Abstract
A new series of N-sec/tert-butyl 2-arylbenzimidazole derivatives was synthesised in 85–96% yields within 2–3.5 min by condensing ethyl 3-amino-4-butylamino benzoate with various substituted metabisulfite adducts of benzaldehyde under focused microwave irradiation. The benzimidazole analogues were characterised using 1H NMR, 13C NMR, high resolution MS and melting points. Evaluation of antiproliferative activity of the benzimidazole analogues against MCF-7 and MDA-MB-231 revealed several compounds with unexpected selective inhibitions of MDA-MB-231 in micromolar range. All analogues were found inactive towards MCF-7. The most potent inhibition against MDA-MB-231 human breast cancer cell line came from the unsubstituted 2-phenylbenzimidazole 10a.
Introduction
Breast cancer ranks as the top cancer in women, amounting to almost one-fifth of all female cancers. It is also the second leading cause of cancer mortality in women.Citation1–3 Apart from anti-oestrogen and radiation therapies and surgery, chemotherapy remains the mainstay treatment for breast cancer.
In oestrogen receptor positive (ER+) breast cancer, tamoxifen has made substantial reductions in the mortality rate in both early and advanced breast cancer patients for almost three decades.Citation4 Long term use of tamoxifen, however, is of limited success due to resistance in patients.Citation5 In contrast, patients with oestrogen-independent (ER-) breast cancer are often unresponsive to current treatments with poorer prognosis than hormone dependent breast cancer patients, thus they receive more aggressive chemotherapy. Despite considerable progress made in the discovery of novel chemotherapeutic agents for example imatinib, paclitaxel, ixabepilone; many current anti-cancer agents are still beseted with clinical problems such as toxicity, resistance and debilitating adverse effects.Citation6–8 Therefore, there is a clear need for new anti-cancer agents with enhanced selectivity towards breast cancer.
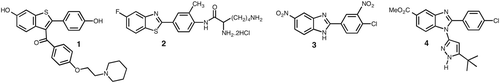
Small molecule inhibitors are the cornerstone of existing anti-cancer agents, and serve as lead compounds for many new chemotherapeutic agents.Citation1,Citation2,Citation8 Recently several structurally related bicyclic privileged scaffoldsCitation9,Citation10 were reported to exhibit strong inhibitory effects on breast cancer cell lines, for instance benzothiophene 1Citation11, benzothiazole 2Citation12, benzoxazoleCitation13 and benzimidazoles 3, 4Citation14,Citation15 ().
Of particular interest to us is the benzimidazole core, which exhibits a wide range of biological and pharmacological activitiesCitation16 including against several viruses such as HIVCitation17, human cytomegalovirus (HCMV)Citation18; Chlamydia bacteriaCitation19; as topoisomerase inhibitorsCitation20, angiotensin inhibitorCitation21, antitumour and anticancer agents.Citation11,Citation22–25 The proton pump inhibitor Omeprazole, and the antihelmintic Albendazole are examples of benzimidazoles with established clinical use.Citation16 The remarkable biological significance of benzimidazole derivatives together with their close structural resemblance to benzimidazole analogues 3 and 4 () led us to surmise that this series of 2-arylbenzimidazole derivatives could exert anti-proliferative effects towards breast cancer cell lines. Thus in this paper, we report microwave assisted preparation of a new series of N-sec/tert-butyl-2-arylbenzimidazole derivatives and the evaluation of their antiproliferative activity against hormone dependent (MCF-7) and hormone independent (MDA-MB-231) breast cancer cell lines.
Materials and methods
Microwave-assisted syntheses were performed using CEM Discover™ microwave synthesizer. Melting points were measured on a Stuart SMP10 instrument and are uncorrected. Compounds 6–8 have been synthesised as previously described.Citation15,Citation26,Citation27 Preparative thin layer chromatography (PLC) was performed using Merck 60 GF254 silica gel coated (1 mm) on glass plates (20 × 20 cm). TLC experiments were performed on alumina-backed silica gel 40 F254 plates (Merck, Darmstadt, Germany). Visualisation of TLC plates was performed under UV light and aided by KMnO4 and iodine staining. Routine NMR (1H and 13C) spectra were recorded on a Bruker 300, 400 and 500 MHz instruments in CDCl3. Acquisition of high resolution mass measurements of the compounds was performed on an Agilent 6520 Quadrupole Time of Flight Mass Spectrometer (Agilent Technologies, Santa Clara, CA, USA) operating in the MS mode. All commercially available starting materials and solvents are used without further purification.
General procedure for synthesis of benzimidazoles 10a-e, 11a-e
Ethyl-3-amino-4-(sec-/tert-butylamino) benzoates were prepared according to previous procedure.Citation27 Thus, a solution of ethyl-3-amino-4-(sec-/tert-butylamino) benzoate (200 mg, 0.84 mmol) and sodium bisulfite adduct of benzaldehyde (353 mg, 1.68 mmol) in DMF (1 ml) was irradiated under microwave conditions at 130°C for 2–3.5 min. The reaction mixture was then diluted in EtOAc (20 mL) and washed with H2O (20 mL). The organic layer was collected and dried over Na2SO4. The solvent was removed under reduced pressure to afford the crude product.
Ethyl 1-sec-butyl-2-phenyl-1H-benzimidazole-5-carboxylate (10a). 1H NMR (CDCl3, 300 MHz), δ (ppm): 0.65 (t, CH3, 3H), 1.44 (t, CH3, 3H), 1.70 (d, CH3, 3H), 1.80–1.93 (m, CH2, 1H), 2.10–2.22 (m, CH2, 1H), 4.42 (q, CH2, 2H), 4.50–4.54 (m, CH, 1H), 7.50–7.58 (m, 3H), 7.61–7.67 (m, 3H), 8.01 (d, J = 9.0 Hz, 1H), 8.56 (s, 1H); 13C NMR (CDCl3, 75 MHz), δ (ppm): 11.22, 14.45, 31.02, 39.04, 55.7, 61.34, 112.32, 123.20, 124.32, 124.85, 128.56, 129.32, 131.26, 137.64, 144.51, 156.24, 167.52; HRMS (ESI/Q-TOF): m/z calcd for C20H22N2O2 (M+H), 323.1754; found 323.1767.
Ethyl 1-sec-butyl-2-(4-fluorophenyl)-1H-benzimidazole-5-carboxylate (10b). 1H NMR (CDCl3, 500 MHz), δ(ppm): 0.64 (t, CH3, 3H), 1.42 (t, CH3, 3H), 1.70 (d, CH3, 3H), 1.85–1.93 (m, CH2, 1H), 2.13–2.22 (m, CH2, 1H), 4.42 (q, CH2, 2H), 4.45–4.49 (m, CH, 1H), 7.21–7.25 (m, 2H), 7.60–7.64 (m, 3H), 7.99–8.01 (dd, J = 8.5, 1.5 Hz), 8.54 (d, J = 1.0 Hz, 1H); 13C NMR (CDCl3, 125 MHz), δ (ppm): 10.94, 14.37, 19.89, 28.09, 55.18, 60.80, 111.70, 115.88, 116.05, 122.51, 123.83, 124.78, 126.80, 131.60, 136.68, 143.30, 155.17, 162.68, 164.68, 167.03; HRMS (ESI/Q-TOF): m/z calcd for C20H21FN2O2 (M+H), 341.1660; found 341.1666.
Ethyl 1-sec-butyl-2-(4-bromophenyl)-1H-benzimidazole-5-carboxylate (10c). 1H NMR (CDCl3, 500 MHz), δ(ppm): 0.64 (t, CH3, 3H), 1.43 (t, CH3, 3H), 1.70 (d, CH3, 3H), 1.86–1.94 (m, CH2, 1H), 2.13–2.22 (m, CH2, 1H), 4.43 (q, CH2, 2H), 4.45–4.47 (m, CH, 1H), 7.52 (d, J = 8.0 Hz, 2H), 7.61 (d, J = 8.5 Hz, 1H), 7.69 (d, J = 8.0 Hz, 2H), 8.00–8.02 (dd, J = 8.5, 1.0 Hz, 1H), 8.55 (d, J = 1.0 Hz, 1H); 13C NMR (CDCl3, 125 MHz), δ (ppm): 10.94, 14.38, 19.91, 28.12, 55.25, 60.83, 111.76, 122.60, 123.94, 124.52, 124.87, 129.61, 131.09, 132.04, 136.71, 143.36, 155.01, 167.00; HRMS (ESI/Q-TOF): m/z calcd for C20H21BrN2O2 (M+H), 401.0859; found 401.0872.
Ethyl 1-sec-butyl-2-(3-nitrophenyl)-1H-benzimidazole-5-carboxylate (10d). 1H NMR (CDCl3, 500 MHz), δ(ppm): 0.70 (t, CH3, 3H), 1.45 (t, CH3, 3H), 1.77 (d, CH3, 3H), 1.92–2.01 (m, CH2, 1H), 2.19–2.28 (m, CH2, 1H), 4.45 (q, CH2, 2H), 4.47–4.51 (m, CH, 1H), 7.67 (d, J = 8.5 Hz, 1H), 7.76–7.79 (m, 1H), 8.03–8.07 (m, 2H), 8.41–8.43 (m, 1H), 8.53–8.54 (m, 1H), 8.58 (d, J = 1.0 Hz, 1H); 13C NMR (CDCl3, 125 MHz), δ (ppm): 11.02, 14.38, 19.99, 28.25, 55.65, 60.94, 111.98, 122.89, 124.41, 124.52, 124.67, 125.28, 130.05, 132.41, 135.52, 136.72, 143.31, 148.31, 153.36, 166.87; Mass ESI m/z 368.3 (M+1). HRMS (ESI/Q-TOF): m/z calcd for C20H21N3O4 (M+H), 368.1605; found 368.1620.
Ethyl 1-sec-butyl-2-(2-hydroxyphenyl)-1H-benzimidazole-5-carboxylate (10e). 1H NMR (CDCl3, 400 MHz), δ(ppm): 0.65 (t, CH3, 3H), 1.45 (t, CH3, 3H), 1.77 (d, CH3, 3H), 1.91–2.02 (m, CH2, 1H), 2.13–2.22 (m, CH2, 1H), 4.413 (q, CH2, 2H), 4.79–4.87 (m, CH, 1H), 6.97–7.02 (m, 1H), 7.13–7.15 (dd, J = 8.3, 1.0 Hz, 1H), 7.35–7.39 (m, 1H), 7.40–7.44 (dd, J = 7.8, 1.5 Hz, 1H), 7.63 (d, J = 8.6 Hz, 1H), 7.69–7.99 (m, 1H), 8.44 (d, J = 1.5 Hz, 1H); 13C NMR (CDCl3, 100 MHz), δ (ppm): 11.21, 14.81, 20.01, 28.54, 56.30, 61.32, 112.59, 114.25, 118.73, 119.57, 121.90, 124.43, 125.56, 128.48, 132.19, 136.65, 142.10, 154.35, 158.40, 167.23; HRMS (ESI/Q-TOF): m/z calcd for C20H22N2O3 (M+H), 339.1703; found 339.1717.
Ethyl 1-tert-butyl-2-p-tolyl-1H-benzimidazole-5-carboxylate (11a). 1H NMR (CDCl3, 300 MHz), δ (ppm): 1.41 (t, CH3, 3H), 1.62 (s, CH3, 9H), 2.42 (s, CH3, 3H), 4.41 (q, CH2, 2H), 7.23 (d, J = 7.8 Hz, 2H), 7.36 (d, J = 8.1 Hz, 2H), 7.73 (d, J = 8.7 Hz, 1H), 7.97–8.00 (dd, J = 8.7, 1.5 Hz, 1H), 8.47 (d, J = 1.5 Hz, 1H); 13C NMR (CDCl3, 75 MHz,), δ (ppm): 14.78, 21.81, 31.89, 59.80, 61.18, 114.57, 122.53, 122.83, 124.62, 128.89, 129.72, 132.71, 138.62, 139.65, 143.35, 155.95, 167.50; HRMS (ESI/Q-TOF): m/z calcd for C21H24N2O2 (M+H), 337.1911; found 337.1922.
Ethyl 1-tert-butyl-2-(4-methoxyphenyl)-1H-benzimidazole-5-carboxylate (11b). 1H NMR (CDCl3, 300 MHz), δ(ppm): 1.41 (t, CH3, 3H), 1.62 (s, CH3, 9H), 3.87 (s, OCH3, 3H), 4.40 (q, CH2, 2H), 6.96 (d, J = 9.0 Hz, 1H), 7.39–7.41(m, 2H), 7.74 (d, J = 9.0 Hz, 1H), 7.96–7.99 (m, 1H), 8.47 (s, 1H); 13C NMR (CDCl3, 75 MHz), δ (ppm): 14.77, 31.87, 55.76, 60.07, 61.21, 114.11, 114.69, 122.39, 123.76, 124.88, 127.25, 131.32, 138.43, 142.67, 155.58, 160.86, 167.37; HRMS (ESI/Q-TOF): m/z calcd for C20H24N2O3 (M+H), 353.1860; found 353.1863.
Ethyl 1-tert-butyl-2-(2-chlorophenyl)-1H-benzimidazole-5-carboxylate (11c). 1H NMR (CDCl3, 500 MHz), δ(ppm): 1.43 (t, CH3, 3H), 1.66 (s, CH3, 9H), 4.43 (q, CH2, 2H), 7.38–7.40 (m, 1H), 7.45–7.50 (m, 3H), 7.79 (d, J = 8.5 Hz), 8.03–8.05 (dd, J = 8.5, 1.5 Hz, 1H), 8.52 (d, J = 1.5 Hz); 13C NMR (CDCl3, 125 MHz), δ (ppm): 14.39, 30.39, 59.45, 60.80, 114.17, 122.64, 123.56, 124.49, 126.46, 129.32, 130.81, 131.44, 134.49, 135.08, 137.67, 143.17, 151.60, 167.00; HRMS (ESI/Q-TOF): m/z calcd for C20H21ClN2O2 (M+H), 357.1365; found 357.1378.
Ethyl 1-tert-butyl-2-(2,4-dichlorophenyl)-1H-benzimidazole-5-carboxylate (11d). 1H NMR (CDCl3, 500 MHz), δ (ppm): 1.42 (t, CH3, 3H), 1.65 (s, CH3, 9H), 4.42 (q, CH2, 2H), 7.37–7.39 (dd, J = 8.5, 2.0 Hz, 1H), 7.43 (d, J = 8.5 Hz, 1H), 7.51 (d, J = 2.0 Hz, 1H), 7.77 (d, J = 9.0 Hz, 1H), 8.03–8.05 (dd, J = 9.0, 1.5 Hz, 1H), 8.50 (d, J = 1.0 Hz); 13C NMR (CDCl3, 125 MHz), δ (ppm): 14.37, 30.45, 59.57, 60.85, 114.23, 122.63, 123.77, 124.70, 126.95, 129.28, 132.17, 133.60, 135.33, 136.32, 137.61, 143.03, 150.44, 166.87; EIMS m/z calcd for C20H20Cl2N2O2 (M+H), 391.1; found 391.1 (100), 392.1 (19), 393.0 (59), 394.1 (14); HRMS (ESI/Q-TOF): calcd (M+H) 391.0974, found 391.2843; (M-Cl+H) calcd 357.1365, found 357.1362.
Ethyl 1-tert-butyl-2-(2-hydroxyphenyl)-1H-benzimidazole-5-carboxylate (11e). 1H NMR (CDCl3, 500 MHz), δ(ppm): 1.47 (t, CH3, 3H), 1.70 (s, CH3, 9H), 4.43 (q, CH2, 2H), 6.53 (d, J = 8.5 Hz), 6.87–6.90 (m, 1H), 7.08–7.11 (m, 1H), 7.15–7.16 (dd, J = 7.5, 1.5 Hz 1H), 7.53 (d, J = 8.5 Hz), 7.61–7.64 (m, 2H); 13C NMR (CDCl3, 125 MHz), δ (ppm): 14.52, 30.21, 60.02, 60.63, 114.04, 119.52, 119.70, 120.27, 122.85, 123.58, 124.04, 130.09, 131.16, 136.90, 141.03, 152.91, 155.40, 166.46; HRMS (ESI/Q-TOF): m/z calcd for C20H22N2O3 (M+H), 339.1703; found 339.1710.
In vitro cytotoxicity assay
MCF-7 and MDA-MB-231 cell lines were purchased from ATCC and were grown, respectively, in DMEM and L-15 supplemented with 10% fetal bovine serum. Cytotoxicity of the compounds was evaluated using Cell Titer 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega), according to manufacturer’s instructions. Briefly, 90 μL of MCF-7 and MDA-MB-231 at respective cell number (MCF-7: 2 × 104 cells/well; MDA-MB-231: 2.5 × 104 cells/well) were seeded in triplicates in 96-well plate and incubated for 24 h. Subsequently, 10 μL of test compounds at various concentrations were added into each well and incubated for 48 h. Following incubation, 20 μL of MTS-PMS solution was added into each well and the plate was incubated 2 h for MCF-7 and 5 h for MDA-MB-231. Cisplatin and DMSO were used as positive and negative controls in this assay. The optical density of each well was determined spectrophotometrically at 490 nm, with 655 nm as reference wavelength. The percentage growth inhibition was calculated according to the following formula:
Percentage of growth inhibition (%) = Absorbance of negative control – Absorbance of test compound Absorbance of negative control
Results and discussion
Microwave-assisted synthesis of 2-arylbenzimidazoles
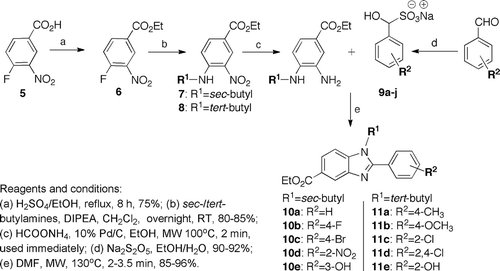
Synthesis of our target benzimidazoles 10 and 11 began with esterification of benzoic acid 5 in the presence of catalytic sulfuric acid in ethanol by refluxing for 8 h to afford ethyl ester 6 in 75% yield (). The benzoate 6 was then treated with sec- or tert-butylamine and N,N-diisopropylethylamine (DIPEA) in dichloromethane overnight to afford compound 7 and 8. Reduction of the aryl nitro 7 using 1.5 equivalent of ammonium formate in 10% Pd/C in ethanol gave a 30% yield; repeating it at 3.5 equivalent doubled the yield to 65%. When this reaction was performed under microwave conditions at 100oC for 2 min, the yields of the diamine intermediate improved significantly to 85%.Citation27 The pleasant smell compound was found to decompose by turning to dark red if kept any longer. Hence, the diamino compound was used immediately in the next step without further purification.
Benzimidazoles are typically prepared by condensing o-phenylenediamine with acid chloride, aldehydes and carboxylic acids.Citation28,Citation29 To effect the condensation of phenylenediamines with carboxylic acids generally require harsh, dehydrating conditions; in contrast, access via aldehydes employs oxidative reagents for example, copper(II) acetate, 1,4-benzoquinone, I2/KI, sodium metabisulfite and, interestingly, air.Citation30–32 Thus, we prefer using bisulfite adducts of aldehyde as this method has been reported to be more efficient and environmental friendly affording good to excellent yield of benzimidazoles when compared to other synthetic methods.Citation15,Citation31 Initial condensation reactions of the unstable substituted o-phenylenediamines with sodium bisulfite adduct of some substituted benzaldehydes, by heating in DMF for 2–4 h, afforded moderate yields of benzimidazoles. The cyclocondensation reaction was further optimised under microwave conditions and then performed on commercially available aldehydes 9a-j to assess the generality of the optimised reaction conditions. We found that this microwave-irradiated cyclocondensation reactions proceeded in 2–3.5 min and afforded excellent yields (85–96%) of 2-arylbenzimidazoles as shown in .
Table 1. Cyclocondensation reaction of aldehydes 9a-j under microwave conditions and analytical data of the benzimidazole products 10a-e and 11a-e.
Pharmacology
The newly synthesised compounds 10a-e and 11a-e were evaluated in in vitro antiproliferative (MTS assay) studies against two human breast cancer cell lines MDA-MB-231 and MCF-7. The inhibitory activities are summarised in . Several compounds were found to exhibit moderate antiproliferative activity towards MDA-MB-231 cell lines. The unsubstituted 2-phenylbenzimidazole 10a emerged as the compound with most potent inhibition against MDA-MB-231 cell line (IC50 value of 29.7 µM) followed by 3-nitrophenyl 10d and 2,4-dichlorophenyl 11d with IC50 of 36.8 µM and 47.6 µM, respectively.
Table 2 Cytotoxicity of sec-/tert-butyl 2-arylbenzimidazoles against human breast cancer cell lines.
In the sec-butyl series, the replacement of the hydrogen on the para-position of 10a with an isosteric fluoro in 10b seems detrimental to its activity. Unlike the fluoro 10b, the 4-bromophenyl derivative 10c (IC50 of 81.5 µM) showed a 2.7-fold reduced activity compared to the unsubstituted 10a. In terms of substitution on the phenyl ring, the activity seen in 2,4-dichloro 11d however, was abolished in its ortho-substituted analogue 11c. Another ortho-substituted phenylbenzimidazoles 10e and 11e also showed no antiproliferative effects against both breast cancer cell lines. This suggests the unlikely role of the 2-substitution in the phenyl ring towards activity. All compounds showed no antiproliferative activity on MCF-7 cell lines (IC50 > 200 µM).
Various substituted benzimidazoles were previously reported to show potent antiproliferative activity on MCF-7 and other breast cancer cell lines; however with little or no selectivity.Citation11,Citation23,Citation33–38 In this study, even though these benzimidazole analogues showed moderate antiproliferative activity, their unexpected selectivity towards MDA-MB-231 is interesting and worth pursuing further. To date, such selectivity remains uncommon for benzimidazole derivatives.Citation22
Conclusions
The synthesis of a new series of N-sec/tert-butyl substituted benzimidazole derivatives was accomplished in excellent yields (85–96%) and short reaction time (2–3.5 min) under optimised microwave conditions. The results of the antiproliferative studies showed that these N-sec/tert-butyl-2-arylbenzimidazoles unexpectedly exhibited antiproliferative activity selective towards MDA-MB-231 breast cancer cell lines, but completely inactive towards MCF-7. The unsubstituted sec-butyl-2-phenylbenzimidazole 10a with IC50 of 29.7 µM is the most potent candidate of this series against MDA-MB-231 cancer cell line. Therefore it serves as the focus of our future studies in the quest for new chemotherapeutic agents with enhanced selectivity towards ER breast cancer.
Acknowledgements
This research was funded by Universiti Sains Malaysia (USM) Research University Grant 1001/PFARMASI/815026, Ministry of Higher Education (MoHE), under the FRGS grants: 203/PFARMASI/671156 and 671159, and Ministry of Science and Technology and Innovation (MOSTI) under the R&D initiative Grant No: 09-05-lfn-meb-004. We are grateful to Dr Mohd Nazri Ismail and Dr Michael Harvey at USM Doping Control Centre for helpful technical discussions and assistance in providing the HRMS service. We thank Ms Nurasyikin Hamzah for NMR and MS sample preparations. N.A. thanks USM for the award of postdoctoral research fellowship.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- World Health Organisation - Breast Cancer: Prevention and Control. Available at http://www.who.int/ cancer/detection/breastcancer/en/. Accessed 23 June 2012.
- Varmus H. The new era in cancer research. Science. 2006;312:1162–1165.
- Rosen ST. Cancer chemoprevention. Bergan RC, ed. New York: Academic Publisher; 2001:137–154.
- Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer 2007;7:46–53.
- Clarke R, Skaar TC, Bouker KB, Davis N, Lee YR, Welch JN et al. Molecular and pharmacological aspects of antiestrogen resistance. J Steroid Biochem Mol Biol 2001;76:71–84.
- Harrison MR, Holen KD, Liu G. Beyond taxanes: a review of novel agents that target mitotic tubulin and microtubules, kinases, and kinesins. Clin Adv Hematol Oncol 2009;7:54–64.
- Komlodi-Pasztor E, Sackett DL, Fojo AT. Inhibitors targeting mitosis: tales of how great drugs against a promising target were brought down by a flawed rationale. Clin Cancer Res 2012;18:51–63.
- Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer 2007;7:713–722.
- Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL et al. Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists. J Med Chem 1988;31:2235–2246.
- Nicolaou KC, Pfefferkorn JA, Roecker AJ, Cao GQ, Barluenga S, Mitchell HJ. Natural Product-like Combinatorial Libraries Based on Privileged Structures. 1. General Principles and Solid-Phase Synthesis of Benzopyrans. J Am Chem Soc 2000;122:9939–9953.
- Dettmann S, Szymanowitz K, Wellner A, Schiedel A, Müller CE, Gust R. 2-phenyl-1-[4-(2-piperidine-1-yl-ethoxy)benzyl]-1H-benzimidazoles as ligands for the estrogen receptor: synthesis and pharmacological evaluation. Bioorg Med Chem 2010;18:4905–4916.
- Bradshaw TD, Stevens MF, Westwell AD. The discovery of the potent and selective antitumour agent 2-(4-amino-3-methylphenyl)benzothiazole (DF 203) and related compounds. Curr Med Chem 2001;8:203–210.
- Shi DF, Bradshaw TD, Wrigley S, McCall CJ, Lelieveld P, Fichtner I et al. Antitumor benzothiazoles. 3. Synthesis of 2-(4-aminophenyl)benzothiazoles and evaluation of their activities against breast cancer cell lines in vitro and in vivo. J Med Chem 1996;39:3375–3384.
- Romero-Castro A, León-Rivera I, Avila-Rojas LC, Navarrete-Vázquez G, Nieto-Rodríguez A. Synthesis and preliminary evaluation of selected 2-aryl-5(6)-nitro- 1H-benzimidazole derivatives as potential anticancer agents. Arch Pharm Res 2011;34:181–189.
- Abonia R, Cortés E, Insuasty B, Quiroga J, Nogueras M, Cobo J. Synthesis of novel 1,2,5-trisubstituted benzimidazoles as potential antitumor agents. Eur J Med Chem 2011;46:4062–4070.
- Spasov A, Yozhitsa I, Bugaeva L, Anisimova V. Benzimidazole derivatives: Spectrum of pharmacological activity and toxicological properties (a review). Pharm Chem J 1999;33:232–243.
- Roth T, Morningstar ML, Boyer PL, Hughes SH, Buckheit RW Jr, Michejda CJ. Synthesis and biological activity of novel nonnucleoside inhibitors of HIV-1 reverse transcriptase. 2-Aryl-substituted benzimidazoles. J Med Chem 1997;40:4199–4207.
- Zhu Z, Lippa B, Drach JC, Townsend LB. Design, synthesis, and biological evaluation of tricyclic nucleosides (dimensional probes) as analogues of certain antiviral polyhalogenated benzimidazole ribonucleosides. J Med Chem 2000;43:2430–2437.
- Keurulainen L, Salin O, Siiskonen A, Kern JM, Alvesalo J, Kiuru P et al. Design and synthesis of 2-arylbenzimidazoles and evaluation of their inhibitory effect against Chlamydia pneumoniae. J Med Chem 2010;53:7664–7674.
- Kim JS, Gatto B, Yu C, Liu A, Liu LF, LaVoie EJ. Substituted 2,5’-Bi-1H-benzimidazoles: topoisomerase I inhibition and cytotoxicity. J Med Chem 1996;39:992–998.
- Kohara Y, Kubo K, Imamiya E, Wada T, Inada Y, Naka T. Synthesis and angiotensin II receptor antagonistic activities of benzimidazole derivatives bearing acidic heterocycles as novel tetrazole bioisosteres. J Med Chem 1996;39:5228–5235.
- Thimmegowda NR, Nanjunda Swamy S, Kumar CS, Kumar YC, Chandrappa S, Yip GW et al. Synthesis, characterization and evaluation of benzimidazole derivative and its precursors as inhibitors of MDA-MB-231 human breast cancer cell proliferation. Bioorg Med Chem Lett 2008;18:432–435.
- Kadri H, Matthews CS, Bradshaw TD, Stevens MF, Westwell AD. Synthesis and antitumour evaluation of novel 2-phenylbenzimidazoles. J Enzyme Inhib Med Chem 2008;23:641–647.
- Camacho J, Barazarte A, Gamboa N, Rodrigues J, Rojas R, Vaisberg A et al. Synthesis and biological evaluation of benzimidazole-5-carbohydrazide derivatives as antimalarial, cytotoxic and antitubercular agents. Bioorg Med Chem 2011;19:2023–2029.
- Starcevic K, Kralj M, Ester K, Sabol I, Grce M, Pavelic K et al. Synthesis, antiviral and antitumor activity of 2-substituted-5-amidino-benzimidazoles. Bioorg Med Chem 2007;15:4419–4426.
- Narendra Babu SN, Abdul Rahim AS, Osman H, Razak IA, Fun HK. Ethyl 4-fluoro-3-nitro-benzoate. Acta Crystallogr Sect E Struct Rep Online 2009;65:o556.
- Arumugam N, Abdul Rahim AS, Abd Hamid S, Rosli MM, Fun HK. Ethyl 3-amino-4-[(2-hydroxyethyl)-amino]benzoate. Acta Crystallogr Sect E Struct Rep Online 2010;66:o2141.
- Yet L. Progress in Heterocyclic Chemistry. Gribble GW, Joule JA, eds. Oxford: Elsevier; 2011:231–266.
- Wright JB. The Chemistry of the Benzimidazoles. Chem Rev. 1951;48:397–541.
- Gogoi P, Konwar D. An efficient and one-pot synthesis of imidazolines and benzimidazoles via anaerobic oxidation of carbon–nitrogen bonds in water. Tetrahedron Lett. 2006;47:79–82.
- Navarrete-Vázquez G, Moreno-Diaz H, Aguirre-Crespo F, León-Rivera I, Villalobos-Molina R, Muñoz-Muñiz O et al. Design, microwave-assisted synthesis, and spasmolytic activity of 2-(alkyloxyaryl)-1H-benzimidazole derivatives as constrained stilbene bioisosteres. Bioorg Med Chem Lett 2006;16:4169–4173.
- Lin S, Yang L. A simple and efficient procedure for the synthesis of benzimidazoles using air as the oxidant. Tetrahedron Lett 2005;46(25):4315–4319.
- Abonía R, Castillo J, Cuervo P, Insuasty B, Quiroga J, Ortà z A, et al. A Simple One-Pot Synthesis of New Imidazol-2-yl-1 H-quinolin-2-ones from the Direct Reaction of 2-Chloroquinolin-3-carbaldehyde with Aromatic o-Diamines. Eur J Org Chem 2010;2:317–325.
- Refaat HM. Synthesis and anticancer activity of some novel 2-substituted benzimidazole derivatives. Eur J Med Chem 2010;45:2949–2956.
- Ramla MM, Omar MA, El-Khamry AM, El-Diwani HI. Synthesis and antitumor activity of 1-substituted-2-methyl-5-nitrobenzimidazoles. Bioorg Med Chem 2006;14:7324–7332.
- Elzahabi HS. Synthesis, characterization of some benzazoles bearing pyridine moiety: search for novel anticancer agents. Eur J Med Chem 2011;46:4025–4034.
- Rashid M, Husain A, MishraR. Synthesis of benzimidazoles bearing oxadiazole nucleus as anticancer agents Eur. J. Med. Chem. 2012;1–38.
- Shaharyar M, Abdullah MM, Bakht MA, Majeed J. Pyrazoline bearing benzimidazoles: search for anticancer agent. Eur J Med Chem 2010;45:114–119.