Abstract
In this study, nine new hydrazide derivatives were synthesized. The reaction of 2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide with various benzaldehydes or acetophenones resulted in N′-substituted-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide derivatives. The structure elucidation of the synthesized and purified compounds was performed by using IR, 1H-NMR, and FAB+-MS spectral data and elemental analyses, respectively. Furthermore, the compounds were screened and evaluated for their antifungal activity against a panel of different human pathogenic Candida strains such as C. albicans, C. glabrata, C. utilis, C. tropicalis, C. krusei, C. zeylanoides and C. parapsilosis using agar diffusion and broth microdilution assays, respectively. Some of the tested compounds showed comparable antifungal activity (MIC = 0.0156–>2 mg/mL) with ketoconazole.
Introduction
Even though Candida spp. are commensally present in the human flora of about 30–60% of healthy individuals, they may become pathological depending on various circumstances related to the host, as an example of systemic disease leading to immunosuppression, and local destructive conditions. In fact, the pathogenicity of Candida spp. is affected by several virulence factors, such as the ability to adhere to epithelial and endothelial cells, germination, extracellular proteinases and phospholipases, and phenotypic switchingCitation1,Citation2.
In the recent years, the incidence of systemic fungal infections has increased remarkably and the main target is still the opportunistic pathogen Candida albicans. A major obstacle in the treatment of C. albicans infections is the spread of antifungal drug resistance mainly in patients chronically subjected to antimycotic therapy, i.e. those treated with broad-spectrum antibiotics, immunosuppressive agents, anticancer, antifungal and anti-AIDS drugsCitation3,Citation4. As known, not only biochemical similarity of the human cell and fungi forms is a handicap for selective activity, but also the easily gained resistance is the main problem encountered in developing safe and effective antifungals. The choice of suitable antifungal agents remains relatively limited, although the advent of the new echinocandin class is effective as the expansion of members of the azole antifungalsCitation5–7.
There are two basic approaches to develop new antifungal drugs: (1) synthesis of analogues, modifications or derivatives of existing compounds for shortening and improving fungal infection treatment, and (2) searching for novel structures for the treatment of fungal infectionCitation8.
Naftifine, terbinafine, and their analogues possessing a naphthalene moiety, which are a major class of non-azole antifungal agents, are known to inhibit the squalene epoxidase enzyme in fungal cellsCitation9,Citation10. Tetrahydronaphthalene derivatives as naphthalene analogues are also well established and known compounds for their antifungal profilesCitation11–14.
On the other hand, it is well known that hydrazide-hydrazone group plays an important role for antimicrobial activityCitation2,Citation15–17.
In the design of new drugs, the development of hybrid molecules through the combination of different pharmacophores, such as the tetrahydronaphthalene and hydrazide moieties in one frame may lead to compounds with interesting antifungal properties and profiles. Thus, in the present work we report in our ongoing antifungal syntheses the combination of these moieties for the first time to the best of our knowledge.
Experimental section
Chemistry
All chemicals were purchased from Sigma-Aldrich Chemical Co. All melting points (m.p.) were determined by Electrothermal 9100 digital melting point apparatus and were uncorrected. The purity of the compounds was routinely checked by thin layer chromatography (TLC) using silica gel 60G (Merck). Spectroscopic data were recorded with the following instruments: IR: Shimadzu IR-435 spectrophotometer, 1H-NMR, Bruker 250 MHz spectrometer; MS-FAB, VG Quattro Mass spectrometer and elemental analyses were performed on a Perkin Elmer EAL 240 elemental analyzer.
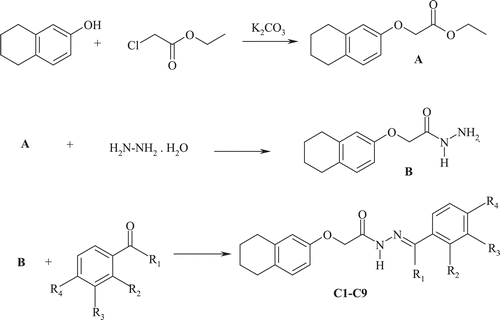
Preparation of ethyl 2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetate (A)
This compound was prepared as starting material in accordance with the method described in the literatureCitation18.
Preparation of 2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (B)
In a flask equipped with a reflux condenser, a mixture of ethyl 2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetate (A) (100 mmol) and hydrazine hydrate (100 mmol) was reacted in ethanol (200 mL). The mixture was then refluxed for 1 h and the resulting solid product was filtered and used without further purificationCitation19.
Preparation of N′-benzylidene-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide and
N′-[1-(phenyl)ethylidene]-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide derivatives (C1–C9)
The reaction of equimolar quantities of hydrazide (B) (5 mmol) with appropriate benzaldehyde/ acetophenone(5 mmol) in isopropyl alcohol resulted in the formation of the title compounds(Scheme 1). Some characteristics of the synthesized compounds are shown in .
Table 1. Some characteristics of the synthesized compounds.
N′-(4-Chlorobenzylidene)-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (C1)
IR (KBr) νmax(cm−1): 3432 (amide N-H), 1681 (amide C=O), 1630–1521 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.70 (4H, s, 2CH2), 2.60–2.80 (4H, m, 2CH2), 4.60 and 5.05 (2H, 2s, O-CH2-CO), 6.60–7.10 (3H, m, Ar-H), 7.50–7.85 (4H, m, Ar-H), 8.05 and 8.35 (1H, 2s, N=CH), 11.65 (1H, brs, N-H). For C19H19ClN2O2 calculated: 66.57 % C, 5.59 % H, 8.17% N; found: 66.60 % C, 5.62 % H, 8.15 % N. MS (FAB) [M + 1]+: m/z 343.
N′-(4-Nitrobenzylidene)-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide
(C2IR (KBr) νmax (cm−1): 3405 (amide N-H), 1687 (amide C=O), 1621–1514 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.65 (4H, s, 2CH2), 2.55–2.80 (4H, m, 2CH2), 4.60 and 5.10 (2H, 2s, O-CH2-CO), 6.55–7.00 (3H, m, Ar-H), 7.90–8.00 (2H, m, Ar-H), 8.20–8.35 (2H, m, Ar-H), 8.10 and 8.40 (1H, 2s, N=CH), 11.80 (1H, brs, N-H). For C19H19N3O4 calculated: 64.58 % C, 5.42% H, 11.89% N; found: 64.61 % C, 5.45 % H, 11.93 % N. MS (FAB) [M + 1]+: m/z 354.
N′-[4-(Dimethylamino)benzylidene]-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (C3)
IR (KBr) νmax (cm−1): 3467 (amide N-H), 1697 (amide C=O), 1605–1507 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.60–1.80 (4H, m, 2CH2), 2.55–2.80 (4H, m, 2CH2), 3.00 (6H, s, N(CH3)2), 4.60 and 5.05 (2H, 2s, O-CH2-CO), 6.55–7.05 (5H, m, Ar-H), 7.45–7.55 (2H, m, Ar-H), 7.90 and 8.20 (1H, 2s, N=CH), 11.30–11.40 (1H, br, N-H). For C21H25N3O2 calculated: 71.77 % C, 7.17% H, 11.96% N; found: 71.80 % C, 7.16 % H, 11.99 % N. MS (FAB) [M + 1]+: m/z 352.
N′-(4-Methoxybenzylidene)-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (C4)
IR (KBr) νmax (cm−1): 3410 (amide N-H), 1703 (amide C=O), 1651–1499 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.70 (4H, s, 2CH2), 2.60–2.80 (4H, m, 2CH2), 3.80 (3H, s, OCH3), 4.60 and 5.10 (2H, 2s, O-CH2-CO), 6.60–6.80 (2H, m, Ar-H), 6.90–7.10 (3H, m, Ar-H), 7.60–7.75 (2H, m, Ar-H), 8.00 and 8.30 (1H, 2s, N=CH), 11.50 (1H, brs, N-H). For C20H22N2O3 calculated: 70.99 % C, 6.55% H, 8.28% N; found: 71.01 % C, 6.51 % H, 8.32 % N. MS (FAB) [M + 1]+: m/z 339.
N′-(4-Methylbenzylidene)-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (C5)
IR (KBr) νmax (cm−1): 3455 (amide N-H), 1689 (amide C=O), 1611–1520 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.65 (4H, s, 2CH2), 2.30 (3H, s, CH3), 2.55–2.80 (4H, m, 2CH2), 4.55 and 5.00 (2H, 2s, O-CH2-CO), 6.65–6.80 (2H, m, Ar-H), 6.95–7.55 (5H, m, Ar-H), 8.05 and 8.35 (1H, 2s, N=CH), 11.40 (1H, brs, N-H). For C20H22N2O2 calculated: 74.51 % C, 6.88% H, 8.69% N; found: 74.55 % C, 6.90 % H, 8.71 % N. MS (FAB) [M + 1]+: m/z 323.
N’-(2-Chlorobenzylidene)-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (C6)
IR (KBr) νmax (cm−1): 3422 (amide N-H), 1690 (amide C=O), 1624–1561 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.70 (4H, s, 2CH2), 2.55–2.75 (4H, m, 2CH2), 4.55 and 5.05 (2H, 2s, O-CH2-CO), 6.65–7.10 (3H, m, Ar-H), 7.35–7.65 (4H, m, Ar-H), 8.10 and 8.35 (1H, 2s, N=CH), 11.55 (1H, brs, N-H). For C19H19ClN2O2 calculated: 66.57 % C, 5.59 % H, 8.17% N; found: 66.58 % C, 5.59% H, 8.19% N. MS (FAB) [M + 1]+: m/z 343.
N′-(3-Nitrobenzylidene)-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (C7)
IR (KBr) νmax (cm−1): 3441 (amide N-H), 1695 (amide C=O), 1620–1517 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.70 (4H, s, 2CH2), 2.60–2.80 (4H, m, 2CH2), 4.55 and 5.05 (2H, 2s, O-CH2-CO), 6.50–6.90 (3H, m, Ar-H), 7.90–8.20 (4H, m, Ar-H), 8.25 and 8.45 (1H, 2s, N=CH), 11.70 (1H, brs, N-H). For C19H19N3O4 calculated: 64.58 % C, 5.42 % H, 11.89% N; found: 64.60 % C, 5.43% H, 11.90% N. MS (FAB) [M + 1]+: m/z 354.
N′-(1-Phenylethylidene)-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (C8)
IR (KBr) νmax (cm−1): 3462 (amide N-H), 1700 (amide C=O), 1641–1510 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.60 (4H, s, 2CH2), 2.20 (3H, s, CH3), 2.55–2.70 (4H, m, 2CH2), 4.60 and 5.10 (2H, 2s, O-CH2-CO), 6.50–6.70 (2H, m, Ar-H), 6.80–6.95 (1H, m, Ar-H), 7.30–7.50 (3H, m, Ar-H), 7.70–7.90 (2H, m, Ar-H), 10.70 (1H, brs, N-H). For C20H22N2O2 calculated: 74.51 % C, 6.88% H, 8.69% N; found: 74.55 % C, 6.90 % H, 8.70 % N. MS (FAB) [M + 1]+: m/z 323.
N′-[1-(4-Hydroxyphenyl)ethylidene]-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (C9)
IR (KBr) νmax (cm−1): 3402 (amide N-H), 1691 (amide C=O), 1637–1531 (C=C and C=N). 1H NMR (250 MHz, DMSO-d6): 1.70 (4H, s, 2CH2), 2.20 (3H, s, CH3), 2.60–2.75 (4H, m, 2CH2), 4.65 and 5.10 (2H, 2s, O-CH2-CO), 6.50–7.00 (4H, m, Ar-H), 7.60–7.90 (3H, m, Ar-H), 9.80 (1H, br, OH), 10.40 and 10.70 (1H, 2brs, N-H). For C20H22N2O3 calculated: 70.99 % C, 6.55% H, 8.28% N; found: 71.02 % C, 6.54 % H, 8.28 % N. MS (FAB) [M + 1]+: m/z 339.
Microbiology
Anticandidal assays and microorganisms
The activities of the compounds (C1–9) were first screened including the standard antifungal ketoconazole by an agar diffusion method using C. albicans (clinical isolate) and C. tropicalis and all active compounds (inhibition zones >9–11 mm, at 2 mg/mL concentration) were further evaluated using the broth microdilution method to identify the minimum inhibitory concentrations (MIC) against all Candida sppCitation20,Citation21.
Microorganisms were obtained from ATCC, NRRL and clinical isolates (Faculty of Medicine, Eskisehir Osmangazi University, Turkey) and were stored in 15% glycerol containing micro-test tubes at -86 °C (strain numbers of microorganisms were given in ). All Candida strains were inoculated on Sabouraud Dextrose Agar (SDA) prior the experiments at 37°C. After sufficient growth, Candida spp. were then transferred to Mueller Hinton Broth (MHB) for further incubation at the same conditions for another 24 h.
Table 2. MIC values (mg/mL) of compounds C1–C9.
Broth microdilution assay
The test compounds (C1–9) and ketoconazole were first dissolved in DMSO which was used to prepare the stock solutions at an initial concentration of 2 mg/mL. Serial dilution series were prepared in 100 µL Mueller Hinton Broth (MHB) with an equal amount of the test samples. The last row was filled only with water as growth control for the microorganism. Overnight grown microorganism suspensions were first diluted in double strength MHB and standardized to 108 CFU/mL (using McFarland No: 0.5) under sterile conditions. Then each microorganism suspension was pipetted into each well and incubated at 37°C for 24 h. Ketoconazole was used as a standard antifungal agent against Candida spp. Sterile distilled water and medium served as a positive growth control. The first well without turbidity was assigned as the minimum inhibitory concentration (MIC, in mg/mL). Average results of separately performed three experiments were given in .
Results and discussions
The starting materials ethyl 2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetate (A) and 2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazide (B) were synthesized in accordance to the method described in the literatureCitation18,Citation19. Aryl aldehydes or aryl ketones were reacted with hydrazide (B) to give the required N’-substituted-2-[(5,6,7,8-tetrahydronaphthalen-2-yl)oxy]acetohydrazides(C1–C9) (). Substitution on arylaldehyde or arylketones played an important role in the title compounds formation step. Namely, the substitution with a strong electron withdrawing group like NO2 on the benzene ring, gave higher yield compared to the other groups.
Formulas of compounds (C1–C9) () were obtained by elemental analyses and their structures were determined by IR, 1H-NMR and FAB+-MS spectral data, respectively. IR data was very informative and provided evidence for the formation of the expected structures. In the IR spectra, some significant stretching bands were observed due to N-H, C=O, and C=N and C=C bonds at 3467–3402, 1703-1681 and 1651–1499 cm−1, respectively.
In the 1H-NMR spectra of the compounds, the C6 and C7 protons of 5,6,7,8-tetrahydronaphthalene were detected at 1.60–1.80 ppm as singlets or multiplets. The C5 and C8 protons of 5,6,7,8-tetrahydronaphthalene were observed at 2.55–2.80 ppm as multiplets. The -O-CH2-CO protons appeared as two singlets at 4.55–4.65 and 5.00–5.10 ppm. The -N=CH- proton of compounds C1-C7 was observed as two singlets at 8.00–8.25 and 8.20–8.45 ppm. The -NH-N= proton was observed as two broad singlet or broad singlet at 10.40–11.65 ppm, respectively. All other aliphatic and aromatic protons were observed at expected regions. The 1H-NMR data were also consistent with the assigned structures. In the 1H-NMR spectra of the compounds; we observed paired peaks for each of the protons -O-CH2-,-N=CH-, and -NH-N= corresponding to E- and Z-forms of the compounds. For each compound, the intensities of these paired peaks differed from others, due to the variable amounts of E- and Z-isomer, which are usually known to be unequal.
The mass spectra MS (FAB) of the compounds showed [M + 1] peaks, in agreement with their molecular formula.
The antifungal activity of the compounds was studied with eight pathogenic fungi. Ketoconazole was used as reference for inhibitory activity against fungi. MIC values were recorded as the minimum concentration (MIC)of a compound that inhibits the growth of tested microorganisms. All of the compounds tested showed good antifungal activity when compared with ketoconazole. The MIC values were generally within the range of 0.0312–>2 mg/mL against all evaluated strains. The results are summarized in .
Among the tested compounds,C2, C6, and C7 were effective against C. albicans (ATCC 90028) when compared with ketoconazole. Especially compound C2 showed strong Candida inhibitory activity. Compound C7 showed a similar level of activity with ketoconazole, whereas C6 showed moderate activity.
Compounds C1, C2, C4, C6, C7 and C9 were relatively effective against C. zeylanoides (NRLL Y-1774). Compound C2 showed strong inhibitory activity such in the case of C. albicans. Compounds C1, C6, and C7 showed a similar level of activity with ketoconazole, whereas compounds C4, and C9 showed moderate activity.
In comparing their MIC values with the reference agent ketoconazole, compounds C1, C2, C4, C6, C7 and C9 were effective against C. glabrata (Clinical Isolate). Compounds C2, and C7 showed a similar level of activity with ketoconazole and C1, C4, C6, and C9 showed moderate activity. On the other hand, the compounds exhibited comparable activities against C. albicans (Clinical Isolate). Compounds C2 and C7 showed moderate activity and the other compounds were found less active than the reference agent.
Compounds C2, C6, C7 showed moderate activity against C. krusei (NRLL Y-7179). From the similar results obtained with C. parapsilosis (NRLL Y-12696), compounds C2 and C7 showed moderate activity, whereas all other compounds showed less activity when compared with ketoconazole.
While compound C2 showed moderate activity against C. utilis (NRRL Y-900), compounds C2, C7 also showed moderate activity against C. tropicalis (NRLL Y-12968) when compared with ketoconazole.
Conclusions
Considering all the results obtained from the in vitro antifungal evaluations, in comparison with reference agent ketoconazole, it is possible to state that the tested C2, C6, and C7 compounds are relatively more active than other tested compounds in this panel. Based on the limited number of compounds evaluated, it appears that 4-nitro (C2), 2-chloro (C6), and 2-nitro (C7) substitutions on the phenyl ring have made a good contribution to the antifungal activity in this series of tetrahydronaphthalene-hydrazide combination. Meanwhile, the substitution with electron withdrawing groups such as NO2 and Cl on the arylidene moiety has contributed an interesting impact on the antifungal activity. It is worthwhile to test the synthesized compounds and their derivatives against other pathogenic fungi and bacteria.
Declaration of interest
The authors are grateful to the Research Foundation of Anadolu University (BAP-1103S060) for financing this research.
References
- Playford EG, Marriott D, Nguyen Q, Chen S, Ellis D, Slavin M, Sorrell TC.Candidemia in non-neutropenic critically ill patients: risk factors for non-albicans Candida spp. Crit Care Med 2008;36:2034–2039.
- Chimenti F, Bizzarri B, Maccioni E, Seci D, Bolasco A, Fioravanti R, Chimenti P, Granese A, Carradori S, Rivenera D, Lilli D, Zicari A, Distinto S. Synthesis and in vitro activity of 2-thiazolylhydrazone derivatives compared with the activity of clotrimazole against clinical isolates of Candida spp. Bioorg Med Chem Lett 2007;17:4635–4640.
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev 1999;12:40–79.
- Schiaffella F, Macchiarulo A, Milanese L, Vecchiarelli A, Costantino G, Pietrella D, Fringuelli R. Design, synthesis, and microbiological evaluation of new Candida albicans CYP51 inhibitors. J Med Chem 2005;48:7658–7666.
- Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin Infect Dis 1998;27:1138–1147.
- Polak A. The past, present and future of antimycotic combination therapy. Mycoses 1999;42:355–370.
- Musiol R, Jampilek J, Buchta V, Silva L, Niedbala H, Podeszwa B et al. Antifungal properties of new series of quinoline derivatives. Bioorg Med Chem 2006;14:3592–3598.
- Rao VS, Srinivas K. Modern drug discovery process. J Bioinform Seq Anal 2011;2: 89–94.
- Nussbaumer P, Dorfstätter G, Leitner I, Mraz K, Vyplel H, Stütz A. Synthesis and structure-activity relationships of naphthalene-substituted derivatives of the allylamine antimycotic terbinafine. J Med Chem 1993;36:2810–2816.
- Gokhale VM, Kulkarni VM. Understanding the antifungal activity of terbinafine analogues using quantitative structure-activity relationship (QSAR) models. Bioorg Med Chem 2000;8:2487–2499.
- Ates-Alagoz Z, Yildiz S, Buyukbingol E. Antimicrobial activities of some tetrahydronaphthalene-benzimidazole derivatives. Chemotherapy 2007;53:110–113.
- Raj AA, Raghunathan R, SrideviKumari MR, Raman N. Synthesis, antimicrobial and antifungal activity of a new class of spiro pyrrolidines. Bioorg Med Chem 2003;11:407–419.
- Suresh M, Lavanya P, Raju KN, Jonnalagadda SB, Rao CV. Synthesis and biological studies of novel 2-(4-substitutedbenzylthio)-5-amino-6-(benzo[d]thiazol-2-yl)-7-(4-chlorophenyl)pyrido-[2,3-d]-pyrimidin-4(3H)-one derivatives. Org Commun 2011;4:33–41.
- Turan-Zitouni G, Özdemir A, Kaplancıklı ZA, Revial G, Demirci F. Synthesis and antimicrobial activity evaluation of new hydrazide derivatives. Turk J Pharm Sci 2011;8:199–206.
- Vicini P, Zani F, Cozzini P, Doytchinova I. Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur J Med Chem 2002;37:553–564.
- Metwally KA, Abdel-Aziz LM, Lashine el-SM, Husseiny MI, Badawy RH. Hydrazones of 2-aryl-quinoline-4-carboxylic acid hydrazides: synthesis and preliminary evaluation as antimicrobial agents. Bioorg Med Chem 2006;14:8675–8682.
- Özdemir A, Turan-Zitouni G, Kaplancikli ZA, İşcan G, Khanc S, Demirci F. Synthesis and the selective antifungal activity of 5,6,7,8-tetrahydroimidazo[1,2-a]pyridinederivatives. Eur J Med Chem 2010;45:2080–2084.
- Sakaguchi H. N-Benzyl-4-alkynyloxybenzenepropanamides, agrochemical fungicides containing them, and plant disease control using them. Jpn Kokai Tokkyo Koho 2005; 66:JP2005314352.
- Turan-Zitouni G, Kaplancikli ZA, Erol K, Kiliç FS. Synthesis and analgesic activity of some triazoles and triazolothiadiazines. Farmaco 1999;54:218–223.
- Janssen AM, Scheffer JJ, Baerheim Svendsen A. Antimicrobial activity of essential oils: a 1976-1986 literature review. Aspects of the test methods. Planta Med 1987;53:395–398.
- NCCLS, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. Approved Standard, second ed., NCCLS document M27-A2 [ISBN 1-56238-469-4].