-yl methylene)-thiazolidine-2,4-dione) (Va)
Yield: 57.10%, mp: 195°C, 1H NMR (CDCl3): δ = 1.26 (t, 3H, J = 7.20 Hz, -CH3), 2.48 (s, 3H, CH3), 3.81 (q, 2H, J = 7.20 Hz, –CH2–), 7.41 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.54 (dd, 1H, J7,8 = 8.80 Hz, J7,5 = 2.40 Hz, 7-H), 7.82 (s, 1H, =CH), 8.06 (d, 1H, J5,7 = 1.20 Hz, 5-H), 8.17 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 316.7 (M + 1, 100), Anal. for C16H13NO4S: Calc. C: 60.94; H: 4.16; N: 4.44; S: 10.17%. Found C: 61.31; H: 4.27; N: 4.51; S: 10.08%.
(Z)-3-Ethyl-5-(6-methyl-4-oxo-4H-chromen-3-yl methylene)-imidazolidine-2,4-dione) (Vb)
Yield: 49.5 %, mp: 249°C, 1H NMR (CDCl3): δ = 1.27 (ψt, 3H, J = 6.80 and 7.60 Hz, –CH3), 2.49 (s, 3H, CH3), 3.67 (q, 2H, J = 7.20 Hz, –CH2–), 6.13 (s, 1H, =CH), 7.41 (d, 1H, J8,7 = 8.80 Hz, 8-H), 7.55 (dd, 1H, J7,8 = 8.40 Hz, J7,5 = 1.60 Hz, 7-H), 8.05 (d, 1H, J5,7 =0.80 Hz, 5-H), 8.11 (s, 1H, 2-H), 10.12 (s, 1H, NH), MS (ESI+) m/z (rel intensity): 299.8 (M + 1, 100), Anal. for C16H14N2O4: Calc. C: 64.42; H: 4.73; N: 9.39%. Found C: 64.21; H: 5.00; N: 9.20%.
(Z)-3-Ethyl-2-ethylsulfanyl-5-(6-methyl-4-oxo-4H-chromen-3-yl methylene)-3,5-dihydro-imidazol-4-one) (Vc)
Yield: 65.5%, mp: 200°C, 1H NMR (DMSO-d6): δ = 1.16 (t, 3H, J = 7.20 Hz, –CH3), 1.44 (ψt, 3H, J = 7.20 Hz, –CH3), 2.45 (s, 3H, CH3), 3.38 (q, 2H, J = 7.20 and 7.60 Hz, –CH2–), 3.56 (q, 2H, J = 6.80 Hz and J = 7.20 Hz, –CH2–), 6.99 (s, 1H, =CH), 7.61 (d, 1H, J8,7 = 8.40 Hz, 8-H), 7.68 (dd, 1H, Jo = 8.40 Hz, Jm = 2.40 Hz, 7-H), 7.93 (dd, 1H, J5,7 = 0.80 Hz, 5-H), 9.59 (s, 1H, 2-H), MS (ESI+) m/z (rel intensity): 343.6 (M + 1, 100), Anal. for C18H18N2O3S: Calc. C: 63.14; H: 5.30; N: 8.18; S: 9.36%. Found C: 62.89; H: 5.57; N: 8.19; S: 9.07%.
Biological activity studies
Insulin releasing activity
Cell culture of INS-1 cells
INS-1 cells, generously provided by Dr. C. Wollheim, Geneva, SwitzerlandCitation21, were grown in plastic culture bottles or micro-wells for 4–6 days (half confluence: 1–2 × 106 cells per mL) in RPMI medium supplemented with 10 % (v/v) fetal calf serum, 100 U of penicillin per mL and 0.1 mg of streptomycin per mL. Cells were seeded at a density of 5 × 105 cells/mL. The medium was changed every 5 days, and the cells were detached from the culture flask with trypsin 1 week after seeding, centrifuged and reseeded as described above. Prior to the experiment cells were washed 2 times and then incubated in Krebs-Ringer buffer containing 10 mM HEPES and 0.5 % bovine serum albumin (KRBH).
Insulin release
To measure insulin secretion, half-confluent cells in micro-wells were incubated for 90 min at 37°C in the aforementioned KRBH buffer. Insulin released into the medium was assayed with a radioimmunoassay using rat insulin (Novo Nordisk, Bagsvaerd, Denmark) as a standard, (mono 125I-Tyr a14)-porcine insulin as the labeled compound (Sanofi-Aventis, Germany) and anti-insulin antibodies from Linco (St. Louis, USA). Each compound had been checked for non-interference with the insulin radioimmunoassay. The data were corrected for the effects of solubilizing compounds (ethanol, DMSO).
Results and discussion
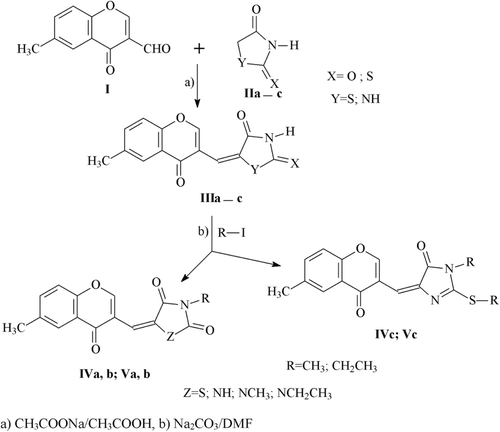
6-Methylchromonyl-2,4-thiazolidinedione compounds (IIIa–c, IVa–c, Va–c) were synthesized according to the synthetic pathway described in .
The condensation of 3-formyl chromone (I) with 2,4-thiazolidinedione, thiazolidine-2,4-dione/imidaz-olidine-2,4-dione/2-thioxo-imidazolidine-4-one (IIa–c) in the presence of sodium acetate/acetic acid glacial by Knoevenagel reaction, led to 6-methylchromonyl-2,4-thiazolidinediones IIIa–c. Compounds IVa–c, Va–c were synthesized by alkylation of compounds IIIa–c with methyl/ethyl iodide in the presence of anhydrous sodium carbonate/DMF.
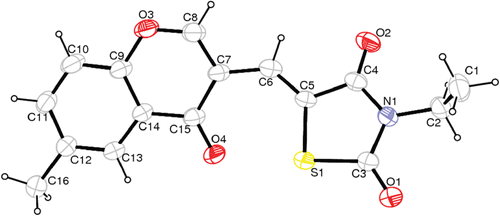
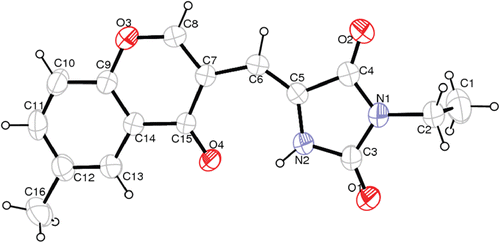
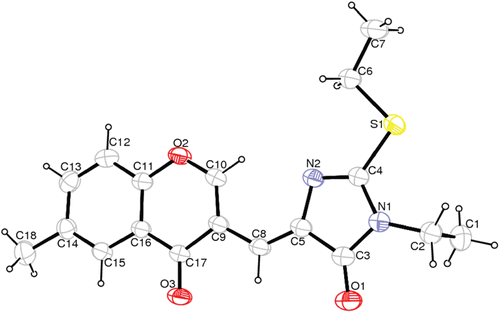
The structure of the synthesized chromonyl compounds was elucidated by, 1H NMR, Mass spectral data and elementary analysis findings. All spectral data were in accordance with assumed structures. In mass spectra, the compounds have M + H ion peak. In 1H NMR spectra, chromone protons were observed between 7.37 and 9.65 ppm; methylene protons for chromonyl-2,4-TZDs, chromonyl-2,4-imidiazolidinediones and chromonyl-2-thioxo-imidazolidine-4-ones were seen 7.82, 6.13–6.58 and 6.37–7.42 ppm as a singlet, respectively. In this study only one isomer of the compounds was obtained. After the appropriate crystals of the compounds Va (CCDC deposition number: 864612), Vb (CCDC deposition number: 864613) and Vc (CCDC deposition number: 864614) were obtained, X-Ray analysis was performed. As a result, the X-ray diffractometric analysis of compound Va (), Vb (), Vc () unambiguously confirmed the Z configuration at the chiral axis. Since all the ethyl alkylated compounds Va–c and methyl alkylated compounds IVa–c were obtained from compounds IIIa–c, we can say that all the synthesized compounds IIIa–c, IVa–c, Va–c have the Z configuration.
Derivatives of chromonyl TZD compounds (IIIa–c, IVa–c, Va–c) were tested comparing with glibenclamide for their insulinotropic activities in INS-1 cells at two different concentrations (). Compounds IIIb, IIIc, IVa–c, Va and Vc (at lower concentration; 0.001 mg/mL) were able to increase insulin release in the presence of 5.6 mmol/L glucose. When all the synthesized compounds’ activities is compared in both concentrations, we saw that at higher concentration (0.01 mg/mL), compounds IVa–c, Vb and Vc were more potent than their lower concentrations. In contrast, compound Va was more active at its lower concentration than higher concentration. Otherwise, compound IIIb possessed similar activity in both concentrations. In this series, the most potent compound is IVa having methyl group at N3 position of TZD ring in both test concentrations.
Table 1. Effects of various compounds on glucose-mediated insulin release from INS-1 cells.*
In our previous studiesCitation9–18, we showed that 2,4-TZD N-acetic acid, acetic acid ethyl ester, benzyl and phenacyl derivatives were more potent than unsubstituted TZDs with regard to their insulin releasing activities. As seen in this study, 2,4-TZD-N-unsubstituted derivatives had also no effect on insulin releasing activity in INS-1 cells. According to these results, it should be pointed out that compared with imidic hydrogen carboxylic acid, carboxylic acid ethyl ester, benzyl, phenacyl, methyl or ethyl groups on the TZD ring at N-3 position played a noticeable role for increasing the insulin releasing activity in INS-1 cells. On the other hand, it was shown that compounds having unsubstituted imidazolidine-2,4-dione and 2-thioxo-imidazolidine-4-one rings were more potent than unsubstituted 2,4-TZDs; in contrast, substituted 2,4-TZDs were more potent than the other compounds having substituted bioisosteric rings. As a result, we can say that instead of imidic hydrogen on the TZD ring at N-3 position lipofilic groups are important for increasing the insulin releasing activity.
Conclusion
We report the synthesis and the in vitro insulin releasing activity of the 6-Methylchromonyl-2,4-thiazolidinedione compounds (IIIa–c,IVa–c,Va–c). We also report X-ray diffractometric analysis of compounds Va, Vb, Vc. These compounds confirmed the Z configuration at the chiral axis. Compounds IIIb, IIIc, IVa–c, Va and Vc (at lower concentration; 0.001 mg/mL) were able to increase insulin release in the presence of 5.6 mmol/L glucose.
In this series, the most potent compound is IVa having methyl group at N3 position of TZD ring in both test concentrations. In conclusion, we can say that instead of imidic hydrogen on the TZD ring at N-3 position lipofilic groups are important for increasing the insulin releasing activity; otherwise, compounds having N-substituted TZD ring are more potent than the other bioisosteric substituted TZD analogs.
Declaration of interest
This work was supported by Research Organization of Ankara University, Turkey (No: 09B3336003).
References
- Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–795.
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med 2004;351:1106–1118.
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008;31:173–175.
- Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. Food and Drug Administration. N Engl J Med 2010;363:1489–1491.
- Jian-ping Y. Challenges in drug discovery for thiazolidinedione substitute. Acta Pharmaceutica Sinica B 2011;1:137–142.
- Kai H, Xuegang L, Xin C, Xiaoli Y, Jing H, Yanan J, Panpan L, Yafei D, Qing J, Qing S, Hejing S. Evaluation of antidiabetic potential of selected traditional Chinese medicines in STZ-induced diabetic mice. J Ethnopharmacol 2011;137: 1135–1142.
- Grazul M, Budzisz E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord Chem Rev 2009;253:2588–2598.
- Bozdag O, Verspohl EJ, Ertan R. Synthesis and hypoglycemic activity of some new flavone derivatives. 2nd communication: 4′-flavonyl-2,4-thiazolidinediones. Arzneimittelforschung 2000;50:539–543.
- Bozdag O, Verspohl EJ, Ertan R. Synthesis and hypoglycemic activity of some new flavone derivatives. 3rd communication: 3′-flavonyl-2,4-thiazolidinediones. Arzneimittelforschung 2000;50:626–630.
- Bozdag-Dündar O, Waheed A, Verspohl EJ, Ertan R. Synthesis and hypoglycemic activity of some new flavone derivatives. 4th communication: 6-flavonyl-2,4-thiazolidinediones. Arzneimittelforschung 2001;51:623–627.
- Tunçbilek M, Bozdag-Dündar O, Ayhan-Kilcigil G, Ceylan M, Waheed A, Verspohl EJ et al. Synthesis and hypoglycemic activity of some substituted flavonyl thiazolidinedione derivatives–fifth communication: flavonyl benzyl substituted 2,4-thiazolidinediones. Farmaco 2003;58:79–83.
- Bozdag-Dündar O, Verspohl EJ, Das-Evcimen N, Kaup RM, Bauer K, Sarikaya M et al. Synthesis and biological activity of some new flavonyl-2,4-thiazolidinediones. Bioorg Med Chem 2008;16:6747–6751.
- Bozdag-Dündar O, Verspohl EJ, Waheed A, Ertan R. Synthesis and antidiabetic activity of some new furochromonyl-2,4-thiazolidinediones. Arzneimittelforschung 2003;53:831–836.
- Bozdag-Dündar O, Ceylan-Unlüsoy M, Verspohl EJ, Ertan R. Synthesis and antidiabetic activity of some new chromonyl-2,4-thiazolidinediones. Arzneimittelforschung 2007;57:532–536.
- Verspohl EJ, Bozdağ-Dündar O, Kaup RM, Bauer K, Ertan R. Insulinotropic activity of chromonyl-2,4-thiazolidinediones. Med Chem Res 2009;18: 665–670.
- Bozdag-Dündar O, Ceylan-Unlüsoy M, Verspohl EJ, Ertan R. Synthesis and antidiabetic activity of novel 2,4-thiazolidinedione derivatives containing a thiazole ring. Arzneimittelforschung 2006;56:621–625.
- Bozdag-Dündar O, Mentese A, Verspohl EJ. Synthesis and antidiabetic activity of some new thiazolyl-2,4-thiazolidinediones. Arzneimittelforschung 2008;58:131–135.
- Ezer M, Yildirim LT, Bayro O, Verspohl EJ, Dundar OB. Synthesis and antidiabetic activity of morpholinothiazolyl-2,4-thiazolidindione derivatives. J Enzyme Inhib Med Chem 2012;27:419–427.
- Lima MC, Costa DL, Goes AJ, Galdino SL, Pitta IR, Luu-Duc C. [Synthesis and antimicrobial activity of chlorobenzyl benzylidene imidazolidinedione derivatives and substituted thiazolidinediones]. Pharmazie 1992;47:182–184.
- Bozdag-Dündar O, Evranos B, Das-Evcimen N, Sarikaya M, Ertan R. Synthesis and aldose reductase inhibitory activity of some new chromonyl-2,4-thiazolidinediones. Eur J Med Chem 2008;43:2412–2417.
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 1992;130:167–178.