Abstract
A novel series of carbazole substituted aminopyrimidines (5a-p) were synthesized and screened for their in vitro urease inhibition and antimicrobial activity. Among the compounds, 4-(2,4-dichlorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5i) was found to be the most potent showing urease inhibitory activity with an IC50 value 19.4 ± 0.43 µM. Compounds 5c, 5g, 5j and 5o showed good activity against all selected bacterial strains and compounds 5b, 5c, 5m and 5o showed good activity against selected fungal strains. All the compounds were subjected for ADME predictions by computational method.
Introduction
Urease inhibitors are considered as new targets for antiulcer drugsCitation1. The activity of bacterial urease has been shown to be important virulence factor in the development of many harmful clinical conditions for human and animal health as well as agricultureCitation2. Bacterial ureases have been reported to be involved in the formation of infectious stones and development of peptic ulcers and stomach cancerCitation3. Ureases also contribute to the development of urolithiasis, pyelonephritis, hepatic encephalopathy, hepatic coma, and urinary catheter encrustationCitation4. In the near past, a number of compounds have been proposed as urease inhibitors to reduce environmental problems and enhance the uptake of urea nitrogen by plantsCitation5–8. The treatment of infections caused by urease producing bacteria may also be possible by urease inhibition. In recent years, a variety of urease inhibitors have been studied, including acetohydroxamic acid, flurofamide, omeprazole, lansoprazole, rabaprazole, 1,4-benzoquinone and its derivatives, and inorganic metal salts, etcCitation9–11. Various heterocyclic compounds were reported as urease inhibitors by several research groups. Khan et al. have synthesized biscoumarins which were shown as urease inhibitorsCitation8. Akhtar et al. have synthesized 3-substituted-4-amino-5-thioxo-1H-4H-1,2,4-triazoles derivatives showing their urease inhibition activityCitation12. Rauf et al. have reported the synthesis and urease inhibition activity of barbituric and thiobarbituric acid derivativesCitation13. More recently, Khan et al. also reported arylidene barbiturates as urease inhibitorsCitation14. Amines derived from deoxybenzoins are also reported as urease inhibitorsCitation15.
The pyrimidine entity is one of the most prominent structures found in nucleic acid chemistry. Pyrimidine derivatives including uracil, thymine, cytosine, adenine, and guanine are fundamental building blocks for deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Vitamin B1 (thiamine) is a well-known example of a naturally occurring pyrimidine that is encountered in our daily livesCitation16,Citation17. Pyrimidine derivatives form a component in a number of useful drugs and are associated with many biological, pharmaceutical and therapeutical activitiesCitation18. Condensed pyrimidine derivatives have been reported as antimicrobialCitation19, analgesicCitation20, antiviral, anti-inflammatoryCitation21, anti-HIVCitation22, antitubercularCitation23, antitumourCitation24, antineoplasticCitation25, antimalarialCitation26, diureticCitation27 and antiplatelet agentsCitation28.
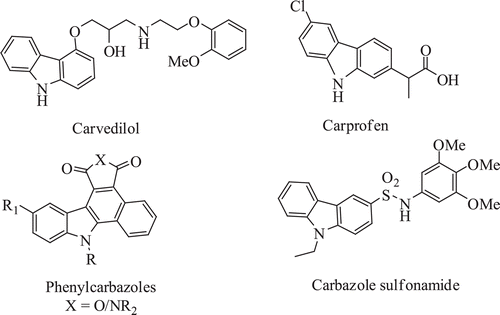
Like pyrimidines, carbazole compounds also exhibits diverse biological properties. Carbazole and its derivatives are the important class of heterocyclic compounds endowed with various pharmacological activities such as anticancer, antimicrobial, antiviral, anti-inflammatory and antioxidant activityCitation29. Carbazole scaffold is present in many drugs such as carvedilol and carprofen (). Carvedilol, an antihypertensive drug, acts as a nonspecific β-adrengic antagonistCitation30. Carprofen, a nonsteroidal anti-inflammatory drug (NSAID), is a selective COX-2 inhibitorCitation31. More recently, a series of phenylcarbazole () molecules have been reported as antitumour agentCitation32, carbazole sulphonamides () are a novel class of antimitotic agents against solid tumoursCitation33. Syutkin et al.Citation34 have reported carbazole containing chalcones and pyrimidines as light emitting diodes.
It was envisaged that these two active pharmacophores, if linked together would generate novel molecular templates which are likely to exhibit interesting biological properties. The above-cited applications pyrimidines and barbiturates prompted us to synthesize a series of carbazole containing pyrimidine derivatives. Owing to this importance and in continuation of our work on synthesis of biologically active compoundsCitation35–37, now we wish to describe the synthesis of carbazole containing aminopyrimidine derivatives (). The newly synthesized compounds were screened for their in-vitro urease inhibition and antimicrobial activity. We also subjected all synthesized compounds for ADME predictions by computational method.
Results and discussion
Chemistry
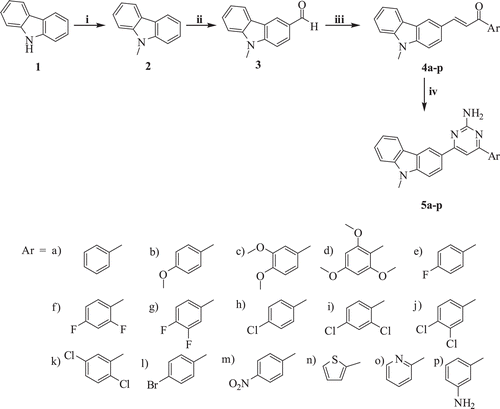
The target compounds were synthesized as shown in . Carbazole (1) on methylation with methyl iodide gave 9-methyl carbazole (2), which on Vilsmeier-Haack formylation gave 3-formyl-9-methylcarbazole (3). The 3-formyl-9-methylcarbazole (3) on Claisen-Schimdt condensation with various aromatic acetophenones and heteroaromatic acetophenones in aqueous sodium hydroxide afforded compounds 4a-pCitation37. This on further treatment with guanidine hydrochloride in presence of NaH in DMF gave the target compounds 5a-p. Compounds have been characterized by IR, 1H NMR and mass spectroscopy. In the 1H NMR spectra of these compounds, the pyrimidine ring proton was observed downfield as a singlet at δ ~7.80 and NH2 proton appears at δ ~6.65 as a singlet.
Biological evaluation
The urease inhibition activity was carried out according to the literature protocolCitation38 using thiourea as the standard inhibitor having an IC50 value of 21.0 ± 0.14 µM and the results are presented in . Out of 16 screened compounds, compounds 5c, 5f, 5g, 5i, 5j, and 5k were found to be active against urease. Compound 5i proved to be the most potent showing urease inhibitory activity with an IC50 value 19.4 ± 0.23 µM as compared to the standard thiourea. The compounds 5j (IC50 = 23.2 ± 0.58) and 5k (IC50 = 28.1 ± 0.59) exhibited good activity with an IC50 value as compared to that of standard thiourea. Compound 5c with 3,4-dimethoxy substituent was found to be good inhibitor with an IC50 value of 30.7 ± 0.94. Compounds 5f and 5g were displayed moderate urease inhibitory activities with an IC50 value 40.2 ± 0.31 and 45.2 ± 0.63, respectively. From these results of urease inhibitory activities by pyrimidine derivatives, following tentative results can be drawn; it appears that disubstituted halogen compounds were found to inhibit urease enzyme. Dichloro substituted compounds were showing promising urease inhibitory activity than difluoro and dimethoxy substituted compounds. Whereas, monosubstituted compound with electron donating and withdrawing substituent are weak inhibitors of urease. Heteroaryl substituted pyrimidine derivatives (compounds 5n and 5o) are also weak inhibitors of urease.
Table 1. Urease inhibition activity.
The synthesized pyrimidine derivatives were tested for their in vitro antimicrobial against five bacterial and two fungal species using disk diffusion method. Tetracycline and nystatin were used as standards against bacteria and fungi, respectively. The antibacterial activity against two Gram positive bacteria i.e. Bacillus subtilis (BS) and Staphylococcus aureus (SA), and three Gram negative bacteria, i.e. Escherichia coli (EC), Shigella flexenari (SF) and Salmonella typhi (ST) was tested at a sample concentration of 1 mg/mL in DMSO. The antibacterial activity data is tabulated in . The result indicated that among the tested compounds, compounds 5c, 5g, 5j and 5o showed good activity against all selected bacterial strains at concentration of 1 mg/mL as compared to standard drug tetracycline. Compound 5o showed comparable activity as that of standard, against B. subtilis (BS), S. aureus (SA) and S. flexenari (SF). The remaining compounds showed moderate activity against all of the tested bacterial strains except S. typhi (ST).
Table 2. Antimicrobial activity of pyrimidine derivatives.
The antifungal activity of pyrimidine derivatives against two fungal species, i.e. Candida albicans (CA) and Aspergillus niger (AN) was tested at a sample concentration of 1 mg/mL in DMSO using nystatin as standard. The antifungal activity data are tabulated in . The result indicated that among the tested compounds, compounds 5b, 5c, 5m and 5o showed good activity against selected fungal strains at concentration of 1 mg/mL as compared to standard drug nystatin. Compounds 5m and 5o showed comparable activity as that of standard, against C. albicans (CA) and A. niger (AN). The remaining compounds showed moderate activity against C. albicans (CA). While, all remaining compounds showed less activity against A. niger (AN).
The bioavailability of the target compounds was assessed using ADME (absorption, distribution, metabolism, and excretion) prediction methods. In particular, we calculated the compliance of compounds to the Lipinski’s rule of fiveCitation39,Citation40. Predictions of ADME properties for the studied compounds are shown in . The results showed that all the tested compounds comply with the rules. Compounds 5h, 5i, 5j, 5k and 5l have c Log P value more than 5 however, these compounds have molecular weight, hydrogen bond donor, hydrogen bond acceptor within the range of Lipinski rule of five and these compounds have low polar surface area (PSA) and molar volume (MV). Molecular polar surface area (PSA) is a very useful parameter for the prediction of drug transport properties. All the tested compounds have maximum absorption as they are having low PSA and MV. Drug-likeness score (a combined effect of physico-chemical properties, pharmacokinetics and pharmacodynamics of a compound and is represented by a numerical value) was computed by MolSoft (MolSoft 2007) software for the 16 molecules under study. Maximum drug-likeness score was found to be 0.85 for the compound 5b. Hence, these compounds do not pose absorption and permeation difficulty.
Table 3. In silico pharmacological parameters for bioavailability.
Conclusion
A new series of carbazole substituted pyrimidine derivatives were synthesized and screened for their in-vitro urease inhibition and antimicrobial activity. Compounds 5c, 5f, 5g, 5i, 5j, and 5k were found to be active against urease. Compound, 4-(2,4-dichlorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine 5i was found to be the most potent showing urease inhibitory activity with an IC50 value 19.4 ± 0.43 µM. Compounds 5c, 5g, 5j and 5o showed good activity against all selected bacterial strains and compounds 5b, 5c, 5m and 5o showed good activity against selected fungal strains. ADME prediction revealed that the compounds could be considered as good candidates for drug development.
Experimental section
General
All the reagents and solvents used were of analytical grade and were used as supplied unless otherwise stated. Melting points were recorded in open capillaries with electrical melting point apparatus and were uncorrected. IR spectra (KBr disks) were recorded using a Perkin–Elmer 237 spectrophotometer. 1H NMR (200 MHz) spectra were recorded on a Bruker Avance spectrometer using DMSO-d6 as solvent. Mass spectra were recorded on a Shimadzu LCMS-QP 1000 EX. Thin layer chromatography (TLC) was performed on silica gel-coated plates for monitoring the reactions.
General procedure for the synthesis of 4-(9-methyl-9H-carbazol-3-yl)-6-phenylpyrimidin-2-amine (5a-p)
Chalcones 4a-p (1.0 mmol) was dissolved in DMF (15 mL) and guanidine hydrochloride (2 mmol) was added to it. To this reaction mixture, NaH (4.0 mmol) was added and the reaction mixture was heated up to 110°C for 10 h. After completion of reaction (TLC), the reaction mixture was cooled to room temperature and water (15 mL) was added to it, solid product started to separate out. The solid was filtered and purified by column chromatography using chloroform as eluent to afford target compounds 5a-p.
Spectral data for synthesized compounds
Synthesis of 4-(9-methyl-9H-carbazol-3-yl)-6-phenylpyrimidin-2-amine (5a)
Yield: 90%; MP: 234–236°C; IR (KBr, cm−1): 3368, 3325, 3214, 3020, 1641, 1559, 1372, 1216, 1149; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.82 (s, 3H, NCH3), 6.66 (s, 2H, NH2), 7.23 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 4H, ArH), 7.61–7.73 (m, 2H, ArH), 7.80 (s, 1H, ArH), 8.23–8.28 (m, 3H, ArH), 8.40 (d, 1H, J = 8 Hz, ArH), 9.07 (s, 1H, ArH); MS: m/e 351 (M + 1).
4-(4-methoxyphenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5b)
Yield: 87%; MP: 210–212°C; IR (KBr, cm−1): 3368, 3320, 3214, 3019, 1641, 1559, 1372, 1220, 1149, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.80 (s, 3H, OCH3), 3.87 (s, 3H, NCH3), 6.66 (s, 2H, NH2), 6.90 (d, 2H, J = 8 Hz, ArH), 7.23 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 2H, ArH), 7.61–7.73 (m, 2H, ArH), 7.80 (s, 1H, ArH), 8.23–8.28 (m, 2H, ArH), 8.40 (d, 1H, J = 8 Hz, ArH), 9.07 (s, 1H, ArH); MS: m/e 381 (M + 1).
4-(3,4-dimethoxyphenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5c)
Yield: 86%; MP: 218–221°C; IR (KBr, cm−1): 3368, 3320, 3214, 3019, 1641, 1559, 1372, 1220, 1149, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.82 (s, 3H, OCH3), 3.87 (s, 3H, OCH3), 4.00 (s, 3H, NCH3), 7.26 (d, 1H, J = 8 Hz, ArH), 7.38 (t, 1H, J = 8 Hz, ArH), 7.58 (t, 1H, J = 8 Hz, ArH), 7.70 (s, 1H, ArH), 7.81 (d, 1H, J = 8 Hz, ArH), 7.90 (s, 1H, ArH), 8.00–8.08 (m, 2H, ArH), 8.31 (d, 1H, J = 8 Hz, ArH), 8.45–8.50 (m, 1H, ArH), 9.24 (s, 1H, ArH). MS: m/e 411 (M+1).
4-(9-methyl-9H-carbazol-3-yl)-6-(2,4,6-trimethoxyphenyl)-pyrimidin-2-amine (5d)
Yield: 86%; MP: 208–211°C; IR (KBr, cm−1): 3368, 3320, 3214, 3019, 1641, 1559, 1372, 1220, 1149, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.80 (s, 3H, OCH3), 3.85 (s, 6H, OCH3), 4.01 (s, 3H, NCH3), 6.35 (s, 2H, ArH), 6.66 (s, 2H, NH2), 7.38 (t, 1H, J = 8 Hz, ArH), 7.58 (t, 1H, J = 8 Hz, ArH), 7.61–7.70 (m, 1H, ArH), 7.90 (s, 1H, ArH), 8.00–8.08 (m, 2H, ArH), 8.31 (d, 1H, J = 8 Hz, ArH), 9.24 (s, 1H, ArH); MS: m/e 441 (M + 1).
4-(4-fluorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5e)
Yield: 92%; MP: 230–232°C; IR (KBr, cm−1): 3417, 3330, 3296, 3020, 1596, 1566, 1443, 1369, 1212, 1155, 1045, 932, 663; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.85 (s, 3H, NCH3), 6.66 (s, 2H, NH2), 7.23–7.58 (m, 6H, ArH), 7.80 (s, 1H, ArH), 8.23–8.28 (m, 4H, ArH), 9.12 (s, 1H, ArH); MS: m/e 369 (M + 1).
4-(2,4-difluorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5f)
Yield: 87%; MP: 112–116°C; IR (KBr, cm−1): 3417, 3330, 3296, 3020, 1596, 1566, 1443, 1369, 1212, 1155, 1140, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.85 (s, 3H, NCH3), 6.66 (s, 2H, NH2), 7.23 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 3H, ArH), 7.61–7.73 (m, 2H, ArH), 7.80 (s, 1H, ArH), 8.23–8.28 (m, 2H, ArH), 8.40 (d, 1H, J = 8 Hz, ArH), 9.08 (s, 1H, ArH); MS: m/e 387 (M + 1).
4-(3,4-difluorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5g)
Yield: 83%. MP: 190–192°C; IR (KBr, cm−1): 3417, 3330, 3296, 3020, 1596, 1566, 1443, 1369, 1212, 1155, 1140, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.85 (s, 3H, NCH3), 6.66 (s, 2H, NH2), 7.23 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 3H, ArH), 7.61–7.73 (m, 2H, ArH), 7.80 (s, 1H, ArH), 8.23–8.28 (m, 2H, ArH), 8.41 (d, 1H, J = 8 Hz, ArH), 9.07 (s, 1H, ArH); MS: m/e 387 (M + 1).
4-(4-chlorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5h)
Yield: 93%. MP: 220–222°C; IR (KBr, cm−1): 3350, 3019, 2926, 2400, 1597, 1535, 1425, 1123, 1015, 928; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.87 (s, 3H, NCH3), 6.77 (s, 2H, NH2), 7.31 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 5H, ArH), 7.83 (s, 1H, ArH), 8.22–8.38 (m, 3H, ArH), 8.45 (d, 1H, J = 8 Hz, ArH), 9.12 (s, 1H, ArH); MS: m/e 385 (M + 1).
4-(2,4-dichlorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5i)
Yield: 82%; MP: 210–212°C; IR (KBr, cm−1): 3360, 3325, 3019, 2926, 2400, 1597, 1535, 1425, 1123, 1015, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.87 (s, 3H, NCH3), 6.80 (s, 2H, NH2), 7.28 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 5H, ArH), 7.83 (s, 1H, ArH), 8.25–8.38 (m, 3H, ArH), 9.02 (s, 1H, ArH); MS: m/e 419 (M+).
4-(3,4-dichlorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5j)
Yield: 80%; MP: 184–187°C; IR (KBr, cm−1): 3360, 3325, 3019, 2926, 2400, 1597, 1535, 1425, 1123, 1015, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.87 (s, 3H, NCH3), 6.80 (s, 2H, NH2), 7.32 (t, 1H, J = 8 Hz, ArH), 7.48–7.77 (m, 5H, ArH), 7.87 (s, 1H, ArH), 8.30 (d. 1H, J = 7.2 Hz, ArH), 8.37 (s, 1H, ArH), 8.48 (d, 1H, J = 8 Hz, ArH), 9.14 (s, 1H, ArH); MS: m/e 419 (M+).
4-(2,5-dichlorophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5k)
Yield: 86%; MP: 210–212°C; IR (KBr, cm−1): 3360, 3325, 3019, 2926, 2400, 1597, 1535, 1425, 1123, 1015, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.87 (s, 3H, NCH3), 6.80 (s, 2H, NH2), 7.28 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 5H, ArH), 7.83 (s, 1H, ArH), 8.20–8.40 (m, 3H, ArH), 9.02 (s, 1H, ArH); MS: m/e 419 (M+).
4-(4-bromophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5l)
Yield: 82%; MP: 260–262°C; IR (KBr, cm−1): 3365, 3330, 3296, 3020, 1596, 1566, 1443, 1369, 1212, 1155, 1045, 932; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.87 (s, 3H, NCH3), 6.77 (s, 2H, NH2), 7.31 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 5H, ArH), 7.81 (s, 1H, ArH), 8.25–8.37 (m, 3H, ArH), 8.40 (d, 1H, J = 8 Hz, ArH), 9.10 (s, 1H, ArH); MS: m/e 431 (M + 2).
4-(9-methyl-9H-carbazol-3-yl)-6-(4-nitrophenyl)-pyrimidin-2-amine (5m)
Yield: 94%; MP: 144–146°C; IR (KBr, cm−1): 3360, 3320, 3020, 1596, 1566, 1443, 1369, 1212, 1155, 1045, 932; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.85 (s, 3H, NCH3), 6.88 (s, 2H, NH2), 7.28 (t, 1H, J = 8 Hz, ArH), 7.48–7.74 (m, 4H, ArH), 8.02 (s, 1H, ArH), 8.23–8.65 (m, 5H, ArH), 9.11 (s, 1H, ArH); MS: m/e 396 (M + 1).
4-(9-methyl-9H-carbazol-3-yl)-6-(thiophen-2-yl)-pyrimidin-2-amine (5n)
Yield: 80%; MP: 144–146°C; IR (KBr, cm−1): 3360, 3325, 3019, 1595, 1560, 1445, 1369, 1212, 1155, 1045, 928; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.87 (s, 3H, NCH3), 6.77 (s, 2H, NH2), 7.23 (t, 1H, J = 8 Hz, ArH), 7.35 (t, 1H, J = 3.9 and 4.8 Hz, ArH), 7.48–7.60 (m, 3H, ArH), 7.80 (s, 1H, ArH), 7.93 (d, 1H, J = 2.1 Hz, ArH), 8.23–8.28 (m, 2H, ArH), 8.40 (d, 1H, J = 8 Hz, ArH), 9.12 (s, 1H, ArH); MS: m/e 357 (M + 1).
4-(9-methyl-9H-carbazol-3-yl)-6-(pyridin-2-yl)-pyrimidin-2-amine (5o)
Yield: 65%; MP: 161–164°C; IR (KBr, cm−1): 3368, 3325, 3019, 1595, 1560, 1445, 1369, 1212, 1155, 1045, 932; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.87 (s, 3H, NCH3), 6.80 (s, 2H, NH2), 7.29 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 4H, ArH), 7.61–7.73 (m, 2H, ArH), 7.80 (s, 1H, ArH), 8.25–8.38 (m, 2H, ArH), 8.40 (d, 1H, J = 8 Hz, ArH), 9.12 (s, 1H, ArH); MS: m/e 352 (M + 1).
4-(3-aminophenyl)-6-(9-methyl-9H-carbazol-3-yl)-pyrimidin-2-amine (5p)
Yield: 70%; MP: 178–180°C; IR (KBr, cm−1): 3360, 3320, 3020, 2925, 1595, 1560, 1445, 1369, 1212, 1155, 1045, 930; 1H NMR (200 MHz, DMSO-d6, δ in ppm): 3.87 (s, 3H, NCH3), 6.40 (s, 2H, NH2), 6.79 (s, 2H, NH2), 7.23 (t, 1H, J = 8 Hz, ArH), 7.48–7.58 (m, 4H, ArH), 7.61–7.73 (m, 2H, ArH), 7.80 (s, 1H, ArH), 8.23–8.28 (m, 2H, ArH), 8.40 (d, 1H, J = 8 Hz, ArH), 9.09 (s, 1H, ArH); MS: m/e 366 (M + 1).
Urease inhibition assay
Mixture of 25 µL of urease enzyme (Jack bean) solution containing 1 unit of enzyme (1 unit = 0.02 mg of enzyme) was dissolved in phosphate buffer of pH 6.8, 55 µL of buffer containing 100 mM urea, and 5 µL of test compounds (0.5 mM concentration) was incubated at 30°C for 15 min in 96-well plates. Urease activity was determined by measuring ammonia production using the iodophenol methodCitation38. Briefly, 45 µL each of phenol reagent and 70 µL of alkali reagent were added to each well. The increase in absorbance at 630 nm was measured after 50 min, using a microplate reader (Molecular Device, CA, USA). All reactions were performed in triplicate in a final volume of 200 µL. The results (change in absorbance per min) were processed using Soft-Max Pro software (Molecular Device, CA, USA). Thiourea was used as the standard inhibitor and percentage inhibitions were calculated as follow: 100 − (ODtestwell/ODcontrol) × 100.
Antimicrobial activity
Antimicrobial activities of all the synthesized compounds were determined by disc diffusion methodCitation41. All the bacterial and fungal species used were procured from Institute of Microbial Type Culture Collection (IMTCC), Chandigarh, India, and National Collection of Industrial Microorganisms (NCIM), Pune, India, namely, Escherichia coli, Bacillus subtilis, Staphylococcus aureus, Shigella flexenari, Salmonella typhi, Candida albicans, and Aspergillus niger. The test compounds were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 1 mg/mL. 20 mL of sterilized agar media was poured into each presterilized Petri dish. Excess of suspension was decanted and plates were dried by placing in an incubator at 37°C for an hour. About 60 µL of 24-h-old culture suspension were poured and neatly swabbed with the presterilized cotton swabs. 6-mm diameter well was then punched carefully using a sterile cork borer and 30 µL of test solutions were added into each labelled well. Inoculated plates in duplicate were then incubated at 37 ± 0.5°C for antibacterial activity for 24 h and at 25 ± 0.5°C for antifungal activity for 48 h. After incubation, the antimicrobial activity was measured in terms of the zone of inhibition in mm.
Calculation of drug-likeness properties
The physicochemical properties such as molecular weight, c Log P, HBA, HBD, polar surface area, molecular volume and drug likeness of the synthesized compounds are studied from online Osiris property explorer for drug bioavailability of chemical compoundsCitation42.
Acknowledgments
HVC grateful to Council of Scientific and Industrial Research (CSIR), New Delhi, India for financial assistance in the form of SRF.
Declaration of interest
The authors report no conflicts of interest.
References
- Onoda Y, Takido M, Magaribuchi T, Tamaki H. Effects of 12-sulfodehydroabietic acid monosodium salt (TA-2711), a new anti-ulcer agent, on gastric mucosal lesions induced by necrotizing agents and gastric mucosal defensive factors in rats. Jpn J Pharmacol 1990;52:631–638.
- Mobley HL, Island MD, Hausinger RP. Molecular biology of microbial ureases. Microbiol Rev 1995;59:451–480.
- Montecucco C, Rappuoli R. Living dangerously: how Helicobacter pylori survives in the human stomach. Nat Rev Mol Cell Biol 2001;2:457–466.
- Mobley HL, Hausinger RP. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev 1989;53:85–108.
- Hauck RD. (1984). Nitrogen in Crop Production. In: Hauck RD, ed. Madison, WI: American Society of Agronomy, 551.
- Andrews RK, Dexter A, Blakeley RL, Zerner B. Jack bean urease (EC 3.5.1.5) VIII. On the inhibition of urease by amides and esters of phosphoric acid. J Am Chem Soc 1986;108:7124–7125.
- McCarty GW, Bremner JM, Lee JS. Inhibition of plant and microbial ureases by phosphoroamides. Plant Soil 1990;127:269–283.
- Khan KM, Iqbal S, Lodhi MA, Maharvi GM, Ullah Z, Choudhary MI et al. Biscoumarin: new class of urease inhibitors; economical synthesis and activity. Bioorg Med Chem 2004;12:1963–1968.
- Amtul Z, Rahman AU, Siddiqui RA, Choudhary MI. Chemistry and mechanism of urease inhibition. Curr Med Chem 2002;9:1323–1348.
- Kosikowska P, Berlicki L. Urease inhibitors as potential drugs for gastric and urinary tract infections: a patent review. Expert Opin Ther Pat 2011;21:945–957.
- You ZL, Xian DM, Zhang M, Cheng XS, Li XF. Synthesis, biological evaluation, and molecular docking studies of 2,5-substituted-1,4-benzoquinone as novel urease inhibitors. Bioorg Med Chem 2012;20:4889–4894.
- Akhtar T, Hameed S, Khan KM, Choudhary MI. Syntheses, urease inhibition, and antimicrobial studies of some chiral 3-substituted-4-amino-5-thioxo-1H,4H-1,2,4-triazoles. Med Chem 2008;4:539–543.
- Rauf A, Ahmed F, Qureshi AM, Rehman A-U, Khan A, Qadir MI et al. Synthesis and urease inhibition studies of barbituric and thiobarbituric acid derived sulphonamides. J Chin Chem Soc 2011;58:528–537.
- Khan KM, Ali M, Wadood A, Zaheer-ul-Haq, Khan M, Lodhi MA et al. Molecular modeling-based antioxidant arylidene barbiturates as urease inhibitors. J Mol Graph Model 2011;30:153–156.
- Li HQ, Xiao ZP, Yin-Luo, Yan T, Lv PC, Zhu HL. Amines and oximes derived from deoxybenzoins as Helicobacter pylori urease inhibitors. Eur J Med Chem 2009;44:2246–2251.
- Lagoja IM. Pyrimidine as constituent of natural biologically active compounds. Chem Biodivers 2005;2:1–50.
- Mishra R, Tomar I. Pyrimidine: The molecule of diverse biological and medicinal importance. Internat J Pharma Sci Res 2011;2:758–771.
- Patel R, Desai K, Chikhalia K. Synthesis and biological activity of some 2,4,6-trisubstituted-1,3,5-s–triazines. J Ind Chem Soc 2003;80:138–140.
- Chaudhary A, Sharma PK, Verma P, Kumar N, Dudhe R. Microwave assisted synthesis of novel pyrimidine derivatives and investigation of their analgesic and ulcerogenic activity. Med Chem Res 2012;21:3629–3645.
- Rangappa KS, Kallappa MH, Ramya VS, Mallinath HH. Analgesic, anti-pyretic and DNA cleavage studies of novel pyrimidine derivatives of coumarin moiety. Euro J Med Chem 2010;45:2597–2605.
- Amr AE, Nermien MS, Abdulla MM. Synthesis, reactions, and anti-inflammatory activity of heterocyclic systems fused to a thiophene moiety using citrazinic acid as synthon. Monatsh Chem 2007;138:699–707.
- Fujiwara N, Nakajima T, Ueda Y, Fujita H, Kawakami H. Novel piperidinylpyrimidine derivatives as inhibitors of HIV-1 LTR activation. Bioorg Med Chem 2008;16:9804–9816.
- Ballell L, Field RA, Chung GA, Young RJ. New thiopyrazolo[3,4-d]pyrimidine derivatives as anti-mycobacterial agents. Bioorg Med Chem Lett 2007;17:1736–1740.
- Wagner E, Al-Kadasi K, Zimecki M, Sawka-Dobrowolska W. Synthesis and pharmacological screening of derivatives of isoxazolo[4,5-d]pyrimidine. Eur J Med Chem 2008;43:2498–2504.
- Jean-Damien C, David B, Ronald K, Julian G, Pan L, Robert D. Vertex Pharmaceuticals Incorporated, USA, PCT. Int Appl 2002;WO 0222608.
- Gorlitzer K, Herbig S, Walter RD. Indeno[1,2-d]pyrimidin-4-yl-amine. Pharmazie 1997;52:670–672.
- Kashyap SJ, Sharma PK, Garg VK, Dudhe R, Kumar N. Review on synthesis and various biological potential of thiazolopyrimidine derivatives. J Adv Sci Res 2011;2:18–24.
- Giridhar R, Tamboli RS, Ramajayam R, Prajapati DG, Yadav MR. Assessment of antiplatelet activity of 2-aminopyrimidines. Eur J Med Chem 2012;50:428–432.
- Knölker HJ, Reddy KR. Isolation and synthesis of biologically active carbazole alkaloids. Chem Rev 2002;102:4303–4427.
- Noguchi N, Nishino K, Niki E. Antioxidant action of the antihypertensive drug, carvedilol, against lipid peroxidation. Biochem Pharmacol 2000;59:1069–1076.
- Zall A, Kieser D, Höttecke N, Naumann EC, Thomaszewski B, Schneider K et al. NSAID-derived γ-secretase modulation requires an acidic moiety on the carbazole scaffold. Bioorg Med Chem 2011;19:4903–4909.
- Routier S, Mérour JY, Dias N, Lansiaux A, Bailly C, Lozach O et al. Synthesis and biological evaluation of novel phenylcarbazoles as potential anticancer agents. J Med Chem 2006;49:789–799.
- Hu L, Li ZR, Li Y, Qu J, Ling YH, Jiang JD et al. Synthesis and structure-activity relationships of carbazole sulfonamides as a novel class of antimitotic agents against solid tumors. J Med Chem 2006;49:6273–6282.
- Syutkin RV, Abashev GG, Shklyaeva EV, Kudryavtsev PG. New carbazole-containing chalcones and pyrimidines based thereon: synthesis and electrochemical study. Russ J Org Chem 2011;47:530–536.
- Bandgar BP, Sarangdhar RJ, Viswakarma S, Ahamed FA. Synthesis and biological evaluation of orally active prodrugs of indomethacin. J Med Chem 2011;54:1191–1201.
- Bandgar BP, Jalde SS, Korbad BL, Patil SA, Chavan HV, Kinkar SN et al. Synthesis and antioxidant, cytotoxicity and antimicrobial activities of novel curcumin mimics. J Enzyme Inhib Med Chem 2012;27:267–274.
- Bandgar BP, Adsul LK, Lonikar SV, Chavan HV, Shringare SN, Patil SA et al. Synthesis of novel carbazole chalcones as radical scavenger, antimicrobial and cancer chemopreventive agents. J Enzyme Inhib Med Chem 2012. DOI: 10.3109/14756366.2012.663365.
- Khan I, Ali S, Hameed S, Rama NH, Hussain MT, Wadood A et al. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur J Med Chem 2010;45:5200–5207.
- Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997;23:3–25.
- Hou T, Wang J, Zhang W, Xu X. ADME evaluation in drug discovery. 6. Can oral bioavailability in humans be effectively predicted by simple molecular property-based rules? J Chem Inf Model 2007;47:460–463.
- Bandgar BP, Patil SA, Korbad BL, Nile SH, Khobragade CN. Synthesis and biological evaluation of β-chloro vinyl chalcones as inhibitors of TNF-α and IL-6 with antimicrobial activity. Eur J Med Chem 2010;45:2629–2633.
- Drug-likeness and molecular property prediction. Available at: http://molsoft.com/mprop/.