Abstract
This study was meant to determine the inhibitory activity of tannins and flavonoid compounds from Geranium robertianum, Helleborus purpurascens and Hyssopus officinale plant polyphenol rich extracts against urease and α-chymotrypsin. The G. robertianum, H. purpurascens and H. officinale extracts were purified and concentrated by microfiltration and ultrafiltration. Phenolic compounds including flavonoids and tannins have been linked to many pharmacological activities. Thus, the polyphenolic content of the extracts was assessed by UV–Vis spectroscopy and HPLC. The concentrated extracts enriched in polyphenolic compounds (flavonoids, tannins and phenolic acids) showed a significant inhibition against urease from jack bean (over 90%), whereas in case of the α-chymotrypsin, they proved to have an inhibition below 54%. The results of this support the use of G. robertianum, H. purpurascens and H. officinale polyphenolic extracts as potential sources of urease inhibitors. Among the three plant extracts tested, H. officinale polyphenolic extracts exhibited a high inhibitory activity (92.67%) against urease and low inhibition (19.6%) against α-chymotrypsin and could be considered as possible remedy in ulcer treatment.
Introduction
Plants have always played a major role in the treatment of human and animal diseases as a therapeutic source for traditional medicine. After extensive research all over the world, it has been discovered that natural products have the potential to control the overactivity of many enzymes. Urease (urea amidohydrolase; EC 3.5.1.5) is a nickel-containing enzyme that catalyzes the hydrolysis of urea to ammonia and carbamate. Urease is known to be one of the major causes of pathologies induced by Helicobacter pylori as this allows bacteria to survive at the low pH of the stomach. It therefore plays an important role in the pathogenesis of gastric and peptic ulcersCitation1–3, apart from cancer as wellCitation4–6. Many urease inhibitors have been described in the past, such as hydroxamic acids, which were found to be potent and specific inhibitors of urease activities for the plant and bacterial origin enzymesCitation7. A particular interest is directed on the novel biological properties of exploring polyphenolsCitation8,Citation9.
α-Chymotrypsin (EC 3.4.21.1) is a serine protease enzyme, which acts to alleviate ulcers and digesting the polypeptide of damaged tissueCitation10. Recently, serine protease inhibitors from potato and other plants have also been reported to have inhibitory effects on tumor cell growthCitation11,Citation12. Such inhibitors have potential therapeutic use.
Herbal medicines become important factors in the treatment of gastroduodenal diseases, as they prove themselves to be free of side effects and are less expensive than synthetic drugs. Clinical research has confirmed the efficiency of several plants for the treatment of gastroduodenal diseasesCitation13,Citation14. The medicinal properties of many plants are mainly assigned to the flavonoids present, but they may be influenced also by other compounds such as coumarins, alkaloids, terpenoids, tannins, phenolic acids and antioxidant micronutrients, e.g. Cu, Mn and ZnCitation15,Citation16.
Previous research has shown that the active compounds from plants, polyphenolic compounds (flavonoids and fenolic acids) and tannins, act synergistically in healing of gastric ulcers. They protect the gastric mucosa against a variety of ulcerogenic agents via several mechanisms of action, mainly free-radical scavenging and antioxidant properties, increased mucus production, antisecretory action and inhibition of the H. pylori growthCitation17. Tannins prevent ulcer development due to their protein precipitating and vasoconstricting effectsCitation18.
Some of them have been favorably reconsidered for their biological behavior in pharmacological and medical applications, due to their ability to inhibit the enzymes involved in the digestive processes of living beings. Recently, many polyphenols, flavones and catechins were reported to prove urease inhibitory activityCitation19–22.
Geranium robertianum L. (Geraniaceae) and Hyssopus spp. (Labiatae) are common species in Europe, Asia and North Africa. Geranium robertianum and H. officinale have been used for the treatment of stomach ache and gastric ulcer in the traditional medicine. Helleborus species are evergreen, rhizomatous plants belonging to the family Ranunculaceae and it is an old medicinal plant. The main sapogenin of the roots and rhizomes of Helleborus species is used in medicine, especially to treat ulcersCitation23.
Information about urease and α-chymotrypsin inhibitors in Geranium spp., Helleborus spp. and Hyssopus spp. is very limited.
This study was carried out to initiate research for newer pharmacophores with emphasis toward urease and α-chymotrypsin. Hence, polyphenolic extracts of the above-mentioned plants were screened to detect urease and α-chymotrypsin inhibitors and subjected to phytochemical analysis in order to identify the potential inhibitory phytoconstituents.
Materials and methods
Preparation and concentration of extracts
The extracts were obtained by ultrasound-assisted extraction using a Transsonic-460/H ultrasonic bath (Elma, Singen, Germany). The procedure involved ambient temperature (20 ± 2°) and ultrasound-assisted extraction of grounded plant material with cold distilled water as solvent, for a period of 1 h. The herbal mass concentration in the solvent was 60 g L−1.
The extracts were successively filtered through Whatman 1 (Medium-fast) filter paper and microfiltered through a 0.45 μm pore size membrane (Millipore, Billerica, MA), in order to remove any fine solid particles, which could initiate membrane fouling during ultrafiltration (UF). All extracts were then filtered through a 30 000 Da molecular weight cut-off UF membrane which retained the proteins and polysaccharides. Experiments to obtain the rich-polyphenols extracts were also carried out on the UF, using a membrane with cut-off 1000 Da. These experiments were carried out in a KMS Laboratory Cell CF-1 type cross-flow lab-scale filtration unitCitation24.
Fourier transform infrared spectrophotometer
Fourier transform infrared (FT-IR) spectroscopy was used to identify the characteristic functional groups in the extract. A Tensor 27 Brucker FT-IR spectrometer (Bruker Co., Karlsruhe, Germany) was used to record IR spectra. A potassium bromide microdisk was prepared from finely ground dried powder aqueous extracts and UF fractions of 1 mg of vegetable sample with 100 mg of KBr. The scanning wavelength of infrared was at 4000–400 cm−1 at a resolution of 4 cm−1.
Determination of polyphenolic compounds
Determination of the total phenolic content
The total phenolic content in extracts was determined using Folin–Ciocalteu reagent, as earlier describedCitation25. Gallic acid was used as standard to perform the calibration. The total phenolic content was expressed as gallic acid equivalents (GAE) in milligram per liter of extracts.
Determination of flavonoids content
The total flavonoid content was determined according to Lin method (aluminum chloride-based method), with slight modificationsCitation26. Quercetin was used as standard, and results were expressed as milligram quercetin equivalents (QE) per liter. Total flavonoid contents were obtained subsequent to interpolation of the measured absorbance value for extracts to calibration curve, defined by the regression equation of the quercetin (y = 0.00989x + 0.01975, R2 = 0.9977).
Determination of tannins content
The method designed by Makkar et al.Citation27 slightly modified was used for the indirect determination of total tannins as the difference in total phenolic content before and after tannin precipitation from the tannin-containing extract with insoluble polyvinylpyrrolidone. Results were expressed as microgram of tannic acid (TA) equivalents per milliliter extract.
High performance liquid chromatography–mass spectrometry screening of extracts content in polyphenols and flavones
The high performance liquid chromatography–diode array detection–mass spectrometry system used was a Shimadzu system (Shimadzu Corporation, Kyoto, Japan) equipped with two pumps LC-20ADsp, a column oven CTO-20AC, a degasser DGU-20A5, an autosampler and a photodiode array detector SPD-M20A operating at wavelengths between 220 and 500 nm The system was coupled to a MS detector Shimadzu 2010A equipped with standard LCQ ESI interface. UV and MS data were acquired and processed using operating system LCMS Solution software.
The used method was a reverse-phase high performance liquid chromatography, with a column. KROMASIL C18, inner diameter 2.1 mm, length 100 mm, in binary gradient. The mobile phase consisted of component A (water acidified with 1% formic acid, pH 3.00) and B (acetonitrile acidified with 1% formic acid). The gradient elution started with 5% B and changed to 50% B in 50 min, then reached 5% B in 5 min. The flow rate was 0.1 mL/min. The column was maintained at 20 °C. The used injection volume was 20 mL. The MS experiments were performed using the standard in negative mode, the calibration tuning being achieved prior determination. Identification of compounds by HPLC–MS analysis was based on retention time and ratio mass/charge fragments criteria, subsequent comparison between HPLC–MS chromatograms and MS spectra for extracts with those obtained for standard. The quantitative determinations were made by interpolation of the peak area corresponding to specific retention times with the calibration curves obtained for used standards, namely caffeic acid, gallic acid, coumaric acid, ferulic acid, chlorogenic acid, rosmarinic acid, rutin, quercetin and kaempferol.
Urease inhibition assay
The urease inhibition assay was determined using Nessler’s reagentCitation26. A solution consisting of 0.25 mL urease (0.1 mg/mL) from jack bean, 2 mL of Tris–HCl buffer (pH 8.0) and 0.25 mL urea (60 mM) were incubated with 100 μL of the test compounds at 30 °C for 20 min. After this interval, the reaction is stopped by the addition of 1 mL of 10% trichloroacetic acid and then 0.5 mL Nessler’s reagent was added. A standard curve was drawn using a series of concentrations of ammonium sulfate. The absorbance was measured at 436 nm. The percentage inhibition was calculated from the formula:
Thiourea was used as the standard inhibitor.
α-Chymotrypsin inhibition assay
The α-chymotrypsin activity inhibition by the G. robertianum, H. purpurascens and H. officinale polyphenolic extracts was evaluated using a spectrophotometric method of Ee et al.Citation28 with some modification. The polyphenolic extracts were mixed with 0.1 mL α-chymotrypsin from bovine pancreas (16 units/mL of 80 mM Tris–HCl buffer pH 7.8) and incubated for 20 min at 25 °C prior to addition to a 2.9 mL reaction volume containing 1.18 mM BTEE (1.4 mL) and 80 mM Tris Tris–HCl buffer solution with 20 mM CaCl2 pH 7.8 (1.5 mL) pre-warmed to the same temperature. The increase in absorbance was continuously monitored at 256 nm for 3 min. Ki was obtained by the reciprocal plotting of the reaction velocity via inhibitor concentration under different substrate concentrations (0.6–2 mM).
Experiments to evaluate the degree of α-chymotrypsin inhibition by the polyphenolic extracts were carried out for 20 min at 25 °C, and subsequently the residual enzymatic activity was measured. The percentage inhibition was calculated as follows:
where E is the activity of the enzyme without test compound and S the activity of enzyme in the presence of the test compound.
Statistical analysis
The measurements were performed in triplicate and for statistical processing Excel 2007 was used, standard deviation (SD) was <10%.
Results and discussion
Comparing the FT-IR spectra () of different samples, we can notice that they contained the same types of chemical constituents as shown in FT-IR spectra similarity. The analyzed polyphenolic extracts are complex mixtures, therefore their FT-IR spectrum shows substantial overlap of various compounds. Each band represents an overall overlap of some characteristic absorption peaks of functional groups occurring in the sample.
Figure 1. FT-IR spectra of: G. robertianum polyphenolic extract (1), H. purpurascens polyphenolic extract (2) and H. officinale polyphenolic extract (3).
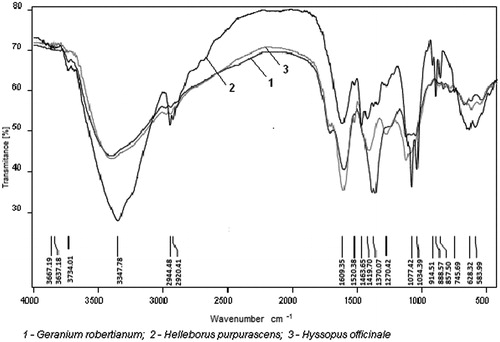
shows the FT-IR spectra of the main peaks at ∼3400 cm−1 (O–H stretching), 2970–2920 cm−1 (C–H stretching), ∼1600 cm−1 (C=C and C=O stretching), ∼1400 cm−1 (aromatic ring stretching) and ∼1118 cm−1 (C–O stretching) present in all these tested samples. The sharp absorption peak at 1600 cm−1 (G. robertianum extracts), 1602 cm−1 (H. purpurascens extracts) and 1604 cm−1 (H. officinale extracts) is assigned to C=O stretching vibration from the central heterocyclic ring and are ascribable to the presence of glycosilated tannins or flavonoids. Peaks between 1650 and 1450 cm−1 proved the presence of aromatic rings. The absorption bands assigned to bending vibration of C–C bonds from phenolic groups appearing in the 1550–1400 cm−1 regionsCitation29. The peaks from 910 to 740 cm−1 can be assigned to the C–H bond vibration in the phenolic rings. The peaks in the proximity of 1118 cm−1 can be assigned to C–O stretching vibrations of the CH2OH group.
The IR spectrum of G. robertianum and H. officinale polyphenolic extracts showed peaks at 1710 and 1713 cm−1 indicating the presence of lactone ring, proving the presence of flavonoids or coumarins.
The absorption bands in the 1200–800 cm−1 regions are useful to identify the polysaccharides with different structures and compositionCitation28.
The G. robertianum polyphenolic extract showed an increase in intensity of the 1707, 1358, 1381 and 1358 cm−1 absorption bands, caused by the higher phenolic compounds (tannins and flavonoids) content.
Total phenolic compounds (TPC) content of the analyzed extracts was ranging from 463.1 ± 8.4 to 859.4 ± 9.8 mg GAE/L (). Comparing the extracts with high polyphenolic compounds (phenolic acids, flavonoids and tannins) content, H. officinale polyphenolic extract (859.4 ± 9.8 mg GAE/L) showed the highest content of TPC, while H. purpurascens polyphenolic extracts (463.08 ± 8.4 mg GAE/L) showed the lowest TPC value.
Table 1. Contents of total phenolics, total flavonoids and tannins in Geranium spp., Helleborus spp. and Hyssopus spp. polyphenolic extracts.
Helleborus purpurascens polyphenolic extract showed the highest tannin contents, between the three analyzed extracts plant.
Biological active compounds were identified by HPLC–MS according to their specific retention times and absorbance spectra, considering as reference the values of retention times obtained for standard compounds, namely for gallic acid, caffeic acid, ferulic acid, ellagic acid, rutin, quercetin and kaempferol. shows the main analytical performances for the analyzed extracts, in terms of: retention times, equation of linear response and limit of detection. From the table, it could be seen that quite good linearity and high sensitivity was obtained. The contents of polyphenols and flavones in analyzed extracts are shown in , obtained by interpolation of the area corresponding to the specific peak with equation of regression. Gallic acid, rutin and luteolin are occurring in all of the analyzed extracts, while quercetin was not identified. Ellagic acid was found only in G. robertianum and H. purpurascens extracts; kaempferol was found only in G. robertianum extracts and coumaric acid was found only in H. officinale. Caffeic and ferulic acids were found in H. purpurascens and H. officinale polyphenolic extracts. It could be observed that as a result of the UF process, the contents of caffeic and ferulic acids significantly increased.
Table 2. Retention time, regression equations, correlation coefficients, linear ranges and LD for phenolic compounds from Geraniumv spp., Helleborus spp. and Hyssopus spp. extracts.
Table 3. Phenolic acids and flavonoid contents in G. robertianum, H. purpurascens and H. officinale polyphenolic extracts.
This study was meant to determine the inhibitory activity of tannins and flavonoid compounds from three medicinal plant polyphenol rich extracts against urease and α-chymotrypsin, enzymes related to gastroduodenal diseases. The G. robertianum, H. purpurascens and H. officinale polyphenolic extracts inhibitory activity against jackbean urease is presented in . The polyphenolic extracts showed a significant inhibition against jack bean urease. The results indicate that H. purpurascens polyphenolic extract revealed a strong inhibitory activity against urease with a 95.6% inhibition value. Furthermore, considerable inhibitory activities against urease were exhibited by H. officinale polyphenolic extract (92.67%) and G. robertianum concentrated extract (91.96%).
Figure 2. Urease inhibition activity of Geranium spp., Helleborus spp. and Hyssopus spp. polyphenolic extracts (concentration of each herbal extract: 1.5 mg/mL).
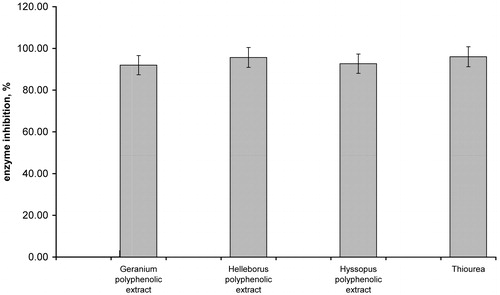
Urease is a nickel metalloenzyme, for which the Ni+2 ions at the catalytic active site are essential for enzyme activity. The hydroxyl groups presented on aromatic ring of polyphenol molecule may be responsible for the inhibitory activity by coordinating with the nickel (active site) of the enzymeCitation30.
Flavonoids can also chelate metal ionsCitation31–33 and thus inhibit urease activity; quercetin, kaempferol, apigenin and luteolin have been reported to exhibit inhibitory activity against ureaseCitation18.
The TPCs content in H. purpurascens polyphenolic extract was lower, but this extract was rich in tannins, while G. robertianum polyphenolic extracts showed a high value for TPC, but the lowest tannin content value.
A decreased activity of the urease when it is associated with tannins contents has also been recorded in other studiesCitation34,Citation35.
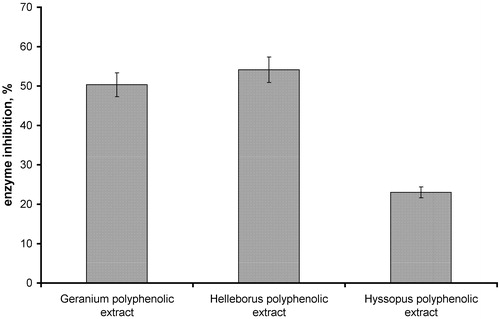
All of the analyzed extracts showed a moderate α-chymotrypsin inhibitory activity (). Helleborus purpurascens polyphenolic extract showed the strongest inhibition of α-chymotrypsin (53.9%), while polyphenol rich H. officinale extract showed a low activity (<20% inhibition).
Figure 3. α-Chymotrypsin inhibition activity of Geranium spp., Helleborus spp. and Hyssopus spp. polyphenolic extracts (concentration of each herbal extract: 1.5 mg/mL).
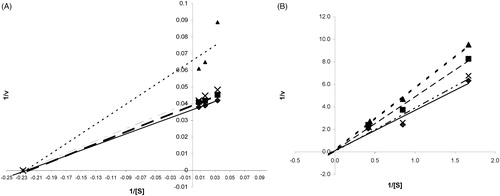
shows the effect of the initial urea concentration on the hydrolysis rate. As can be seen from , the reaction velocity was lower in the presence of enzyme inhibitors (polyphenolic extracts) as compared to the controlled one (in the absence of inhibitors), for the same substrate concentration.
Figure 4. The effect of polyphenolic extracts on kinetic plots of urease (A) and α-chymotrypsin (B).
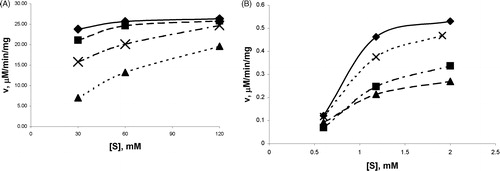
The Helleborus polyphenolic extracts had the highest inhibiting effect on urease and α-chymotrypsin compared to the other two studied plant extracts.
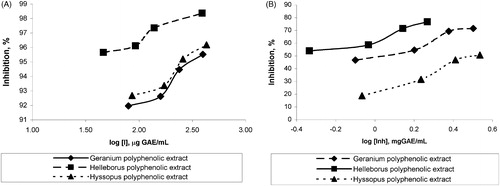
A dose-dependent urease and α-chymotrypsin inhibitory effect were observed with increasing of the extract’s concentration, related to the polyphenols content, suggesting a non-competitive type of inhibition for urease and α-chymotrypsin (). Plots of percent inhibition versus log concentration of polyphenols from extracts showed typical sigmoidal dose response curves ().
Conclusions
Concentrated extracts rich in polyphenolic compounds showed a significant urease inhibition activity (over 90%), but displayed moderate inhibition on α-chymotrypsin. The enzyme inhibitory effect was the result of the synergistic effect of polyphenolic compounds present in the extracts.
The result of this study suggests that G. robertianum, H. purpurascens and H. officinale polyphenolic extracts are potential sources of urease inhibitors. Our studies demonstrated an appreciable urease (92.67%) and a weak α-chymotrypsin (19.6%) inhibitory activity of H. officinale polyphenolic extracts and could be of some potential curing antiulcer agents.
Declaration of interest
This research was supported by the Romanian Executive Agency for Higher Education, Research, Development and Innovation Funding – PN 62 076/2008. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology 1992;102:720–7
- Forman D, Newell DG, Fullerton F, et al. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. Brit Med J 1991;302:1302–5
- Parsonnet J, Hansen S, Rodriguez L, et al. Helicobacter pylori infection and gastric lymphoma. New Engl J Med 1994;330:1267–71
- Kikuchi S, Wada O, Nakajima T, et al. Serum anti-Helicobacter pylori antibody and gastric carcinoma among young adults. Cancer J 1995;75:2789–93
- Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. New Engl J Med 1991;325:1127–31
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. New Engl J Med 2001;345:784–9
- Odake S, Nakahashi K, Morikawa T, et al. Inhibition of urease activity by dipeptidyl hydroxamic acids. Chem Pharm Bull 1992;40:2764–8
- Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori associated gastritis and primary B cell gastric lymphoma. Lancet 1991;338:1175–6
- Tamura K. Urease inhibitor, ammonia smell suppressant and diaper rash preventing agent. Japanese Patent JP 2,004,091,338 A/25.03.2004
- Coblentz A, Coblentz DR. Treatment of peptic ulcer with chymotrypsin and an antibiotic. Mil Med 1966;131:150–4
- Blanco-Aparicio C, Molina MA, Fernández-Salas E, et al. Potato carboxypeptidase inhibitor, a T-knot protein, is an epidermal growth factor antagonist that inhibitors tumor cell growth. J Biol Chem 1998;273:12370–7
- Kennedy AR, Szuhaj BF, Newberne PM, Billings PC. Preparation and production of a cancer chemopreventive agent, Bowman-Birk inhibitor concentrate. Nutr Cancer 1993;19:281–302
- Kanner J, Lapidot T. The stomach as a bioreactor: dietary lipid peroxidation in the gastric fluid and the effects of plant derived antioxidants. Free Radical Bio Med 2001;31:1388–95
- Gurbuz I, Akyuz C, Yesilada E, Sener B. Anti-ulcerogenic effect of Momordica charantia L. fruits on various ulcer models in rats. J Ethnopharmacol 2000;71:77–82
- Cechinel Filho V, Yunes RA. Strategies for obtaining pharmacologically active compounds from medicinal plants: concepts about structural modification for improve the activity. Quim Nova 1998;21:99–105
- Czinner E, Hagymasi K, Blazovics A, et al. The in vitro effect of Helichysi flos on microsomal lipid peroxidation. J Ethnopharmacol 2001;77:31–5
- Mota KS, Dias GE, Pinto ME, et al. Flavonoids with gastroprotective activity. Molecules 2009;14:979–1012
- Aguwa CN, Nwako SO. Preliminary studies of the root extracts of Nauclea latifolia smith for anti ulcer properties. Niger J Pharm Sci 1988;4:16–23
- Matsubara S, Shibata H, Ishikawa F, et al. Suppression of Helicobacter pylori induced gastritis by green tea extract in mongolian gerbils. Biochem Biophys Res Commun 2003;310:7159
- Shabana S, Kawai A, Kai K, et al. Inhibitory activity against urease of quercetin glycosides isolated from Allium cepa and Psidium guajava. Biosci Biotech Biochem 2010;74:878–80
- Perveen S, El-Shafae AM, Al-Taweel A, et al. Antioxidant and urease inhibitory C-glycosylflavonoids from Celtis africana. J Asian Nat Prod Res 2011;13:799–804
- Uddin N, Siddiqui BS, Begum S, et al. Bioactive flavonoids from the leaves of Lawsonia alba (Henna). Phytochem Lett 2011;4:454–8
- Isaac O. Medicine containing the main sapogenin of helleborus. US Patent 3,956,491/11.05.1976
- Paun G, Neagu E, Tache A, et al. Application of the nanofiltration process for concentration of polyphenolic compounds from Geranium robertianum and salvia officinalis extracts. Chem Biochem Eng Q 2011;25:453–60
- Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol 1999;299:152–78
- Lin J-Y, Tang C-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem 2007;101:140–7
- Makkar HPS, Bluemmel M, Borowy NK, Becker K. Gravimetric determination of tannins and their correlations with chemical and protein precipitation methods. J Sci Food Agr 1993;61:161–5
- Ee KY, Zhao J, Rehman A, Agboola S. Characterisation of trypsin and α-chymotrypsin inhibitors in Australian wattle seed (Acacia victoriae Bentham). Food Chem 2008;107:337–43
- Kulshrestha Y, Husain Q. Direct immobilization of peroxidase on DEAE cellulose from ammonium sulphate fractionated proteins of bitter gourd (Momordica charantia). Enzyme Microb Tech 2006;38:470–7
- Özacar M, Soykan C, Ayhan Şengil İ. Studies on synthesis, characterization and metal adsorption of mimosa and valonia tannin resins. J Appl Polym Sci 2005;102:786–97
- Kačuráková M, Capek P, Sasinková V, et al. FT-IR study of plant cell wall model compounds: pectic polysaccharides and hemicelluloses. Carbohyd Polym 2000;43:195–203
- Xiao Z-P, Shi D-H, Li H-Q, et al. Polyphenols based on isoflavones as inhibitors of Helicobacter pylori urease. Bioorg Med Chem 2007; 15:3703–10
- Mira L, Fernandez MT, Santos M, et al. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res 2002;36:1199–208
- Lohan OP, Lall D, Vaid J, Negi SS. Utilization of oak tree (Quercus incana) fodder in cattle rations and fate of oak-leaf tannins in the ruminant system. Indian J Anim Sci 1983;53:1057–63
- Makkar HP, Singh B, Dawra RK. Effect of tannin-rich leaves of oak (Quercus incana) on various microbial enzyme activities of the bovine rumen. Brit J Nutr 1988;60:287–96