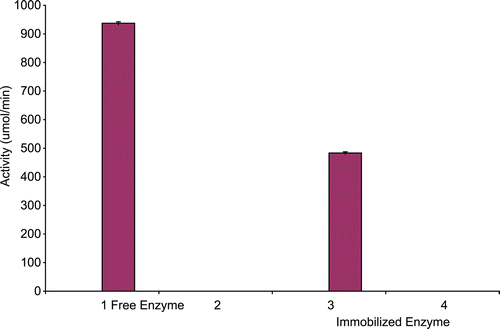Abstract
Cytosine deaminase (CD) from Aspergillus parasiticus, which has half-life of 1.10 h at 37°C, was stabilized by immobilization on calcium alginate beads. The immobilized CD had pH and temperature optimum of 5 and 50°C respectively. The immobilized enzyme also stoichiometrically deaminated Cytosine and 5-fluorocytosine (5-FC) with the apparent KM values of 0.60 mM and 0.65 mM respectively, displaying activation energy of 10.72 KJ/mol. The immobilization of native CD on calcium alginate beads gave the highest yield of apparent enzymatic activity of 51.60% of the original activity and the enzymatic activity was lost exponentially at 37°C over 12 h with a half-life of 5.80 h. Hence, the operational stability of native CD can be improved by immobilization on calcium alginate beads.
Keywords::
Introduction
Cytosine deaminase (CD) can deaminate Cytosine to uracil and 5-fluorocytosine (5-FC) to 5-fluorouracil (5-FU)Citation1. The ability of CD to convert nontoxic 5FC into toxic 5FU antimetabolite and its absence in mammalian cells has been exploited in enzyme-prodrug gene therapy and shown to control tumour growthCitation2. The Escherichia coli CD although stable, has high KM for 5-FC while yeast CD has low KM for 5-FC but is less stableCitation3. Cytosine deaminase from Baker’s yeast was stabilized through immobilization by various techniques. When the yeast CD was immobilized on epoxy-acrylic resin, the highest yield of apparent enzymatic activity was about 80% of the starting activity,and the half-life became of 10 days, though the enzyme lost activity exponentially at 37°C over a few weeks. With lower apparent enzymatic activity of 20–67% and 21%, and half-lives of 15 and 24 days respectively, formyl-cellulofine and CNBr-activated sepharose 4B gave a good stability. The apparent K′M for Cytosine and 5FC of the CD immobilized on epoxy-acrylic resin were 30 and 4 mM respectively which were much larger than those of the free enzyme, about 3 and 1 mM respectivelyCitation4.
Intratumoural conversion of 5FC into 5-fluorouracil (5FU) by locally implanted capsules containing CD followed by systemic administration of 5FC to induce antineoplastic activity at a local site with minimal toxicity was investigated by Nishiyama et al.Citation5 In vitro studies showed that 5FC combined with CD capsule, made of cellulose tubing, induced significant growth-inhibitory effects on the cultured glioma cells. There was a sufficient delivery of 5FC to the tumour cells of the rats with 5FU appearing in the tumour reaching the level of 8.0 µg/g at 2 h and staying at more than 1.0 µg/g at between 1 and 6 h resulting in significant reduction in the tumour growthCitation5.
However, the use of CD from E. coli has two problems: first, the, high KM for 5-FC, second, the difficulty of culturing the bacteria on a large scale to obtain sufficient activityCitation4. Cytosine deaminase from A. parasiticus was purified 387.73 folds with an overall yield of 13%, the half-life being 1.10 h at 37°CCitation6. This study is aimed at improving the half-life of A. parasiticus CD by immobilization on calcium alginate beads and to characterize the immobilized CD.
Materials and methods
Materials
Chemicals
All the chemicals used in this study were of analytical grade and purchased from various sources.
Organism
The A. parasiticus used in this study was isolated and identified by a mycologist using internet resources.
Methods
Inoculation
Spores of A. parasiticus were harvested from 5 day old potato-dextrose agar (PDA) slants by washing with sterile 0.2% tween 80. The spores were used to inoculate the mineral salt medium containing: distilled water 1.0 L, glucose 10 g, KH2PO4 5.0 g, MgSO4.7H2O 1.0 g, NaCl 0.5 g, NaNO3 2 g, 0.5 g peptone and pH adjusted to 5.5 with 0.1 M HCl/NaOH as described by refs Citation7,Citation8.
Enzyme assay
Cytosine deaminase activity was assayed as described by Ipata and CercignaniCitation9. Enzyme activity was measured by direct spectrophotometric assay from the fall in absorbance at 286 nm following conversion of 4-amino to 4-keto compounds. The following aliquots (0.3 mL of 0.5 mM Tris–HCl buffer pH 7.2, 0.5 mL 0f 0.5 mM cytosine) were dispensed into 1 cm quartz cuvettes. This was followed by the addition of 0.2 mL of enzyme preparation. The solutions were mixed and incubated in a spectrophotometer at 286 nm. Change in absorbance was recorded for 5–10 mins as a function of the activity of CD.
Cytosine Deaminase Assay Using 5-Fluorocytosine as Substrate This was done as described by Nishiyama et al.Citation5; Mahan et al.Citation10
Enzyme activity was measured spectrophotometrically at 255 and 290 nm following conversion of 5-FC to 5-FU.
The following concentrations; 0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5 and 4.0 mM 5-fluorocytosine were dispensed into separate tubes. This was followed by the addition of 0.3 mL of 0.5 mM Tris–Hcl buffer, pH7.2, 0.5 mL of 5FC, and 0.2 mL of enzyme. The mixture was shaken thoroughly and incubated at 37°C. One aliquot (50 µL) of the reaction mix was taken every minute over a 15 min time period and the reaction terminated in 2.45 mL 0.1 N HCl. Spectrophotometric readings were taken at OD255 (OD at which 5FU absorbs) and OD290 (OD at which 5FC absorbs). Then absorbance ratio of OD255/290 was used to measure amount of 5FU formed.
Immobilization of CD on calcium alginate
Cytosine deaminase (0.006 mg/mL) was immobilized on calcium alginate as described below. Exactly 1.5 g of Sodium alginate was suspended in 50 mL of distilled water and autoclaved at 121°C for 15 min. After cooling to room temperature 3.5 mL of the enzyme were added and mixed thoroughly and allowed to stand for 10 mins. The enzyme-alginate mixture was carefully pumped through a sterile syringe dropwise into a beaker containing 250 mL of sterile 0.12 M Calcium chloride for the formation of the beads. The beads were kept in solution for 1 h at 4°C to ensure complete precipitation.
Kinetics and characterization of immobilized CD
V′max, K′M, pH optimum and temperature stability studies of the immobilized enzyme were performed.
Statiscal analysis
Results are mean ± standard deviation for triplicate determination.
Results
Native and immobilized CD activities
The specific activities of native and immobilized CD is presented in . The native enzyme had a specific activity of 937.30 µmol/min/mg. Following immobilization on calcium alginate, the specific activity dropped to 483.33 µmol/min/mg representing 51.60% decrease of apparent enzymatic activity.
pH optimum of immobilized cytosine deaminase
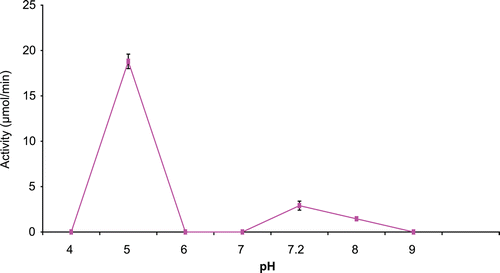
The effect of various pHs on immobilized CD is as shown in . The calcium alginate immobilized enzyme had an optimum pH of 5 as opposed to the native enzyme that had a pH optimum of 7.2.
Effects of temperature on CD catalyzed reactions
Temperature optimum of immobilized CD
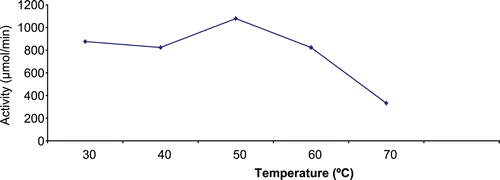
depicts the effect of temperature on immobilized enzyme. Following the exposure of the immobilized enzyme to various degrees of temperature, maximum apparent enzymatic activity was recorded at the temperature of 50°C.
Operational stability for immobilized CD
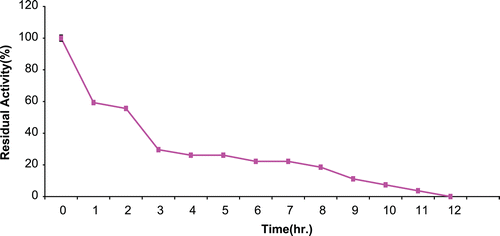
The result of operational stability for the immobilized CD at 37°C is presented in . Percentage residual activity following 2 h. of incubation was about 56%. This dropped to about 26% after 5 h. The t1/2 for the immobilized CD was 5.8 h.
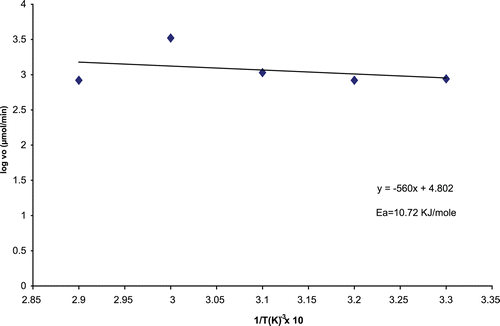
Apparent activation energy (Ea’) for immobilized CD
represents the Arrhenius plot for the determination of Ea’ of the immobilized A. parasiticus CD. The Ea’ of the immobilized CD was calculated to be 10.72 KJ/mole.
Kinetics of immobilized CD with cytosine and 5-FC as substrates
describes the kinetic properties of CD immobilized on calcium alginate beads. Apparent K′M and V′max of the immobilized enzyme using cytosine as substrate was 0.60 mM and 3.27 µmol/min with Kcat of 545 ± 5.60 S−1 while the apparent K′M and V′max of the immobilized CD using 5-FC as substrate was 0.65 mM and 0.024 µmol/min with Kcat of 4.00 S−1.
Table 1. Kinetics of immobilized A. parasiticus CD with cytosine and 5-fluorocytosine as substrates.
Discussion
Earlier, we were able to purify and characterize CD from A. parasiticus. The purified CD had pH and temperature optimum of 7.2 and 45°C respectively. The native enzyme also had an Ea of 8.4 KJ/mole with KM values of 0.19 mM and 0.30 mM for cytosine and 5-FC respectively. The operational stability of CD at 37°C was also determined. The percentage residual activity after 2 h was 33% with t1/2 of 1.10 hCitation6.
Immobilization of the native CD on calcium alginate produced beads of 141.0 mm3. The yield of apparent enzymatic activity following immobilization was 54.7%. The aim of the immobilization was to improve the stability of the native enzyme. The result of the operational stability of immobilized CD at 37°C reveals that the immobilized enzyme retained residual activity of 56% after 2 h of incubation with t1/2 of 5.8 h. The t1/2 of 5.8 h for the immobilized CD compared to that of the native enzyme (1.1 h) shows that the native CD can be stabilized by immobilization. The buffering effect of the carrier against the conditions of the microenvironment might have contributed to the stabilization of the enzyme activity. The pH optimum of the immobilized CD was 5 as opposed to 7.2 for the native enzyme. The drop in pH could be as a result of the polycationic nature of the carrier. Polycationic supports expel protons from the enzyme’s microenvironments and thereby lowering the pH of the bulk phase from which pH measurements are usually done.
The temperature optimum of the immobilized CD was 50°C compared with 40–45°C for the free enzyme. The increase in the optimum temperature following immobilization could be due to the enhanced physical protection provided by the solid support. Increased stability following immobilization is advantageous since this enzyme is intended for use in cancer chemotherapy. The immobilized enzyme had an activation energy of 10.72 KJ/mole, higher than that of the free enzyme (8.4 KJ/mole). This implies that substrate molecules reacting with the immobilized CD must possess higher Ea than when reacting with the free CD. The activation energy following immobilization may be less than, same as or greater than that of the native enzyme.
The apparent Michaelis constant, K′M, for cytosine and 5FC of the immobilized A. parasiticus cytosine deaminase were 0.60 mM and 0.65 mM respectively, which were higher than those of the free enzyme, about 0.19 mM and 0.30 mM respectively. Katsuragi et al.Citation4, also reported larger K′M for cytosine and 5FC (30 and 4 mM respectively) when cytosine deaminase from Baker’s yeast was immobilized on Eupergit C compared with those of the free enzyme (3 and 1 mM respectively). The increase in K′M of the immobilized enzyme could be due to the electrostatic field of the carrier, diffusional restrictions and interaction with carrier or deactivation due to immobilization. Apparent K′M decreases when the carrier has opposite charge with that of the substrate and increases when the carrier and the substrate have similar charge.
Conclusion
Immobilization of the A. parasiticus CD on calcium alginate beads improved the operational stability of the enzyme at 37°C by about 8-fold with superior kinetic properties than both yeast and E. coli CD. Therefore, cytosine deaminase from A. parasiticus is a good candidate for comparison with yeast and E. coli CD; and for subsequent deployment in cancer therapy either as capsule implant at the site of the tumour or in suicide gene therapy.
Declaration of interest
The authors report no declarations of interest.
References
- Ireton GC, McDermott G, Black ME, Stoddard BL. The structure of Escherichia coli cytosine deaminase. J Mol Biol 2002;315:687–697.
- Hamaji Y, Fujimori M, Sasaki T, Matsuhashi H, Matsui-Seki K, Shimatani-Shibata Y et al. Strong enhancement of recombinant cytosine deaminase activity in Bifidobacterium longum for tumor-targeting enzyme/prodrug therapy. Biosci Biotechnol Biochem 2007;71:874–883.
- Kievit E, Bershad E, Ng E, Sethna P, Dev I, Lawrence TS et al. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res 1999;59:1417–1421.
- Katsuragi T, Shibata M, Sakai T, Tonomura K. Stabiliation of cytosine deaminase from Baker’s yeast by immobilization. Agric Biol Chem 1989;53:1515–1523.
- Nishiyama T, Kawamura Y, Kawamoto K, Matsumura H, Yamamoto N, Ito T et al. Antineoplastic effects in rats of 5-fluorocytosine in combination with cytosine deaminase capsules. Cancer Res 1985;45:1753–1761.
- Zanna H, Nok AJ, Ibrahim S, Inuwa HM. Purification and characterization of Aspergillus parasiticus cytosine deaminase for possible deployment in suicide gene therapy. Adv Biol Chem 2012;2:152–159.
- Haq IU, Ali S, Qadeer MA. Influence of dissolved oxygen concentration on intracellular pH for regulation of Aspergillus niger growth rate during citric acid fermentation in a stirred tank bioreactor. Int J Biol Sci 2005;1:34–41.
- Zanna H, Nok AJ, Ibrahim S, Inuwa HM. Screening some Aspergillus fungi for cytosine deaminase activity. Int J Biotechnol Biochem 2011;7:397–403.
- Ipata PL, Cercignani G. Cytosine and cytidine deaminase from yeast. Meth Enzymol 1978;51:394–400.
- Mahan SD, Ireton GC, Knoeber C, Stoddard BL, Black ME. Random mutagenesis and selection of Escherichia coli cytosine deaminase for cancer gene therapy. Protein Eng Des Sel 2004;17:625–633.