Abstract
An efficient and simple microwave assisted synthesis of sulfonamide derivatives incorporating the pyridazine moiety has been developed. These sulfonamides were used for the preparation of new heterocyclic compounds via reaction with different reagents using a microwave irradiation technique. The structures of the newly synthesized compounds were confirmed on the basis of FTIR, 1H and 13C-NMR, mass spectral techniques and elemental analyses. Some of the new synthesized compounds were assayed for their in vitro antibacterial activity against Gram-positive bacteria, Staphylococcus aureus and Staphylococcus epidermidis, Gram-negative bacteria, Escherichia coli and Klebsiella pneumonia and antifungal activity against Aspergillus fumigatus and Candida albicans. Most of the new compounds showed significant antibacterial and antifungal activity.
Introduction
Pyridazines are an important class of heterocycles, that are known for a wide range of biological activitiesCitation1–6 and have been the subject of extensive researchCitation7. The sulfonamides are one of the least expensive drug series to prepare and this factor largely accounts for their extensive use that spans a host of biological properties including antibacterialCitation8, anticarbonic anhydraseCitation9,Citation10, diureticCitation9,Citation11, hypoglycemicCitation12, antithyroidCitation13 and antiproton activitiesCitation14–16.
In addition, they are used in urinary tract infections, meningitis, streptococcal, pharyngites, bacillary, dysentery, trachoma, cancroids, malaria, toxoplasmosis, nocardiases and conjunctivitisCitation17,Citation18.
A large number of sulfonamide derivatives have recently been reported to show substantial antitumor activity, both in vitro and/or in vivo. Some of these derivatives are currently being evaluated in clinical trials, and there is much optimism that they may lead to novel alternative anticancer drugs, devoid of the side effects of the presently available pharmacological agentsCitation19.
Some of biologically active sulfonamide drugs are shown in .
A heterocyclic nucleus is present as the core structural component in an array of drug categories such as antimicrobial, anti-inflammatory, analgesic, antiepileptic, antiviral, antineoplastic, antihypertensive antimalarial, antidepressant, antihistaminic, antioxidant, antitubercular, antidiabetic, antiobesity and immunomodulatory agentsCitation20.
In continuation of our research program toward the synthesis of biological active heterocyclic based on the pyridazine moietyCitation7 and sulfonamidesCitation21,Citation22, we have attempted to increase the microbial activity of the pyridazine component by introducing the sulfonamide moiety in the pyridazine skeleton.
Results and discussion
Chemistry
As depicted in , we have been exploring the reactivity of pyridazine compounds toward constructing sulfonyl chloride derivatives. An attempted reaction of 4-(2-methoxybenzyl)-6-arylpyridazin-3(2H)-thione with thionyl chloride and hydrogen peroxide in presence of acetonitrileCitation23 in a simple reaction resulted in products which did not show any band around 1100 cm−1 in the IR spectra. Instead, a band around 1300 cm−1 characteristic of SO2 was found, indicating the formation of 4-(2-methoxybenzyl)-6-arylpyridazin-3-sulfonylchloride (1a,b*18).
This spectral observation led us to think in terms of a possible nucleophilic attack with different amines, involving the thermodynamically favored amide bond formation leading to sulfonamide derivatives (2a–p) and . These structures were established by IR, 1H-NMR, 13C-NMR and elemental analysis.
Table 1. Sulfonamide derivatives (2a-p).
The 1H-NMR spectrum of compound (2a) shows the following: δ 8.79 (s, 2H, NH2), 7.52–7.02 (m, 4H, Ar-H), 6.66 (s, 1H, CH, hetero), 3.80 (s, 2H, CH2), 3.73 (s, 3H, OCH3 of CH3O-Ar) and 2.36 (s, 3H, CH3) ppm. 13C-NMR spectrum of compound (2a) shows the following: δ 162.12, 160.51, 158.75, 132.70, 130.11, 127.16, 126.89, 122.73, 121.01, 114.23, 56.27, 28.38 and 25.19.
To the best of our knowledge, this is the expected elimination of HCl (a reaction similar to our earlier work on other nitrogen nucleophilesCitation24. A reaction of 4-(2-methoxybenzyl)-6-arylpyridazin-3-sulfonamides (2a,b) with 3-chloro-4-(2-methoxybenzyl)-6-phenylpyridazine under microwave irradiation resulted in compounds (3a,b) . The 1H-NMR spectrum of compound (3a) shows the following: δ 8.35 (s, 1H, NH), 7.83–7.05 (m, 13H, 3Ar-H), 6.92–6.85 (s, 2H, 2CH, hetero), 4.21–3.99 (s, 4H, 2CH2), 3.75–3.52 (s, 6H, 2OCH3 of 2CH3O-Ar) and 2.52 (s, 3H, CH3) ppm.
Scheme 2. (i) 3-Chloropyridazine derivatives/acetic acid/M.W. (ii) Benzaldehyde/acetic acid/M.W. (iii) Acetic acid derivatives/M.W. (iv) Thiosemicarbazide/acetic acid/M.W. (v) Succinic anhydride/toluene/M.W.
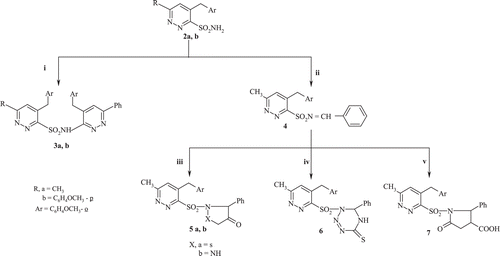
It has been reported that the formation of Schiff bases via reaction between amino group of sulfonamides and benzaldehyde must be carried out in presences of catalystsCitation25. In the present study, we report a simple and efficient method for the synthesis of the Schiff base (4), which has not hitherto been reported, via reaction of the amino group of the sulfonamide group with benzaldehyde. Thus microwave irradiation of a mixture of 4-(2-methoxybenzyl)-6-methylpyridazin-3-sulfonamide (2a) and benzaldehyde in presence of few drops of acetic acid yielded the corresponding Schiff base 4-(2-methoxybenzyl)-N-benzylidene-6-methylpyridazin-3-sulfonamide (4) in good yield .
The IR spectrum of compound (4) lacks any bands around 3134 cm−1 corresponding to the NH of NH2 group, which confirms Schiff base formation.
With a view to introducing heterocyclic moieties of expected biologically activity such as isothiazole, pyrazole, 1,2,3,5-tetrazine and pyrrolin-2-one we reacted a number of double nucleophiles such as thioglycolic acid, glycine, thiosemicarbazide and succinic anhydride with 4-(2-methoxybenyl)-N-benzylidene-6-methylpyridazin-3-sulfonamide (4) to give 2-[4-(2-methoxybenzyl)-6-methylpyridazin-3-yl)sulfonyl]-3-phenyl isothiazolidin-4-one (5a), 1-[4-(2-methoxybenzyl)-6-methylpyridazin-3-yl-sulfonyl]-5-phenylpyrazolidin-4-one (5b), 1-[4-(2-methoxy- benzyl)-6-methylpyridazin-3-yl-sulfonyl]-6-phenyl-5,6-dihydro-1,2,3,5-tetrazine-4(1H)-thione (6) and 1-[4-(2-methoxybenzyl)-6-methylpyridazin-3-yl-sulfonyl]-5-oxo-2-phenylpyrrolidin-3-carboxylic acid (7), respectively .
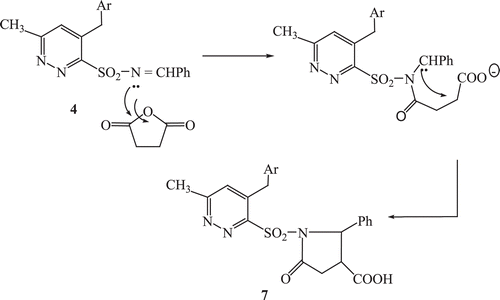
A probable mechanistic pathway for the formation of the pyrrolin-2-one derivative (7) is given in .
In the proposed mechanism the driving force for the nucleophilic attack, is the by ring opening followed by the ring closure leading to formation of the pyrrolin-2-one derivative (7).
Furthermore, nucleophilic attack of 4-(2-methoxybenzyl)-N-(2-hydroxyethyl)-6-(4-methoxy- phenyl) pyridazin-3-sulfonamide (2f) on carbon disulphide can lead to the generation of 3-[4-(2-methoxybenzyl)-6-(4-methoxyphenyl) pyridazin-3-yl-sulfonyl] oxazolidine-2-thione (8) . The 1H-NMR spectrum of compound (8) shows the following: 7.35–7.05 (m, 8H, 2Ar-H), 6.97 (s, 1H, CH hetero) 3.99 (s, 2H, CH2), 3.79 (t, 2H, CH2 hetero) 3.36(t, 2H, CH2 hetero) and 2.39–2.26(s, 6H, 2OCH3 of CH3O-Ar) ppm.
Biological evaluation (in vitro antimicrobial measurement)
The in vitro antimicrobial potency of some of the newly synthesized compounds was compared to the reference drugs Sulfamethoxazole and Fluconazol against Gram-negative Escherichia coli (ATCC-25922) and S. flexeniri, Gram-positive Staphylococcus aureus (ATCC-25923), Bacillus cereus, and fungi Aspergillus flavus and Candida albicans (ATCC 10231) strains.
The antibacterial activity of the synthesized compounds was tested using an agar well-diffusion methodCitation26. The agar-diffusion method was used for the determination of the preliminary antibacterial and antifungal activity. The results were recorded for each tested compound as the average diameter of inhibition zones (IZ) of bacterial or fungal growth around the disks in mm in . Compounds inhibiting the growth of one or more of the above microorganisms were further tested for their MIC and were determined by broth dilution techniqueCitation27. The MIC (mg/mL) and inhibition zone diameters values are recorded in . The inhibition zone diameters values cited in between brackets are attributed to the tested original concentration (1 mg/mL) as a preliminary test. The results depicted in revealed that most of tested compounds displayed variable inhibitory effects on the growth of the tested Gram-positive and Gram-negative bacterial strains, and also against fungal strains. A close investigation of the MIC values indicates that sulfonamide derivative 8j (MIC 2.5 µg/mL) against E. coli, was equipotent with the reference and antifungal C. albicans, also depicted (MIC 5 µg/mL).
Table 2. Minimal inhibitory concentrations (MIC, μg/mL) and inhibition zones (mm) of the new synthesized compounds.
The results of the MIC studies are furnished in . Among the screened samples, the data showed the following:
Antimicrobial activity
Compounds 2(b-j,l,m), 3(b), 4, (5a,b) and 7 were active against E. coli.
Compounds 2(b,d-j), 3(b), 4, 5(a,b) and 7 were active against Klebsiella pneumoniae
Compounds 2(b,e,g,k,l), 4, 5(a),7 and 8 were active against S. aureus.
Compounds 2(j,l), 5(a) and 7 were active against Staphylococcus epidermidis.
Antifungal activity
Compounds 2(b,f,g,j,l), 4, (5a,b) and 7 were active against C. albicans.
Compounds 2(f), 4, 5(a) and 7 were active against Aspergillus fumigatus.
Materials and methods
Chemistry
All melting points are uncorrected and were determined on a Gallenkamp instrument. Infrared spectra were measured on Perkin-Elmer spectrophotometer model 1430 using potassium bromide pellets and frequencies are reported in cm−1. The 1H-NMR and 13C-NMR were measured on Varian Genini-300 MHz spectrometer and chemical shifts (δ) are in ppm. The mass spectral (m/z) values were measured on an HP model GC MS-QPL000EX (Shimadzu) at 70-eV mass spectrometer. Elemental analyses were carried out at the Microanalytical Centre, Cairo University. Antimicrobial activity evaluations were carried out at the Basic Science Department, Faculty of Applied Medical Science, October 6th University, October City, Egypt. An Explorer Automated Microwave Synthesis Workstation (CEM) was used for synthesis of the new compounds.
Preparation of 4-(2-methoxybenzyl)-6-methylpyridazin-3-sulfonylchlorides (1a,b*)
A mixture of 4-(2-methoxybenzyl)-6-methylpyridazin-3-thione (0.1 mole) and thionyl chloride (0.1 mole), hydrogen peroxide (0.3 mole) in acetonitrile was stirred for 1 h. The reaction mixture was filtered off and crystallized from benzene to give the corresponding 1a.
4-(2-Methoxybenzyl)-6-methylpyridazin-3-sulfonylchloride 1a
Off-white solid, yield 80%, mp 100°C; IR (KBr pellet): 1578 for (C=N) and 1378 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz): δ 7.35–6.95 (m, 4H, Ar-H), 6.64 (s, 1H, CH hetero), 4.10 (s, 2H, CH2), 3.99 (s, 3H, OCH3 of CH3O-Ar) and 2.54 (s, 3H, CH3) ppm, 13C-NMR (DMSO, 300 MHz) δ: 179.57, 158.17, 151.30, 151.19, 131.76, 129.34, 126.59, 125.84, 121.41,111.97, 56.26, 34.49, 21.27. Anal. C13H13ClN2O3S (312.70); calcd: C, 49.93; H, 4.19; Cl, 11.34; N, 8.96; S, 10.23; found: C, 50.32; H, 3.99; Cl, 11.65; N, 9.21; S, 10.57 . MS: m/z 312 (M+).
General procedure for preparation of 4-(2-methoxybenzyl)-6-arylpyridazin-3-sulfonamides (2a-p)
A mixture of 4-(2-methoxybenzyl)-6-arylpyridazin-3-sulfonylchloride (1a,b18) (0.01 mole) and appropriate amines (0.01 mole) in 10 mL of pyridine was refluxed for 3 h. The reaction mixtures were washed with dilute HCl to remove excess of pyridine, filtered off and dried. The solid products were crystallized from benzene to give (2a-p).
4-(2-Methoxybenzyl)-6-methylpyridazin-3-sulfonamide 2a
Yellow crystals, yield 78%; 229–230°C; IR(KBr pellet): 3134 for (NH of NH2), 1598 for (C=N) and 1375 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz): δ 8.79 (s, 2H, NH2), 7.52–7.02 (m, 4H, Ar-H), 6.66 (s, 1H, CH hetero), 3.8 (s, 2H, CH2), 3.73 (s, 3H, OCH3 of CH3O-Ar) and 2.36 (s, 3H, CH3) ppm 13C-NMR (DMSO, 300 MHz) δ: 162.12, 160.51, 158.75, 132.70, 130.11, 127.16, 126.89, 122.73, 121.01, 114.23, 56.27, 28.38, 25.19. Anal. C13H15N3O3S (293.28); calcd: C, 53.24; H, 5.16; N, 14.33; S, 10.91; found: C, 53.55; H, 4.98; N, 14.81; s, 11.03. MS: m/z 293 (M+).
4-(2-Methoxybenzyl)-6-(4-methoxyphenyl) pyridazin-3-sulfonamide 2b
Yellow crystals, yield 70%, mp 188–189°C; IR (KBr pellet): 3186 for (NH of NH2), 1601 for (C=N) and 1369 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz): δ 9.23 (s, 2H, NH2), 7.37–6.96 (m, 8H, 2Ar-H), 6.89 (s, 1H, CH hetero), 3.81 (s, 2H, CH2) and 3.73–3.70 (s, 6H, 2OCH3 of 2CH3O-Ar) ppm. Anal. C19H17N3O4S (385.37); calcd: C, 59.21; H, 4.97; N, 10.91; S, 8.30. found: C, 58.92; H, 4.65; N, 11.00; S, 8.72. MS: m/z 385 (M+).
4-(2-Methoxybenzyl)-6-(4-methyl)-N-methylpyridazin)-3-sulfonamide 2c
Golden yellow, crystals, yield 59%, mp 222–223°C; IR (KBr pellet): 3433 for (NH), 1598 for (C=N) and 1373 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 10.35 (quartet, 1H, NH of NHCH3), 7.25–7.02 (m, 4H, Ar-H), 6.86 (s, 1H, CH hetero), 3.98 (s, 2H, CH2), 3.82 (s, 3H, OCH3 of CH3O-Ar), 2.47 (d, 3H, CH3 of CH3NH-) and 2.30 (s, 3H, CH3) ppm. 13C-NMR (DMSO, 300 MHz) δ: 179.57, 158.18, 151.19, 131.76, 129.34, 126.59, 125.84, 121.41, 111.97, 56.26, 34.50, 21.29, 21.28. Anal. C14H17N3O3S (307.31); calcd: C, 54.71; H; 5.58; N, 13.68 found: C, 55.03; H, 5.32; N, 14.00. MS: m/z 307 (M+).
4-(2-Methoxybenzyl)-6-(4-methoxyphenyl)-N-methylpyridazin-3-sulfonamide 2d
Golden yellow crystals, yield 45%, mp 198–200°C; IR (KBr pellet): 3427 for (NH), 1605 for (C=N) and 1377 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 9.89 (q, 1H, NH of –NHCH3), 7.45–7.12 (m, 8H, 2Ar-H), 6.68 (s, 1H, CH hetero), 4.13 (s, 2H, CH2), 3.56 (s, 6H, 2OCH3 of 2CH3O-Ar) and 3.37 (d, 3H, CH3 of CH3NH-) ppm. Anal. C20H21N3O4S (399.40); calcd: N, 10.52; S, 8.01 found: N, 10.34, S, 8.42. MS: m/z 399 (M+−3).
4-(2-Methoxybenzyl)-N-(2-hydroxyethyl)-6-methylpyridazin-3-sulfonamide 2e
Yellow crystals, yield 75%, mp 218–220°C; IR (KBr pellet): 3426 for (OH), 3133 for (NH), 1598 for (C=N) and 1375 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 8.80 (t, 1H, NH), 8.23 (t, 1H, OH), 7.59–7.28 (m, 4H, Ar-H), 6.84 (s, 1H, CH hetero), 4.21 (s, 2H, CH2), 3.92 (quartet, 2H, CH2), 3.65 (s, 3H, OCH3 of CH3 O-Ar), 2.98 (quartet, 2H, CH2) and 2.52 (s, 3H, CH3) ppm; Anal. C15H19N3O4S (337.33), calcd: N, 12.46; S, 9.49; found: N, 12.75; S, 9.21. MS: m/z 337 (M++1).
4-(2-Methoxybenzyl)-N-(2-hydroxyethyl)-6-(4-methoxyphenyl) pyridazin-3-sulfonamide 2f
Yellow crystals, yield 72%, mp 162-164°C; IR (KBr pellet): 3423 for (OH), 3145 for (NH), 1605 for (C=N) and 1369 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 10.03 (t, 1H, NH), 9.32 (t, 1H, OH), 7.47–7.03 (m, 8H, 2Ar-H), 6.63 (s, 1H, CH hetero), 4.13(s, 2H, CH2), 3.82 (q, 2H, CH2), 3.73–3.50 (s, 6H, 2OCH3 of 2CH3O-Ar), and 2.84 (quartet, 2H, CH2) ppm. 13C-NMR (DMSO, 300 MHz) δ: 165.23, 160.92, 159.57, 132.92, 130.12, 128.25, 126.8, 122.83, 121.57, 116.51, 60.23, 56.20, 45.50, 34.31, 25.39. Anal. C21H23N3O5S (429.42); calcd: C; 58.73; H, 5.40; N, 9.79; S, 7.45; found: C, 59.00; H, 50.08; N; 10.12; S, 7.85. MS: m/z 429 (M+).
1-[4-(2-Methoxybenzyl)-(6-methylpyridazin-3-yl)sulfonyl] urea 2g
Yellow crystals, yield 60%, mp. 228–229°C; IR (KBr pellet): 3413 for (NH), 3133 for (NH of NH2), 1656 for (C=O), 1588 for (C=N) and 1378 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz): δ 12.57 (s, 1H, NH), 8.00 (s, 2H NH2), 7.28–6.91 (m, 4H, Ar-H), 6.61 (s, 1H, CH hetero), 3.96 (s, 2H, CH2), 3.74 (s, 3H, OCH3 of CH3O-Ar) and 2.49 (s, 3H, CH3) ppm. Anal. C14H16N4O4S (336.31); calcd: N, 16.66; S, 9.52; found N, 16.25; S, 9.72. MS: m/z 336 (M+ +1).
1-[4-(2-Methoxybenzyl)-6-(4-methoxyphenyl)pyridazin-3-yl-sulfonyl] urea 2h
Yellow crystals, yield 71%, mp. 178–179°C; IR (KBr pellet): 3425 for (NH), 3210 for (NH of NH2), 1662 for (C=O), 1603 for (C=N) and 1369 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 11.87 (s, 1H, NH), 8.32 (s, 2H, NH2), 7.58–7.10 (m, 8H, 2Ar-H), 6.82 (s, 1H, CH hetero), 4.11 (s, 2H, CH2) and 3.82–3.65 (s, 6H, 2OCH3 of 2CH3O-Ar) ppm. Anal. C20H20N4O5S (428.40); calcd: C, 56.07; H, 4.71; found C, 55.89; H, 4.42. MS: m/z 428 (M+).
4-(2-Methoxybenzyl)-N-(2,4-dimethylpyrimidin-5-yl)-6-methylpyridazin-3-sulfonamide 2i
Yellow crystals, yield 55%, mp 206–208°C; IR(KBr pellet): 3220 for (NH), 1605 for (C=N) and 1375 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 13.52 (s, 1H, NH), 7.39–7.12 (m, 4H, Ar-H), 6.91–6.81 (s, 2H, 2CH hetero), 3.81 (s, 2H, CH2), 3.73 (s, 3H, OCH3 of CH3O-Ar), and 2.50–2.35 (s, 9H, 3CH3) ppm. Anal. C19H21N5O3S (399.41); calcd: C, 57.13; H, 5.30; N, 17.54; found; C, 57.54; H, 5.11; N, 17.09. MS: m/z 399(M+).
4-(2-Methoxybenzyl)-N-(2,4-dimethylpyrimidin-5-yl)6-(4-methoxyphenyl)pyridazin-3-sulfonamide 2j
Yellow crystals, yield 60%, mp. 188–190°C; IR (KBr pellet): 3195 for (NH), 1599 for (C=N) and 1378 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 14.84 (s, 1H, NH), 7.68–7.03 (m, 8H, 2Ar-H), 6.95- 6.89 (s, 2H, CH hetero), 4.06 (s, 2H, CH2), 3.79–3.48 (s, 6H, 2OCH3 of 2CH3O-Ar) and 2.51–2.35 (s, 6H, 2CH3) ppm. Anal. C25N25N5O4S (491.50) calcd: C, 61.09; H, 5.13; N, 14.25; found; C, 60.54; H, 5.18; N, 14.09. MS: m/z 491 (M+ + 1).
4-(2-Methoxybenzyl)-6-methyl-N-(pyrimidin-4-yl) pyridazin-3-sulfonamide 2k
Golden yellow crystals, yield 65%, mp. 212–214°C; IR (KBr pellet): 3133 for (NH), 1598 for (C=N) and 1378 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 11.46 (s, 1H, NH), 7.31–7.02 (m, 4H, Ar-H), 6.94–6.91 (d, 2H, 2CH hetero), 6.85–6.80 (s, 2H, 2CH hetero), 4.01(s, 2H, CH2), 3.74 (s, 3H, OCH3 of CH3O-Ar) and 2.50 (s, 3H, CH3) ppm. Anal. C17H17N5O3S (371.36); calcd: N, 18.86; S, 8.62, found: N, 18.52; S, 9.51, MS: m/z 371 (M+ −2).
4-(2-Methoxybenzyl)-6-(4-methoxyphenyl)-N-(pyrimidin-4-yl) pyridazin-3-sulfonamide 2l
Yellow crystals, yield 63%, mp. 190–192°C; IR (KBr pellet): 3230 for (NH), 1610 for (C=N) and 1375 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 10.88 (s, 1H, NH), 7.57–7.06 (m, 8H, 2Ar-H), 6.92–6.89 (d, 2H, 2CH hetero), 6.82–6.63 (s, 2H, 2CH hetero), 4.05 (s, 2H, CH2) and 3.85–3.71 (s, 6H, 2OCH3 of 2CH3O-Ar) ppm. Anal. C23H21N5O4S (463.45); calcd: N, 15.11; S, 6.90; found: N, 14.97; S, 7.12. MS: m/z 463 (M+).
4-(2-Methoxybenzyl)-N-(1,5-dimethyl-3-oxo-2-phenylpyrazolidin-4-yl)-6-methyl- pyridazin-3-sulfonamide 2m
Orange crystals, yield 57%, mp. 210–212°C; IR (KBr pellet): 3133 for (NH), 1654 for (C=O), 1597 for (C=N) and 1378 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 8.92 (d, 1H, NH), 7.85–7.18 (m, 9H, 2Ar-H), 6.99–6.91 (d, 2H, 2CH hetero), 6.78 (s, 1H, CH hetero), 3.87 (s, 2H, CH2), 3.52 (s, 3H, CH3 of N-CH3), 3.40 (s, 3H, OCH3 of CH3O-Ar), 2.35 (s, 3H, CH3) and 2.01 (d, 3H, CH3) ppm. Anal. C24H27N5O4S (481.51); calcd: C, 59. 86; H, 5.65; N, 14.55; found: C, 60.02; H, 5.29, N, 14.32. MS: m/z 481 (M+).
4-(2-Methoxybenzyl)-N-(1,5-dimethyl-3-oxo-2-phenylpyrazolidin-4-yl)-6-(4-methoxyphenyl)pyridazin-3-sulfonamide 2n
Orange crystals, yield 49%, mp. 188–190°C; IR (KBr pellet): 3115 for (NH), 1634 for (C=O), 1604 for (C=N) and 1380 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 14.32 (d, 1H, NH), 8.94–6.98 (m, 13H, 3Ar-H), 6.91–6.85 (d, 2H, 2CH hetero), 6.66 (s, 1H, CH hetero), 3.96 (s, 2H, CH2), 3.76 (s, 3H, CH3 of N-CH3), 3.57 (s, 6H, 2OCH3 of 2CH3O-Ar) and 2.49 (d, 3H, CH3) ppm. 13C-NMR (DMSO, 300 MHz) δ: 179.94, 161.26, 160.95, 159.48, 156.62, 152.30, 148.38, 146.34, 141.89, 138.65, 130.78, 129.99, 128.16, 127.68, 126.75, 125.45, 124.81, 120.55, 114.62, 111.04, 55.38, 40.34, 39.78, 38.94, 34.47. Anal. C30H31N5O5S (573.60); calcd: C, 62.81; H, 5.45; N, 12.21; found: C, 62.45; H, 5.72; N, 12.41. MS: m/z 573 (M).
4-(2-Methoxybenzyl)-6-methyl-N-(thiazol-5-yl)pyridazin-3-sulfonamide 2o
Golden yellow crystals, yield 67%, mp 220–222°C; IR (KBr pellet): 3395 for (NH), 1594 for (C=N) and 1375 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 12.59 (s, 1H, NH), 7.59–7.22 (m, 4H, Ar-H), 7.10–6.82 (s, 3H, 3CH hetero), 4.11 (s, 2H, CH2), 3.69 (s, 3H, OCH3 of CH3O-Ar) and 2.48 (s, 3H, CH3) ppm. Anal. C16H16N9O3S2 (376.33); calcd: N, 14.89; S, 17.01; found: N, 15.12; S, 16.92. MS: m/z 376 (M).
N-[4-(2-Methoxybenzyl)-6-(4-methoxyphenyl)pyridazin-3-yl]thiazole-5-sulfonamide 2p
Pale yellow crystals, yield 68%, mp. 178–180°C; IR (KBr pellet): 3223 for (NH), 1603 for (C=N) and 1380 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 11.98 (s, 1H, NH), 7.86–7.13 (m, 8H, 2Ar-H), 7.08–6.81 (s, 3H, 3CH hetero), 3.98 (s, 2H, CH2) and 3.82–3.66 (s, 6H, 2OCH3 of 2CH3O-Ar) ppm. Anal. C22H20N4O4S2 (468.42); calcd: N, 11.96; S, 13.66; found: N, 12.13; S, 13.45. MS: m/z 468 (M+).
General procedure for preparation of 4-(2-methoxybenzyl)-N-[4-(2-methoxybenzyl)-6-phenylpyridazin-3-yl]-6-aryl-pyridazin-3-sulfonamide 3a,b
A mixture of 4-(2-methoxybenzyl)-6-arylpyridazin-3-sulfonamide (2a,b) (0.01 mole) and 3-chloropyridazine derivatives (0.01 mole) where dissolved in a few drops of acetic acid. The resulting mixture was irradiated by microwave at 200°C for 2 min. The solid product was collected and crystallized from benzene to give (3a,b).
4-(2-Methoxybenzyl)-N-[4-(2-methoxybenzyl)-6-phenylpyridazin-3-yl]-6-methyl-pyridazin-3-sulfonamide 3a
Yellow needles, yield 70%, mp. 232–233°C; IR (KBr pellet): 3133 for (NH), 1567 for (C=N) and 1406 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 8.35 (s, 1H, NH), 7.83–7.05 (m, 13H, 3Ar-H), 6.92–6.85 (s, 2H, 2CH hetero), 4.21–3.99 (s, 4H, 2CH2), 3.75–3.52 (s, 6H, 2OCH3 of 2CH3O-Ar) and 2.52 (s, 3H, CH3) ppm. Anal. C31H29N5O4S (567.59); calcd: C, 65.59; H, 5.15; N, 12.34; S, 5.64; found: C, 65.08; H, 5.23; N, 11.91; S, 5.45; MS: m/z 567 (M+).
4-(2-Methoxybenzyl)-N-(4-(2-methoxybenzyl)-6-phenylpyridazin-3-yl)-6-(methoxyphenyl) pyridazine-3-sulfonamide 3b
Yellow needles, yield 67%, decomposition starts 240°C, mp. 300 C) IR(KBr pellet): 3220 for (NH), 1602 for (C=N) and 1385 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 9.01 (s, 1H, NH), 8.11–6.99 (m, 17H, 4Ar-H), 6.89–6.78 (s, 2H, 2CH hetero), 4.01–3.88 (s, 4H, 2CH2) and 3.92–3.45 (s, 9H, 3OCH3 of 3CH3O-Ar) ppm. Anal. C37H33N5O5S (659.68); calcd: C, 67.36; H, 5.04; N, 10.62; S, 4.85; found: C, 67.08; H, 5.23; N, 11.01; S, 4.45; MS: m/z 659 (M+).
General procedure for preparation of 4-(2-methoxybenzyl)-N-benzylidene-6-methylpyridazin-3-sulfonamide 4
A mixture of 4-(2-methoxybenzyl)-6-methylpyridazin-3-sulfonamide (2a) (0.01 mole) and benzaldehyde (0.01 mole) were dissolved in a few drops of acetic acid. The resulting mixture was irradiated by microwave at 200°C for 2 min. The solid product was collected and crystallized from benzene to give (4).
Yellow solid, yield 78%, mp. 178–180°C; IR (KBr pellet): 1610 for (C=N) and 1370 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 8.35 (s, 1H, CH of -N=CH-), 7.83–7.05 (m, 9H, 2Ar-H), 6.92 (s, 1H, CH hetero), 4.21 (s, 2H, CH2), 3.75 (s, 3H, OCH3 of CH3O-Ar) and 2.52 (s, 3H, CH3) ppm. 13C-NMR (DMSO, 300 MHz) δ: 178.58, 157.19, 150.18, 130.75, 128.34, 125.60, 124.90, 120.43, 111.01, 55.32, 40.33, 40.05, 39.78, 39.50, 39.21, 38.94, 38.66, 33.26, 20.32. Anal. C20H19N3O3S (381.38); calcd: C, 62.98; H, 5.02; N, 11.02; found: C, 63.31; H, 5.42; N, 11.39; MS: m/z 381 (M+).
General procedure for preparation of 2-[4-(2-methoxybenzyl)-6-methylpyridazin-3-yl-sulfonyl]-3-phenylisothiazolidin-4-one 5a or 5-phenylpyrazolidin-4-one 5b
A mixture was formed from 4-(2-methoxybenzyl)-N-benzylidene-6-methylpyridazin-3-sulfonamide (4) (0.01 mole) and (0.01 mole) of the appropriate acetic acid derivatives. The resulting mixture was irradiated by microwave at 200 C for 2 min. The solid product was collected and crystallized from benzene to give (5a,b).
2-[4-(2-Methoxybenzyl)-6-methylpyridazin-3-yl-sulfonyl]-3-phenylisothiazolidin-4-one 5a
Reddish brown solid, yield 83%, mp. 214–216°C; IR (KBr pellet): 1755 for (C=O), 1598 for (C=N) and 1407 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 7.35–6.92 (m, 9H, 2Ar-H), 6.89 (s, 1H, CH hetero), 6.63(s, 1H, CH hetero), 3.93 (s, 2H, CH2), 3.77 (s, 2H, CH2 hetero), 3.55 (s, 3H, OCH3 of CH3O-Ar) and 2.50 (s, 3H, CH3) ppm. Anal. C22H21N3O4S2 (455.42); calcd: N, 9.23; S, 14.05; found: N, 9.52; S, 13.85. MS: m/z 455 (M+).
1-[4-(2-Methoxybenzyl)-6-methylpyridazin-3-yl-sulfonyl]-5-phenylpyrazolidin-4-one 5b
Dark brown solid, yield 59%, mp. 128–130°C, IR (KBr pellet): 3133 for (NH), 1653 for (C=O), 1596 for (C=N) and 1407 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 8.65 (t, 1H, NH), 7.86–7.08 (m, 9H, 2Ar-H), 6.95 (s, 1H, CH hetero), 4.62 (s, 1H, CH hetero), 3.91 (s, 2H, CH2), 3.72 (d, 2H, CH2), 3.51 (s, 3H, OCH3 of CH3O-Ar) and 2.49 (s, 3H, CH3) ppm. 13C-NMR (DMSO, 300 MHz) δ: 178.62, 157.20, 150.34, 150.17, 130.77, 128.34, 125.60, 124.91, 120.43, 110.99, 55.31, 40.34, 40.06, 39.78, 39.50, 39.23, 38.95, 38.67, 33.54, 20.31. Anal. C22H22N4O4S (438.44); calcd: C, 60.26; H, 5.06; N, 12.78; S, 7.30; found C, 60.56; H, 4.92; N, 13.03; S, 7.05. MS: m/z 438 (M+ −3).
General procedure for preparation of 1-[4-(2-methoxybenzyl)-6-methylpyridazin-3-yl-sulfonyl]-6-phenyl-5,6-dihydro-1,2,3,5-tetrazine-4(1H)-thione 6
A mixture of 4-(2-methoxybenzyl)-N-benzylidene-6-methylpyridazin-3-sulfonamide (4) (0.01 mole) and (0.01 mole) of thiosemicarbazide was dissolved in a few drops of acetic acid. The resulting mixture was irradiated by microwave at 200°C for 2 min. The solid product was collected and crystallized from benzene to give (6).
Brown solid, yield 45%, mp. 80–82°C; IR (KBr pellet): 3134 for (NH), 1598 for (C=N) and 1409 for (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 10.85 (s, 1H, NH), 8.52 (s, 1H, CH of -N=CH-), 8.02–7.10 (m, 9H, 2Ar-H), 6.89 (s, 1H, CH hetero), 3.99 (s, 2H, CH2), 3.81 (s, 3H, OCH3 of CH3O-Ar) and 2.39 (s, 3H, CH3) ppm. Anal. C21H20N6O3S2 (468.43); calcd: N, 17.95; S, 13.66; found: N, 18.09; S, 13.53; MS: m/z 468 (M+).
General procedure for preparation of 1-[4-(2-methoxybenzyl)-6-methylpyridazin-3-yl-sulfonyl] 5-oxo-2-phenyl pyrrolidin-3-carboxylic acid 7
Method A
A mixture of 4-(2-methoxybenzyl)-N-benzylidene-6-methylpyridazin-3-sulfonamide (4) (0.01 mole) and (0.01 mole) succinic anhydride was refluxed for 24 h in 20-mL toluene. The solid product was obtained after evaporation and crystallized from benzene to give (7).
Method B
A mixture of 4-(2-methoxybenzyl)-N-benzylidene-6-methylpyridazin-3-sulfonamide (4) (0.01 mole) and (0.01 mole) of succinic anhydride was dissolved in a few drops of toluene. The resulting mixture was irradiated by microwave at 200°C for 2 min. The solid product was collected and crystallized from benzene to give (7).
Golden yellow crystals, yield 52%, mp. 128–130°C. IR (KBr pellet): 3485 for (OH of −COOH), 1725 for (C=O), 1605 for (C=N) and 1385 for (SO2) cm−1. 1H-NMR (DMSO, 300 `MHz) δ: 12.34 (s, 1H, OH of −COOH), 7.81–7.02 (m, 9H, 2Ar-H), 6.99 (s, 1H, CH hetero), 6.52 (d, 1H, CH hetero), 5.81 (quartet, 1H, CH hetero), 5.01 (d, 2H, CH2 hetero), 4.03 (s, 2H, CH2), 3.82 (s, 3H, OCH3 of CH3O-Ar) and 2.50 (s, 3H, CH3) ppm. Anal. C24H23N3O6S (481.45); calcd: C, 59.87; H, 4.82; N, 8.73; S, 6.65; found: C, 60.12; H, 5.03; N, 8.59; S, 6.25. MS: m/z 481 (M+).
General procedure for preparation of 3-[4-(2-methoxybenzyl)-6-(4-methoxyphenyl)pyridazin-3-yl-sulfonyl]oxazolidine-2-thione 8
A mixture of 4-(2-methoxybenzyl)-N-(2-hydroxyethyl)-6-(4-methoxyphenyl) pyridazin-3-sulfonamide (2f) (0.01 mole) and (0.01 mole) of carbon disulfide were dissolved in a few drops of pyridine. The resulting mixture was irradiated by microwave at 200°C for 2 min. The solid product was collected and crystallized from benzene to give (8).
Greenish yellow solid, yield 50%, mp. 158–160°C; IR (KBr pellet): 1604 for (C=N) and (SO2) cm−1. 1H-NMR (DMSO, 300 MHz) δ: 7.35–7.05 (m, 8H, 2Ar-H), 6.97 (s, 1H, CH hetero) 3.99 (s, 2H, CH2), 3.79 (t, 2H, CH2 hetero) 3.36 (t, 2H, CH2 hetero) and 2.39–2.26 (s, 6H, 2OCH3 of CH3O-Ar) ppm. Anal. C22H21N3O5S2 (471.42); calcd: C, 56.05; H, 4.49; N, 8.92; S, 13.58; found: C, 56.45; H, 4.52; H, 4.52; N, 9.22; S, 14.00; MS: m/z 471 (M+).
Antimicrobial evaluation:
Most of the newly synthesized compounds in addition to the reference drugs Sulfamethoxazole and Fluconazol were screened for their antibacterial and antifungal activities. For antibacterial studies micro-organisms employed included Gram-negative E. coli and S. flexeniri, Gram-positive S. aureus and Bacillus cereus. For antifungal screening, Aspergillus flavus and Candida albicans were employed. Both microbial studies were assessed by minimum inhibitory concentration (MIC) using the agar well diffusion techniqueCitation26.
For the antibacterial assay, a standard inoculum (10.5 CFU/m) was distributed on the surface of sterile nutrient agar plates with a sterile glass spreader, whereas for the antifungal assay a loopful of a particular fungal strain was transferred to 3 mL of saline to give a suspension of the corresponding species. Then 0.1 mL of the spore suspension was distributed on the surface of sterile Sabouraud dextrose agar plates. Six mm diameter wells were punched in the agar media and filled with 100 µL (500 µg/mL in DMSO) of the test compounds previously sterilized through 0.45 sterile membrane filtersCitation28. The plates were kept at room temperature for 1 h and then incubated at 37°C for 24 h for bacteria and 30°C for 4 days for fungi.
The antimicrobial activities were evaluated by measuring the inhibition zone diameters (mm). Commercial antibiotic discs were used as positive reference standards to determine the sensitivity of the strains.
Determination of MIC of the synthesized compounds
Compounds inhibiting the growth of one or more of the above microorganisms were further tested for their MIC and were determined by broth dilution techniqueCitation27. The nutrient broth and the yeast extract broth media, which contained 1 mL of different concentrations of the tested compounds (2.5, 5, 10, 15, 20, 25 µg/mL) were inoculated with the microbial strains. The bacterial cultures were incubated for 24 h at 37°C, whereas the fungal cultures were incubated at 30°C for 48 h. The growth was monitored spectrophotometrically. The lowest concentration required to arrest the microbial growth was regarded as MICs (µg/mL).
Conclusion
In the present work, we report a convenient microwave-enhanced, high speed, short, and economic way for the synthesis of a new series of sulfonamide and sulfonamide derivatives incorporating the pyridazine moiety which is used as the key material for further transformations. We present our study illustrating pyridazine moiety reactivity towards some nucluphiles with the aim of preparing new antimicrobial and antifungal agents. All the newly tested compounds showed good antimicrobial and antifungal activity towards different strains.
Acknowledgement
The authors are indebted to Dr. Nashwa A. Ahmed, Basic Science Department, Faculty of Applied Medical Science, October City, Egypt for help with antimicrobial activity measurements. The authors alone are responsible for the content and writing of the article.
Declaration of Interest: The authors report no conflict of interest.
References
- Frank H, Heinisch G. Pharmacologically active pyridazines. In: Ellis GP, West GB (Eds). Progress in Medicinal Chemistry. Elsevier; Amsterdam, 1990, 1–49.
- Li CS, Brideau C, Chan CC, Savoie C, Cleaveau D, Charleson S, Gordon R, Greig G, Cauthier JY, Lau CK, Riendeau D, Therien M, Wong E, Prasit P. Pyridazinones as selective cyclooxygenase-2 inhibitors. Bioorg Med Chem Lett 2003;13:597–600.
- Giblin GMP, Bit RA, Brown SH, Chaignot HM, Chowdhury A, Chessel IP, Clayton NM, Coleman T, Hall A, Hammond B, Hurst DN, Michel A, Naylor A, Novelli R, Scocctti T, Spalding D, Tang SP, Wilson AW, Wilson R. The discovery of 6-[2-(5-chloro-2-{[(2,4-difluorophenyl)methyl]oxy}phenyl)-1-cyclopenten-1-yl]-2-pyridinecarboxylic acid, GW848687X, a potent and selective prostaglandin EP1 receptor antagonist for the treatment of inflammatory pain. Bioorg Med Chem Lett 2007;17:385–389.
- Dorsch D, Mederski WWKR, Osswald M, Devant RM, Schmitges C-JJS, Christadler M, Wilm C. Pyridazinones with a pendant acylsulfonamide moiety as endothelin receptor antagonists. Bioorg Med Chem 1997;7:275–280.
- Nomoto Y, Takai H, Ohno T, Nagashima K, Yao K, Yamada K, Kubo K, Ichimura M, Mihara A, Kase H. Studies of cardiotonic agents. 8. Synthesis and biological activities of optically active 6-(4-(benzylamino)-7-quinazolinyl)-4,5-dihydro-5-methyl-3(2H)-pyridazinone (KF15232). J Med Chem 1996;39:297–303.
- Barbaro R, Betti I, Botta M, Corelli F, Giannaccini G, Maccari I, Manetti F, Strappaghetti G, Corsano S. Synthesis, biological evaluation, and pharmacophore generation of new pyridazinone derivatives with affinity toward α(1)- and α(2)-adrenoceptors. J Med Chem 2001;44:2118–2132.
- Kandile NG, Mohamed MI, Zaky H, Mohamed HM. Novel pyridazine derivatives: Synthesis and antimicrobial activity evaluation. Eur J Med Chem 2009;44:1989–1996.
- Husain A, Ahmad A, Mujeeb M, Akhter M. New amides of sulphonamides: synthesis and biological evalution. Chil J Chem Soc 2010;55:74–77.
- Husain A. Amide derivatives of sulfonamides and isoniazid: synthesis and biological evaluation. Acta Pol Pharm 2009;66:513–521.
- Drews J. Drug discovery: a historical perspective. Science 2000;287:1960–1964.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors and their therapeutic potential. Exp Opin Ther Patents 2000;10:575–600.
- Supuran CT, Scozzafava A. Carbonic anhydrase inhibitors. Curr Med Chem-Immunol Endocr Metabol Agents 2001;1:61–97.
- Maren TH. Relatons between structure and biological activity of sulfonamides. Annu Rev Pharmacol Toxicol 1976;16:309–327.
- Boyd AE 3rd. Sulfonylurea receptors, ion channels, and fruit flies. Diabetes 1988;37:847–850.
- Thornber CW. Isosterism and molecular modification in drug design. Chem Soc Rev 1979;8:563–580.
- Ogden RC, Flexner CW. ( Eds.) Protease Inhibitors in AIDS Therapy. New York: Marcel Dekker, 2001.
- Scozzafava A, Mastrolorenzo A, Supuran CT. Agents that target cysteine residues of biomolecules and their therapeutic potential. Exp Opin Ther Patents 2001;11:765–787.
- Scozzafava A, Supuran CT. Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with MMP inhibitory properties act as efficient inhibitors of CA isozymes I, II, and IV, and N-hydroxysulfonamides inhibit both these zinc enzymes. J Med Chem 2000;43:3677–3687.
- Casini A, Scozzafava A, Mastrolorenzo A, Supuran LT. Sulfonamides and sulfonylated derivatives as anticancer agents. Curr Cancer Drug Targets 2002;2:55–75.
- Dua R, Shrivastava S, Sonwane SK, Srivastava SK. Pharmacological significance of synthetic heterocycles scaffold: a review. Advan Biol Res 2011;5:120–144.
- Zaky HT, Mohamed MI, Nail AM, Kandile NG. Synthesis of some novel biologically active sulphonamides. Egypt J Chem 2004;47:321–331.
- Mohamed MI. Synthesis and antimicrobial activity of new pyridazinyl sulfonamide derivatives. Bulg Chem Commun 2007;39:152–158.
- Bahrami K, Khodaei MM, Soheilizad M. Direct conversion of thiols to sulfonyl chlorides and sulfonamides. J Org Chem 2009;74:9287–9291.
- Mohamed MI, Zaky HT, Mohamed HM, Kandile NG. Novel heterocyclic systems of pyridazines. Afinidad 2005;62:48–56.
- Zolfigola MA, Khazaeia A, Moosavi-Zarea AR, Zareb A. 3-Methyl-1-sulfonic acid imidazolium chloride as a new, efficient and recyclable catalyst and solvent for the preparation of N-sulfonyl imines at room temperature. J Iran Chem Soc 2010;7:646–651.
- Ahmad S, Rathish IG, Bano S, Alam MS, Javed K. Synthesis and biological evaluation of some novel 6-aryl-2-(p-sulfamylphenyl)-4,5-dihydropyridazin-3(2H)-ones as anti-cancer, antimicrobial, and anti-inflammatory agents. J Enzyme Inhib Med Chem 2010;25:266–271.
- Tading H, Mohamed E, Asres K, Gebre T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol 2005;100:168–175.
- Karthikeyan SM, Prasad JD, Mahalinga M, Holla SB, Kumari SB. Antimicrobial studies of 2,4-dichloro-5-fluorophenyl containing oxadiazoles. Eur J Med Chem 2008;43:25–31.


![Scheme 4. Synthesis of 3-[4-(2-methoxybenzyl)-6-(4-methoxyphenyl)pyridazin-3-yl-sulfonyl] oxazolidine-2-thione (8).](/cms/asset/10500cc5-4070-4431-a6ca-dd5af68710f7/ienz_a_737787_f0005_b.gif)