Abstract
1,3-Dicarbonyl derivatives of methylaminobenzene-sulfonamide were synthesized and their inhibitory effects on the activity of purified human carbonic anhydrase (hCA) I and hCA II were evaluated. hCA I and hCA II from human erythrocytes were purified by a simple one-step procedure by using Sepharose 4B-L-tyrosine-sulfanilamide affinity column. Our results show that the synthesized compounds inhibited the activity of carbonic anhydrase (CA) I and CA II. Among them, 2b and 2e were found to be the most active (IC50 = 2.12 and 2.52 µM) for hCA I and hCA II, respectively.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are zinc metalloenzymes that assist a very simple physiological reaction, rapid interconversion of carbon dioxide and water into protons and bicarbonate ionsCitation1. The zinc ion that is essential for catalysis is found within the active site of the most CAs. The reaction is involved in many pathological and physiological processes that include respiration and transport of CO2 and bicarbonate between metabolizing tissues and lungs, biosynthetic reactions, electrolyte secretion in various tissues and organs, pH and CO2 homeostasisCitation2–4.
Up to now, 16 human CA (hCA) isoforms have been identified exhibiting significant differences in catalytic activity, subcellular localization and tissues expression. They play important roles in many of physiological processes such as several biochemical pathways, cell proliferation, intra- and extracellular pH homeostasis and differentiation, and modulation of neuronal transmissionCitation5,Citation6. In human, CAs are found in a variety of tissues such as kidneys, lungs, eyes, skins, nerves systems and gastrointestinal tractCitation7. The different isozymes are found in different part of cell, and hCA I and hCA II are localized in the cytosolCitation2. Biological activities of this metalloenzyme family have several medicinal applications such as treatment of glaucoma, diuretics and management of several neurological disorders, whereas several agents are in clinical evaluations as antiobesity or antidrugsCitation8.
There are abundant number of molecules containing sulfonamide moietyCitation9. The compounds have several applications such as modified oligonucleotidesCitation10, amines synthonsCitation11, peptidomimeticsCitation12, chiral auxilliariesCitation13 and therapeuticsCitation14. Sulfonamide derivatives are well known pharmaceutical agents and have gained much attention due to their diverse biological activities in pharmaceutical as well as in agricultural areasCitation15. Some of 1,3-diketone compounds and their barbituric and thiobarbituric derivatives were prepared and urease inhibition studies were reportedCitation16,Citation17. The sulfonamide compounds have a number of biological activities such as antibacterialCitation18, insulin releasingCitation19, anti-inflammatoryCitation20, antitumorCitation21 and CA inhibitoryCitation22.
Sulfonamides are the best known inhibitors of CA enzymes and used for the treatment of glaucoma in medicinal chemistryCitation23. Acetazolamide (AAZ), dorzalamide (DZA) and brinzolamide (BRZ) are sulfonamide derivatives and used in the treatment of glaucoma. However, the drugs have side effects such as numbness and tingling in the fingers and toes, blurred vision, kidney stones, an increase in urination, upset stomach, dry eye and headache or dizzinesCitation24,Citation25.
In this study, 1,3-dicarbonyl derivatives of methylaminobenzene-sulfonamide were synthesized and their inhibitory effects on the activity of purified human hCA I and hCA II were evaluated.
Materials and methods
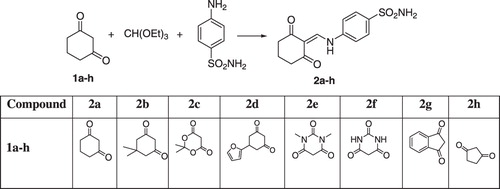
The compounds were prepared from a mixture of sulfanilamide, 1,3-dicorbonyl compounds and triethyl [orthoformate] in ethanol by refluxing for 3 h and shown in . The prepared compounds were characterized by 1H NMR, 13C NMR, IR and elemental analysis.
General procedure
Melting points were taken on a Yanagimoto Barnstead Electrothermal (Surrey, UK) micro-melting point apparatus and were uncorrected. IR spectra were measured on a Shimadzu Prestige-21 (200 VCE) spectrometer (Kyoto, Japan). 1H and 13C NMR spectra were measured on spectrometer at Varian Infinity Plus 300 and at 75 Hz (California), respectively. 1H and 13C chemical shifts were referenced to the internal deuterated solvent. The elemental analysis was carried out with a Leco CHNS-932 instrument (St. Joseph, MI). Flash column chromatography was performed using Merck silica gel 60 (230–400 mesh ASTM) (Darmstadt, Germany). All chemicals were purchased from Merck (Darmstadt, Germany), Alfa Easer (Ward Hill, MA) and Sigma-Aldirch (Taufkirchen, Germany).
Synthesis of 1,3-dicarbonyl substituted methylaminobenzene-sulfonamide derivatives were prepared according to .
General procedure for the synthesis of 1,3-dicarbonyl substituted methylaminobenzene-sulfonamide derivatives
A mixture of sulfanilamide (18.25 g, 0.106 mol), 1,3-dicarbonyl compound (0.127 mol) and triethyl [orthoformate] (16 mL) in ethanol (100 mL) was refluxed for 3 h. After cooling in room temperature, the product was filtered, washed with cold ethanol and air dried.
4-((2,6-Dioxocyclohexylidene)methylamino)benzene-sulfonamide (2a)
Yield 85.8%, m.p. 270.2 °C; IR (KBr, ν, cm−1): 3296 (NH2), 3203 (NH), 3072 (=C–H, aromatic), 1653 (C=O); 1H NMR (300 MHz, DMSO-d6, ppm): 12.62 (1H, d, –NH, j = 13.5), 8.56 (1H, d, =CH, j = 13.5), 7.84 (2H, d, =CH, j = 8.7), 7.71 (2H, d, =CH, j = 8.7), 7.41 (–NH2), 2.42 (2H, t, –CH2), 2.39 (2H, t, –CH2), 1.96 (2H, m, –CH2), 13C NMR (75 MHz, DMSO-d6, ppm): 200.7, 196.4, 150.6, 142.0, 141.6, 128.0, 119.3, 111.0, 38.3, 37.9, 19.7. Anal. Calcd for C13H14N2O4S (%): C, 53.05; H, 4.79; N, 9.52; O, 21.74; S, 10.89. Found (%): C, 53.18; H, 4.82; N, 9.59; O, 21.80; S, 10.96.
4-((4,4-Dimethyl-2,6-dioxocyclohexylidene)methylamino)benzene-sulfonamide (2b)
Yield 87.2%, m.p. 305.4 °C; IR (KBr, ν, cm−1): 3277 (NH2), 3203 (NH), 3065 (=C–H, aromatic), 1666 (C=O); 1H NMR (300 MHz, DMSO-d6, ppm): 12.65 (1H, d, –NH, j = 13.5), 8.50 (1H, d, =CH, j = 13.5), 7.80 (2H, d, =CH, j = 7.9), 7.58 (2H, d, =CH, j = 7.9), 7.28 (NH2), 2.38 (2H, s, –CH2), 2.36 (2H, s, –CH2), 1.02 (6H, s, –CH3), Citation13C NMR (75 MHz, DMSO-d6, ppm): 199.9, 195.9, 150.0, 141.8, 141.5, 128.1, 118.9, 109.7, 51.7, 51.4, 39.2, 31.3, 28.5. Anal. Calcd for C15H18N2O4S (%): C, 55.88; H, 5.63; N, 8.69; O, 19.85; S, 9.95. Found (%): C, 55.99; H, 5.7; N, 8.71; O, 19.93; S, 9.98.
4-((2,2-Dimethyl-4,6-dioxo-1,3-dioxan-5-ylidene)methylamino)benzene-sulfonamide (2c)
Yield 80.6%, m.p. 263.2 °C; IR (KBr, ν, cm−1): 3365 (NH2), 3269 (NH), 3075 (=C–H, aromatic), 1631 (C=O); 1H NMR (300 MHz, DMSO-d6, ppm): 11.25 (1H, d, –NH, j = 13.9), 8.64 (1H, d, =CH, j = 13.9), 7.88 (2H, d, =CH, j = 8.7), 7.45 (2H, d, =CH, j = 8.7), 7.22 (NH2), 1.86 (6H, s, –CH3), Citation13C NMR (75 MHz, DMSO-d6, ppm): 164.4, 163.3, 153.9, 141.9, 127,8, 119.9, 105,0, 88.5, 39.3, 27.2. Anal. Calcd for C13H14N2O6S (%): C, 47.85; H, 4.32; N, 8.58; O, 29.42; S, 9.83. Found (%): C, 47.99; H, 4.39; N, 8.64; O, 29.47; S, 9.89.
4-((4-(Furan-2-yl)-2,6-dioxocyclohexylidene)methylamino)benzene-sulfonamide (2d)
Yield 75.3%, m.p. 278.3 °C; IR (KBr, ν, cm−1): 3321 (NH2), 3180 (NH), 3082 (=C–H, aromatic), 1596 (C=O); 1H NMR (300 MHz, DMSO-d6, ppm): 12.63 (1H, –NH, j = 13.6), 8.53 (1H, d, =CH, j = 13.6), 7.83 (2H, d, =CH, j = 8.7), 7.70 (2H, d, =CH, j = 8.7), 7.52 (NH2), 6.41 (1H, s, =CH), 6.38 (1H, d, =CH, j = 3.1), 6.16 (1H, d, =CH, j = 3.1), 3.51–3.59 (1H, m, –CH), 2.68–2.95 (4H, m, –CH2), Citation13C NMR (75 MHz, DMSO-d6, ppm): 198.7, 194.6, 156.8, 150.6, 142.7, 141.9, 141.7, 128.0, 119.4, 115.1, 110.6, 105.7, 42.3, 42.0, 30.7. Anal. Calcd for C17H16N2O5S (%): C, 56.66; H, 4.47; N, 7.77; O, 22.20; S, 8.90. Found (%): C, 56.73; H, 4.52; N, 7.79; O, 22.26; S, 8.92.
4-((1,3-Dimethyl-2,4,6-trioxo-tetrahydropyrimidin-5(6H)-ylidene)methylamino)benzene-sulfonamide (2e)
Yield 87.6%, m.p. 294.5 °C; IR (KBr, ν, cm−1): 3307 (NH2), 3190 (NH), 3062 (=C–H, aromatic), 1633 (C=O); 1H NMR (300 MHz, DMSO-d6, ppm): 11.98 (1H, d, –NH, j = 13.9), 8.61 (1H, d, =CH, j = 8.7), 7.86 (2H, d, =CH, j = 8.7), 7.65 (2H, d, =CH, j = 13.9), 7.43 (NH2), 3.21 (6H, s, N–CH3), Citation13C NMR (75 MHz, DMSO-d6, ppm): 164.5, 162.4, 152.2, 151.95, 141.7, 141.6, 128.0, 119.3, 94.2, 28.3, 27.6. Anal. Calcd for C13H14N4O5S (%): C, 46.15; H, 4.17; N, 16.56; O, 23.64; S, 9.48. Found (%): C, 46.21; H, 4.24; N, 16.59; O, 23.68; S, 9.52.
4-((2,4,6-Trioxo-tetrahydropyrimidin-5(6H)-ylidene)methylamino)benzene-sulfonamide (2f)
Yield 89.3%, m.p. >350 °C; IR (KBr, ν, cm−1): 3346 (NH), 3209 (NH2), 3105 (NH), 3042 (=C–H, aromatic), 1643 (C=O); 1H NMR (300 MHz, DMSO-d6, ppm): 11.95 (1H, d, –NH, j = 13.7), 11.12 (1H, –NH), 10.97 (1H, –NH), 8.64 (1H, d, =CH, j = 13.7), 7.84 (2H, d, =CH, j = 8.8), 7.72 (2H, d, =CH, j = 8.8), 7.41 (NH2), Citation13C NMR (75 MHz, DMSO-d6, ppm): 166.7, 164.1, 151.7, 151.3, 141.85, 141.4, 128.0, 119.2, 94.3. Anal. Calcd for C11H10N4O5S (%): C, 42.58; H, 3.25; N, 18.06; O, 25.78; S, 10.33. Found (%): C, 42.68; H, 3.29; N, 18.16; O, 25.98; S, 10.55.
4-((1,3-Dioxo-1H-inden-2(3H)-ylidene)methylamino)benzene-sulfonamide (2g)
Yield 90.5%, m.p. 307.8 °C; IR (KBr, ν, cm−1): 3367 (NH2), 3278 (NH), 3052 (=C–H, aromatic), 1651 (C=O); 1H NMR (300 MHz, DMSO-d6, ppm): 11.25 (1H, d, –NH, j = 13.8), 8.38 (1H, d, =CH, j = 13.8), 7.84 (2H, d, =CH), 7,80 (2H, d, =CH), 7.78 (2H, t, =CH), 7.72 (2H, t, =CH), 7.39 (NH2), Citation13C NMR (75 MHz, DMSO-d6, ppm): 191.9, 189.6, 144.5, 142.4, 141.1, 140.2, 140.2, 134.9, 127.8, 122.5, 122.2, 119.0, 107.3. Anal. Calcd for C16H12N2O4S (%): C, 58.53; H, 3.68; N, 8.53; O, 19.49; S, 9.77. Found (%): C, 58.65; H, 3.75; N, 8.61; O, 19.57; S, 9.96.
4-((2,5-Dioxocyclopentylidene)methylamino)benzene-sulfonamide (2h)
Yield 78.7%, m.p. 290.7 °C; IR (KBr, ν, cm−1): 3296 (NH2), 3203 (NH), 3076 (=C–H, aromatic), 1593 (C=O); 1H NMR (300 MHz, DMSO-d6, ppm): 11.95 (1H, d, –NH, j = 13.2), 8.27 (1H, d, =CH, j = 13.2), 7.80 (2H, d, =CH, j = 6.7), 7.77 (2H, d, =CH, j = 6.7), 7.36 (NH2), 2.39 (2H, d, –CH2), 2.36 (2H, d, –CH2). Citation13C NMR (75 MHz, DMSO-d6, ppm): 205.4, 201.9, 147.2, 141.8, 127.8, 119.6, 109.7, 34.5, 33.9. Anal. Calcd for C12H12N2O4S (%): C, 51.42; H, 4.32; N, 9.99; O, 22.83; S, 11.44. Found (%): C, 51.56; H, 4.36; N, 9.91; O, 22.94; S, 11.50.
Preparation of haemolysate and purification from blood red cells
Blood samples (25 mL) were taken from healthy human volunteers. They were anticoagulated with acid-citrate-dextrose, centrifuged at 2000g for 20 min at 4 °C and the supernatant was removed. The packed erythrocytes were washed three times with 0.9% NaCl and then haemolysed in cold water. The ghosts and any intact cells were removed by centrifugation at 2000g for 25 min at 4 °C, and the pH of the haemolysate was adjusted to pH 8.5 with solid Tris-base. The 25 mL haemolysate was applied to an affinity column containing L-tyrosine-sulfonamide-Sepharose-4BCitation26 equilibrated with 25 mM Tris–HCl/0.1 M Na2SO4 (pH 8.5). The affinity gel was washed with 50 mL of 25 mM Tris–HCl/22 mM Na2SO4 (pH8.5). The hCA isozymes were then eluted with 0.1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6), which recovered hCA I and hCA II, respectively. Fractions of 3 mL were collected and their absorbance measured at 280 nm.
CA enzyme assay
CA activity was measured by the Maren method based on the determination of the time required for the pH to decrease from 10.0 to 7.4 due to CO2 hydrationCitation19. The assay solution was 0.5 M Na2CO3/0.1 M NaHCO3 (pH 10.0) and phenol red was added as the pH indicator. CO2-hydratase activity (enzyme units (EU)) was calculated by using the equation t0 − tc/tc where t0 and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
In vitro inhibition studies
For the inhibition studies of sulfonamide, different concentrations of these compounds were added to the enzyme. Activity percentage values of CA for different concentrations of each sulfonamide were determined by regression analysis using Microsoft Office 2000 Excel (New York, NY). CA enzyme activity without a sulfonamide solution was accepted to be 100%.
Results and discussion
For evaluating the physiologically relevant hCA isozymes hCA I and hCA II inhibitory activity, several 1,3-dicarbonyl derivatives of methylaminobenzene-sulfonamide were subjected to CA inhibition assay with CO2 as substrate.
The prepared compounds were characterized by 1H NMR, Citation13C NMR, IR and elemental analysis. From the 1H NMR spectra, the hydrogen attached to the nitrogen resonances between 11.00 and 13.00 ppm, the =CH proton peak comes around 8.50 ppm and NH2 protons are seen between 7.00 and 7.50. From the Citation13C NMR spectra, 1,3-diketone carbonyl and barbiturate carbonyl carbons are observed at around 200 and 160 ppm, respectively. In the infrared spectra of compounds 2a–h, it was possible to observe the absorptions between 3200 and 3400 cm−1 relating to NH stretch, absorptions in 1590–1700 cm−1 from carbonyl moiety stretching.
Sulfonamides are coordinated to the zinc (II) ion within the hCAs active site, whereas its organic scaffold fills the entire enzyme cavity, making an extensive series of van der Waals and polar interactions with amino acid residues both at the bottom, middle and entrance of the active site cavityCitation27.
Inhibitors of CA play an important role in ophthalmology where they are used to reduce elevated intraocular pressure (IOC). Acetazolamide is the most widely used inhibitor and has advantages over the others. It is 20 times less active against CAI than against CAII in erythrocytesCitation28. For evaluating the physiologically relevant hCA isozymes hCA I and hCA II inhibitory activity, several new sulfonamide compounds were subjected to CA inhibition assay with CO2 as substrate. The results showed that all the compounds inhibited the enzyme activity and the inhibition constants against CAs were given in . It is determined that the inhibition values are between 2.12 and 10.89 µM for hCA I and between 2.52 and 18.64 µM for hCA II. Among the compounds, 2b and 2e were found to be the most active for CAs.
Table 1. The IC50 values of the synthesized compounds.
Although sulfonamide compounds are mentioned very potent inhibitor of the cytosolic isoform hCAsCitation29, all the synthesized substituted sulfonamides are moderate inhibitory activity for the hCAs. Substituents on the sulfonamides affect inhibition. In this way, methyl groups in the 2b (2.12 µM for CA I) and 2e (2.52 µM for CA II), and aromatic group in 2g (3.19 and 3.36 µM for hCA I and hCA II, respectively) make them strong inhibitory effects on hCAs compared to the other synthesized sulfonamides.
Although the structures of CA I and CA II are similar, there are several differences in the active sites of amino acids. Particularly, hydrophobic residues (Phe131, Val135 and Leu204) on the surface of CAII play an important stabilizing role when interacting with the hydrophobic groups of sulfonamide inhibitorsCitation30. The sulfonamides inhibit CAs by the coordination of the sulfonamide moiety to the metal ion (Zn+2) from the enzyme active site, and participation in hydrophobic and hydrophilic interactions of the organic scaffold of the inhibitor with amino acids residues are known to be involved in the binding process of CAsCitation31. It is assumed that the effects are the results of hydrophobic and van der Waals interactions between aromatic/aliphatic part of the inhibitor molecule and active site of amino acid residues. The compounds have a lower affinity to CA I and CA II compared to acetazolamideCitation32 which is used in the treatment of glaucoma.
In conclusion, we report here a series of 1,3-dicarbonyl derivatives of methylaminobenzene-sulfonamide which have been assayed for the inhibition of the physiologically relevant CA isozymes. They inhibit the CAs with the inhibition constants of 2.12–10.89 µM for hCA I and 2.52–18.64 µM for hCA II. Compounds 2b and 2e behaved as a moderate inhibitory activity on hCA I and hCA II with an activation constant of 2.12 and 2.52 µM, respectively. Last but not the least, enzyme inhibition is more important issue for drug design and biochemical applications Citation33–46. The results put forward that new sulfonamide derivatives inhibited the hCA I and hCA II enzyme activity. Therefore, our results suggested that sulfonamide derivatives are likely to be adopted as candidates to treat glaucoma and they may be taken for further evaluation in in vivo studies.
Declaration of interest
This work was supported by Research Fund of the Sakarya University. Project Number: 2012-02-04-033.
References
- Hewett-Emmet D. Evolution and distribution of the carbonic anhydrase gene families. In: Chegwidden WR, Edwrds Y, Carter N, eds. The carbonic anhydrase – new horizons. Basel: Birkhauser Verlag; 2000:29–78
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89
- Vullo D, Voipio J, Innocenti A, et al. Carbonic anhydrase inhibitors. Inhibition of the human cytosolic isozyme VII with aromatic and heterocyclic sulfonamides. Bioorg Med Chem Lett 2005;15:971–6
- Nishimori I, Minakuchi T, Onishi S, et al. Carbonic anhydrase inhibitors. DNA cloning, characterization, and inhibition studies of the human secretory isoform VI, a new target for sulfonamide and sulfamate inhibitors. J Med Chem 2007;50:381–8
- Gitto R, Damiano FM, Mader P, et al. Synthesis, structure-activity relationship studies, and X-ray crystallographic analysis of arylsulfonamides as potent carbonic anhydrase inhibitors. J Med Chem 2012;55:3891–9
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50
- Supuran CT. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med Chem 2011;3:1165–80
- Ekinci D, Çavdar H, Durdagi S, et al. Structure-activity relationships for the interaction of 5,10-dihydroindeno[1,2-b] indole derivatives with human and bovine carbonic anhydrase isoforms I, II, III, IV and VI. Eur J Med Chem 2012;49:68–73
- Lach L, Pasquet M-J, Chabanne M. A general route to unsubstituted N-aryl and heteroarylaminobenzenesulfonamides. Tetrahedron Lett 2011;52:1882–7
- Perrin KA, Huang J, McElroy EB, et al. Defining the interactions between DNA and the exonuclease domain of DNA-polymerases. J Am Chem Soc 1994;116:7427–8
- Campbell JA, Hart D. Tert-butyl [[2-(trimethylsilyl)ethyl]sulfonyl]carbamate – a new reagent for use in mitsunobu reactions. J Org Chem 1993;58:2900–3
- Gennari C, Longari C, Ressel S, et al. Synthesis of chiral vinylogous sulfonamidopeptides (vs-peptides). Eur J Org Chem 1998;6:945–59
- Arya P, Quin HS. Advances in asymmetric enolate methodology. Tetrahedron 2000;56:917–47
- Scozzafava A, Owa T, Mastrolorenzo A, Supuran CT. Anticancer and antiviral sulphonamides. Curr Med Chem 2003;10:925–53
- Mustafa G, Khan IU, Ashraf M, et al. Synthesis of new sulfonamides as lipoxygenase inhibitors. Bioorg Med Chem 2012;20:2535–9
- Rauf A, Ahmed F, Qureshi AM, et al. Synthesis and urease inhibition studies of barbituric and thiobarbituric acid derived sulphonamides. J Chin Chem Soc 2011;58:528–37
- Kozlovskaya TF, Strakov AY, Petrova MV, et al. Reactions of 2-formyldimedone with some nitrogeneous nucleophiles. Latvijas Kimijas Zurnals 1991;2:179–84
- Gadad AK, Mahajanshetti CS, Nimbalkar S, Raichurkar A. Synthesis and antibacterial activity of some 5-guanylhydrazone/thiocyanato-6-arylimidazo[2,1-b]-1,3,4-thiadiazole-2-sulfonamide derivatives. Eur J Med Chem 2000;35:853–67
- Maren TH. A simplified micromethod for the determination of carbonic anhydrase and its inhibitors. J Pharm Exp Ther 1960;130:2629–34
- Li JJ, Anderson D, Burton EG, et al. 1,2-Diarylcyclopentenes as selective cyclooxygenase-2 inhibitors and orally active antiinflammatory agents. J Med Chem 1995;38:4570–8
- Yoshino H, Ueda N, Niijma J, et al. Novel sulfonamides as potential, systemically active antitumor agents. J Med Chem 1992;35:2496–7
- Renzi G, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: topical sulfonamide antiglaucoma agents incorporating secondary amine moieties. Bioorg Med Chem Lett 2000;10:673–6
- Kasımoğulları R, Bülbül M, Arslan BS, Gökçe B. Synthesis, characterization and antiglaucoma activity of some novel pyrazole derivatives of 5-amino-1,3,4-thiadiazole-2-sulfonamide. Eur J Med Chem 2010;45:4769–73
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Sugrue MF. Pharmacology and ocular hypotensive properties of topical carbonic anhydrase inhibitors. Prog Ret Eye Res 2000;19:87–112
- Ozensoy O, Arslan O, Oznur SS. A new method for purification of carbonic anhydrase isozymes by affinity chromatography. Biochemistry (Mosc) 2004;69:216–19
- Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase ınhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44
- Arslan O, Cakır U, Ugras HI. Synthesis of new sulfonamide inhibitors of carbonic anhydrase. Biochemistry (Mosc) 2002;67:1055–7
- Winum JY, Vullo D, Casini A, et al. Carbonic anhydrase inhibitors. Inhibition of cytosolic isozymes I and II and transmembrane, tumor-associated isozyme IX with sulfamates including EMATE also acting as steroid sulfatase inhibitors. J Med Chem 2003;46:2197–204
- Hen N, Bialer M, Yagen B, et al. Anticonvulsant 4-aminobenzenesulfonamide derivatives with branched-alkylamide moieties: X-ray crystallography and inhibition studies of human carbonic anhydrase isoforms I, II, VII, and XIV. J Med Chem 2011;54:3977–81
- Wagner J, Avvaru BS, Robbins AH, et al. Coumarinyl-substituted sulfonamides strongly inhibit several human carbonic anhydrase isoforms: solution and crystallographic investigations. Bioorg Med Chem 2010;18:4873–8
- Arslan O, Küfrevioğlu OI, Nalbantoğlu B. Synthesis and investigation of inhibition effects of new carbonic anhydrase inhibitors. Bioorg Med Chem 1997;5:515–18
- Aydemir T, Kavrayan D. Purification and characterization of glutathione-s-transferase from chicken erythrocyte. Artif Cells Blood Substit Immobil Biotechnol 2009;37:92–100
- Gencer N, Arslan O. Purification human PON1 (Q192) and PON1(R192) isoenzymes by hydrophobic interaction chromatography and investigation of the inhibition by metals. J Chrom B Anal Tech Biomed Life Sci 2009;877:134–40
- Gencer N, Ergün A, Demir D. In vitro effects of some anabolic compounds on erythrocyte carbonic anhydrase I and II. J Enzyme Inhib Med Chem 2012;27:208–10
- Sinan S, Gencer N, Turan Y, Arslan O. In vitro inhibition of the carbonic anhydrase from saanen goat (Capra hircus) with pesticides. Pest Biochem Phys 2007;88:307–11
- Kiranoglu S, Sinan S, Gencer N, et al. In vivo effects of oral contraceptives on paraoxonase, catalase and carbonic anhydrase enzyme activities on mouse. Biolog Pharm Bull 2007;30:1048–51
- Sinan S, Kockar F, Gencer N, et al. Amphenicol and macrolide derived antibiotics inhibit paraoxonase enzyme activity in human serum and human hepatoma cells (HepG2) in vitro. Biochemistry (Mosc) 2006;71:46–50
- Erol K, Gençer N, Arslan M, Arslan O. Purification, characterization, and investigation of in vitro inhibition by metals of Paraoxonase from different sheep breeds. Artif Cells Blood Substit Immobil Biotechnol 2012; In press. DOI: 10.3109/10731199.2012.696065
- Demir D, Gençer N, Er A. Purification and characterization of prophenoloxidase from Galleria mellonella L. Artif Cells Blood Substit Immobil Biotechnol 2012;40:369–77
- Bytyqi-Damoni A, Genç H, Zengin M, et al. In vitro effect of novel β-lactam compounds on xanthine oxidase enzyme activity. Artif Cells Blood Substit Immobil Biotechnol 2012;40:369–77
- Gökçe B, Gençer N, Arslan O, et al. Evaluation of in vitro effects of some analgesic drugs on erythrocyte and recombinant carbonic anhydrase I and II. J Enzyme Inhib Med Chem 2012;27:37–42
- Ekinci D, Al-Rashida M, Abbas G, et al. Chromone containing sulfonamides as potent carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:744–7
- Senturk M, Ekinci D, Goksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2012;27:365–9
- Cankaya M, Aktas M, Kuzucu M, et al. Effects of some drugs on human cord blood erythrocyte carbonic anhydrases I and II: an in vitro study. J Enzyme Inhib Med Chem 2012;27:641–5
- Sayin D, Cakir DT, Gencer N, Arslan O. Effects of some metals on paraoxonase activity from shark Scyliorhinus canicula. J Enzyme Inhib Med Chem 2012;27:595–8