Abstract
Carbonic anhydrases (CAs, EC 4.2.1.1) are inhibited by sulfonamides, inorganic anions, phenols, salicylic acid derivatives (acting as drug or prodrugs). A novel class of CA inhibitors (CAIs), interacting with the CA isozymes I and II (cytosolic) in a different manner, is reported here. Kinetic measurements allowed us to identify thiazolidin-based compounds as submicromolar-low micromolar inhibitors of these two CA isozymes. Molecular docking studies of a set of such inhibitors within CA I and II active site allowed us to understand the inhibition mechanism. This new class of inhibitors bind differently compared to other classes of inhibitors known to date: they were found between the phenol-binding site, filling thus the middle of the enzyme cavity.
Introduction
The carbonic anhydrases (CAs, EC. 4.2.1.1) represent a class of ubiquitous zinc-containing enzymes widespread in the all living organisms, which classically participate in the maintenance of pH homeostasis in mammalians, catalyzing the reversible hydration of CO2 in a two-step reaction to yield HCO3− and H+Citation1. At least sixteen CA isozymes have been described up to now in mammals, the most active ones as catalysts for carbon dioxide hydration being CA IICitation1–3. CA II is found primarily in red blood cells but also in many other secretory tissues of the kidney, lung, eye, etcCitation1–5. CA VI is a secretory isoform that was initially described in the ovine parotid gland, saliva, and normal human serum. Other CA isoforms are found in a variety of tissues where they participate in several important biological processes such as acid-base balance, respiration, carbon dioxide and ion transport, bone resorption, lipogenesis and electrolyte secretionCitation1–5.
Recently, our groups investigated the interaction of CA I and II isozymes with several types of phenols such as the simple phenol, hydroxy-/methoxysubstituted benzoic acids as well as di-/tri-methoxy benzenes, anti-oxidant bisphenols and several of its substituted derivatives, for example, salicyclates and some of their derivativesCitation6–12. In this study, we extend these earlier investigations to thiazolidines, a class of derivatives which have been reported to possess a wide range of biological activities including antibacterial, antitumor, and anti-inflammatory activityCitation13–16.
In the present study, we have purified human CA I and II from fresh blood and examined the in vitro inhibition effects of some thiazolidin derivative compounds mentioned above on these enzymes, using the esterase activity of hCA I and hCA II, with 4-nitrophenyl acetate as substrate.
Materials and methods
CNBr-activated sepharose 4B, protein assay reagents, p-aminobenzene sulfonamide, acetazolamide (AZA), 4-nitrophenylacetate (NPA) and chemicals for electrophoresis were purchased from Sigma-Aldrich Co, Munich, Germany. All other chemicals were of analytical grade and obtained from either Sigma or Merck.
Purification of carbonic anhydrase isozymes, protein determination and SDS polyacrylamide gel electrophoresis
Purification of hCA I and hCA II were performed using affinity chromatography as previously describedCitation17. Protein quantity was determined spectrophotometrically at 595 nm according to the Bradford method during the purification steps, using bovine serum albumin as the standardCitation18. SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli procedureCitation19.
CA inhibition
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25ºC using a spectrophotometer (Shimadzu UV-VIS) according to the method described by Verpoorte et al.Citation20. The enzymatic reaction, in a total volume of 3.0 mL, contained 1.4 mL 0.05 M Tris-SO4 buffer (pH 7.4), 1 mL 3 mM NPA, 0.5 mL H2O and 0.1 mL enzyme solution. A reference measurement was obtained by preparing the same cuvette without enzyme solution. The inhibitory effects of compounds 1–6b were examined. All compounds were tested in triplicate at each concentration used. Different inhibitor concentrations were used. Control cuvette activity in the absence of inhibitor was taken as 100%. For each inhibitor an activity%- [inhibitor] graph was drawn. To determine KI values, three different inhibitor concentrations were tested. In these experiments, NPA was used as substrate at five different concentrations (0.15–0.75 mM). KI-s were obtained from IC50 by the Cheng–Prussoff equationCitation21,Citation22.
Molecular docking
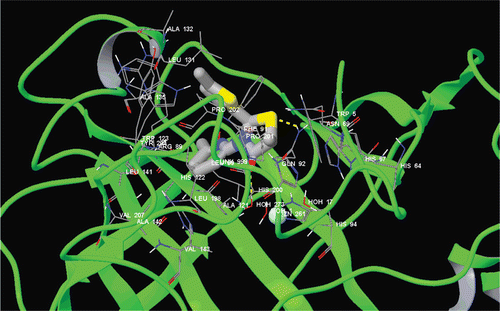
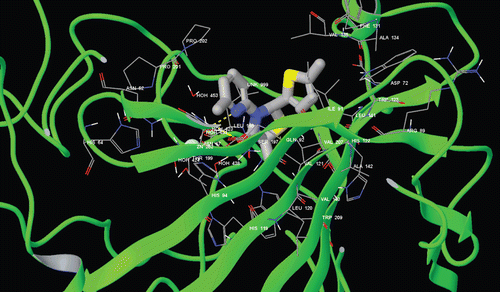
Glide/induced fit docking (IFD) module of Schrodinger molecular modeling package has been used for docking studies ( and ). Extra precision (XP) option is usedCitation23–25.
Results and discussion
There are many studies in the literature on the interactions of different compounds and CAs. The interaction of 1-tosyl pyrrol-2-one derivatives, phenolic and metoxy/bromo phenolic compounds with two human isozymes, CA I–II, has recently been investigatedCitation5,Citation6,Citation26, evidencing several inhibitors. Indeed, the inhibition profile of various isozymes with this class of agents is very variable, with inhibition constants ranging from the millimolar to the sub-micromolar level for many simple pyrrol-2-one-containing moleculesCitation5,Citation6.
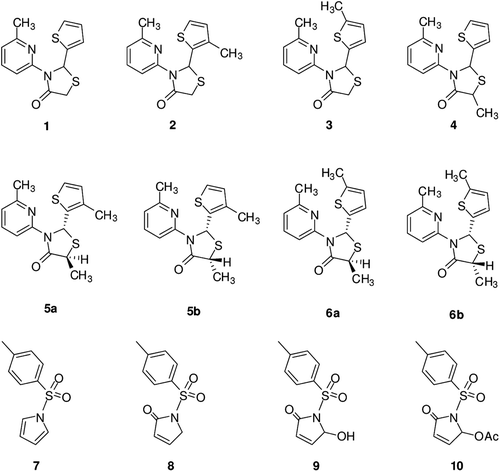
We report here a study on the inhibitory effects of thiazolidin-based compounds of type 1–6b on the esterase activity of hCA I and II. The sulfonamide CAI AZA has been used as controls in our experiments, and for comparison reasons (). Data of show the following regarding inhibition of hCA I and II with compounds 1–6b, by an esterase assay, with 4-NPA as substrate:
Table 1. hCA I and II inhibition data (KI) with compounds 1–10, ZNA and AZA, by an esterase assay with 4-nitrophenylacetate as substrate.
(i) Against the slow cytosolic isozyme hCA I, compounds 7 and 2 behave as weak inhibitors, with KI values of 42.4 μM and 47.5 μM, respectively. Thus, compound 2 was an ineffective hCA I inhibitor. A second group of derivatives compounds 5a, 5b, 8-10 and ZNA showed better inhibitory activity as compared to the previously mentioned compounds, with KI values of 14.6–37.3 µM, (). It is also interesting to note that thiazolidin-4-one derivatives 1, 3, 6a and 6b were better hCA I inhibitors compared to the other compounds investigated here. AZA and ZNA are also medium inhibitors with this assay and substrate against hCA I (KI-s of 14.8 and 36.2 µM, respectively). Kinetic investigations (Lineweaver Burk plots, data not shown) indicate that similarly to phenolic compounds, sulfonamides and inorganic anionsCitation17,Citation26–35, all the investigated compounds act as non-competitive inhibitors with 4-NPA as substrate, i.e. they bind in different regions of the active site cavity as compared to the substrate. However, the binding site of 4-NPA itself is unknown, but it is presumed to be in the same region as that of CO2, the physiological substrate of this enzymeCitation31.
(ii) All compounds had better inhibitory activity against the rapid cytosolic isozyme hCA II (). Compounds 7 and 8 were weak hCA II inhibitors, with KI-s of 37.5 and 23.1 µM, respectively (). The best hCA II inhibitor in this series of derivatives was the bulky 6b (), which with a KI of 0.81 µM, is similar inhibitor ZNA and AZA, a clinically used sulfonamide. It must be stressed that KI-s measured with the esterase method are most of the time in the micromolar range because hCA I and II are weak esterasesCitation26–34.
Although various carbonic anhydrase inhibitors have been identified up to now, it is critically important to explore further classes of potent CAIs in order to detect compounds with a different inhibition profile to find novel applications for the inhibitors of these widespread enzymesCitation35–40.
In silico studies
In this study, to better understand the binding mechanisms of studied molecules, fully flexible docking methodology for both receptor residues at the active site and docked ligands was used. Docking studies are performed using Glide XP-IFD algorithm, which was implemented with the Prime module under Schrodinger molecular modeling packageCitation5,Citation12,Citation23–25. The compounds 1–6b and ZNA were docked at the binding site of the targets (hCA I and hCA II). Glide/IFD docking scores of docked inhibitors at hCA I and II targets and corresponding binding interactions were tabulated in .
Table 2. Molecular docking binding scores of compounds 1–6b and ZNA within the hCA I and II active site.
Conclusions
Compound 6b, ((2S,5R)-5-methyl-3-(6-methylpyridin-2-yl)-2-(5-methylthiophen-2-yl)thiazolidin-4-one) influences the activity of hCA I and II isozymes due to the functional groups although similar structures showed weaker action. Compound 6a ((2S,5S)-5-methyl-3-(6-methylpyridin-2-yl)-2-(5-methylthiophen-2-yl)thiazolidin-4-one) shows relatively lower action although it has the same structure except for the configuration of one methyl group. Thus, the nature of the substituents strongly influences the inhibitory potency of these molecules. Our findings indicate thus another class of possible CAIs of interest, in addition to the well-known sulfonamides/sulfamates, the phenols/bromophenol/diphenols bearing bulky ortho moieties in their molecules. Some of the compounds investigated here showed effective CA inhibitory activity, in the low-micromolar range, by the esterase method which usually gives KI-s an order of magnitude higher as compared to the CO2 hydratase assayCitation31. Probably the inhibition mechanism of these compounds is distinct of the sulfonamides with RSO2NH2 groups and similar to that of the 1-tosyl pyrrol-2-one derivatives binding to a distinct part of the active site than that where sulfonamides bind. These findings point out that substituted thiazolidin-4-one derivatives may be used as leads for generating potent CAIs eventually targeting other isoforms which have not been assayed yet for their interactions with such agents.
Declaration of interest
The authors report no conflicts of interest.
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–181.
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–4350.
- Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, Innocenti A et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem 2008;283:27799–27809.
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229.
- Durdagi S, Sentürk M, Ekinci D, Balaydin HT, Göksu S, Küfrevioglu ÖI et al. Kinetic and docking studies of phenol-based inhibitors of carbonic anhydrase isoforms I, II, IX and XII evidence a new binding mode within the enzyme active site. Bioorg Med Chem 2011;19:1381–1389.
- Alp C, Ekinci D, Gültekin MS, Sentürk M, Sahin E, Küfrevioglu OI. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis of carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–4474.
- Ekinci D, Cavdar H, Talaz O, Sentürk M, Supuran CT. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–3563.
- Ceyhun SB, Sentürk M, Yerlikaya E, Erdogan O, Küfrevioglu OI, Ekinci D. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74.
- Sentürk M, Ekinci D, Göksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2012;27:365–369.
- Ekinci D, Cavdar H, Durdagi S, Talaz O, Sentürk M, Supuran CT. Structure-activity relationships for the interaction of 5,10-dihydroindeno[1,2-b]indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem 2012;49:68–73.
- Cavdar H, Talaz O, Ekinci D. Synthesis of novel mono and bis-indoleconduritol derivatives and their α/β-glycosidase inhibitory effects. Bioorg Med ChemLett 2012 (In Press).
- Balaydin HT, Durdagi S, Ekinci D, Sentürk M, Göksu S, Menzek A. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, methoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2012;27:467–475.
- Andres CJ, Bronson JJ, D’Andrea SV, Deshpande MS, Falk PJ, Grant-Young KA et al. 4-Thiazolidinones: novel inhibitors of the bacterial enzyme MurB. Bioorg Med Chem Lett 2000;10:715–717.
- Grasso S, Chimirri A, Monforte P, Fenech G, Zappalà M, Monforte AM. Compounds with potential antitumor activity. VI–2-Alkyl-3-[2-(1,3,4-thiadiazolyl)]-4-thiazolidinones. Farmaco Sci 1988;43:851–856.
- Look GC, Schullek JR, Holmes CP, Chinn JP, Gordon EM, Gallop MA. The identification of cyclooxygenase-1 inhibitors from 4-thiazolidinone combinatorial libraries. Bioorg Med Chem Lett 1996;6:707–712.
- Fidan I, Kazaz C, Sahin E, Kaban S. Synthesis and spectral investigation of some methyl-substituted 3-(2-pyridyl)-2-(2-thienyl)thiazolidin-4-one derivatives. J Chem Res 2010;5:296–300.
- Ekinci D, Ceyhun SB, Sentürk M, Erdem D, Küfrevioglu OI, Supuran CT. Characterization and anions inhibition studies of an a-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–748.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–254.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–685.
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–4229.
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934;56:658–666.
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 1973;22:3099–3108.
- Schrodinger Suite, Schrodinger, LLC, New York, USA. 2007. (web page: www.schrodinger.com). Accessed on 10/7/2012.
- Sherman W, Day T, Jacobson MP, Friesner RA, Farid R. Novel procedure for modeling ligand/receptor induced fit effects. J Med Chem 2006;49:534–553.
- Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA et al. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J Med Chem 2006;49:6177–6196.
- Talaz O, Cavdar H, Azak H, Durdagi S, Ekinci D. Synthesis of 1,4-bis(indolin-1-ylmethyl) benzene derivatives and their structure-activity relationships for the interaction of human carbonic anhydrase isoforms I and II. Bioorg Med Chem 2012, DOI: 10.1016/j.bmc.2012.09.027.
- Demirdag R, Comakli V, Senturk M, Ekinci D, IrfanKüfrevioglu O, Supuran CT. Purification and characterization of carbonic anhydrase from sheep kidney and effects of sulfonamides on enzyme activity. Bioorg Med Chem 2012, DOI: 10.1016/j.bmc.2012.08.018.
- Alp C, Maresca A, Alp NA, Gültekin MS, Ekinci D, Scozzafava A et al. Secondary/tertiary benzenesulfonamides with inhibitory action against the cytosolic human carbonic anhydrase isoforms I and II. J Enzyme Inhib Med Chem 2012, DOI: 10.3109/14756366.2012.658788.
- Cavdar H, Ekinci D, Talaz O, Saraçoglu N, Sentürk M, Supuran CT. a-Carbonic anhydrases are sulfatases with cyclic diol monosulfate esters. J Enzyme Inhib Med Chem 2012;27:148–154.
- Balaydin HT, Sentürk M, Menzek A. Synthesis and carbonic anhydrase inhibitory properties of novel cyclohexanonyl bromophenol derivatives. Bioorg Med Chem Lett 2012;22:1352–1357.
- Nair SK, Ludwig PA, Christianson DW. Two-site binding of phenol in the active site of human carbonic anhydrase II: structural implications for substrate association. J Am Chem Soc 1994;116:3659–3660.
- Ekinci D, Sentürk M, Küfrevioglu ÖI. Salicylic acid derivatives: synthesis, features and usage as therapeutic tools. Expert Opin Ther Pat 2011;21:1831–1841.
- Ekinci D, Sentürk M, Beydemir S, Küfrevioglu OI, Supuran CT. An alternative purification method for human serum paraoxonase 1 and its interactions with sulfonamides. Chem Biol Drug Des 2010;76:552–558.
- Innocenti A, Casini A, Alcaro MC, Papini AM, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: the first on-resin screening of a 4-sulfamoylphenylthiourea library. J Med Chem 2004;47:5224–5229.
- Balaydin HT, Durdagı S, Ekinci D, Senturk M, Goksu S, Menzek A. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, metoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2012, doi:10.3109/14756366.2011.596836.
- Ekinci D, Al-Rashida M, Abbas G, Sentürk M, Supuran CT. Chromone containing sulfonamides as potent carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:744–747.
- Ekinci D, Kurbanoglu NI, Salamci E, Sentürk M, Supuran CT. Carbonic anhydrase inhibitors: inhibition of human and bovine isoenzymes by benzenesulphonamides, cyclitols and phenolic compounds. J Enzyme Inhib Med Chem 2011, DOI: 10.3109/14756366.2011.621122.
- Ozdemir ZO, Sentürk M, Ekinci D. Inhibition of mammalian carbonic anhydrase isoforms I, II and VI with thiamine and thiamine-like molecules. J Enzyme Inhib Med Chem 2011, DOI: 10.3109/14756366.2011.637200.
- Ekinci D, Karagoz L, Ekinci D, Senturk M, Supuran CT. Carbonic anhydrase inhibitors: in vitro inhibition of a isoforms (hCA I, hCA II, bCA III, hCA IV) by flavonoids. J Enzyme Inhib Med Chem 2011, DOI: 10.3109/14756366.2011.643303.
- Koz O, Ekinci D, Perrone A, Piacente S, Alankus-Caliskan O, Bedir E, Supuran CT. Analysis of saponins and phenolic compounds as inhibitors of α-carbonic anhydrase isoenzymes. J Enzyme Inhib Med Chem 2012, doi:10.3109/14756366.2011.651464.