Abstract
A series of coumarins and benzocoumarins incorporating methyl and hydroxyl moieties in the heterocyclic ring were investigated for the inhibition of the zinc enzyme carbonic anhydrase (CA, EC 4.2.1.1). These coumarins were very weak or ineffective as inhibitors of the house-keeping, offtarget isoforms CA I and II, but showed effective, submicromolar inhibition of the transmembrane, tumor-associated isoforms CA IX and to a slightly less extent, CA XII. The nature and position of the groups substituting the coumarin ring influenced CA inhibitory properties. 4-Methyl-5,7-dihydroydroxycoumarin showed KIs >200 µM against CA I and II, of 0.19 µM against CA IX and of 6.4 µM against CA XII, being thus a selective, efficient inhibitor for the tumor-associated over cytosolic CA isoforms. These compounds are interesting leads for designing isoform-selective enzyme inhibitors.
Introduction
Coumarins are a class of widely spread natural compounds which was only recently revealed to inhibit the zinc enzyme carbonic anhydrase CA (EC 4.2.1.11)Citation1–3. CAs are ubiquitous enzymes in organisms throughout the tree of life, with several genetically distinct families encoding them in prokaryotes and eukaryotesCitation4–11. CAs are inhibited by metal complexing anions, sulfonamides and their isosteres (sulfamates, sulfamides, etc.), phenols and polyamines, which bind either to the metal ion within the enzyme active site or are anchored to the water molecule coordinated to itCitation11. However, the coumarin inhibition mechanism of the α-CAs is totally different and was only recently decipheredCitation1,Citation2. Coumarin derivatives were recently shown to constitute a totally new class and type of CA inhibitors (CAIs), being “prodrugs” which are hydrolyzed within the CA active site with formation of 2-hydroxycinnamic acids.
The coumarins represent an interesting class of CAIs due to several reasons:
The inhibition mechanism is different from those of other CAIs, and due to their binding at the entrance of the enzyme active site, where there is a rather large variability in the 3D structure and amino acid sequence between the various isoforms, may lead to compounds with isoform-selective behaviorCitation1–3.
Coumarins are widespread natural products and are also easily prepared synthetically with a large range of substitution patterns, incorporating moieties of diverse chemical nature, in different numbers of such moieties and in diverse positions of the coumarin ring.
A natural product coumarin, 6-(1S-hydroxy-3-methylbutyl)-7-methoxy-2H-chromen-2-one, isolated from the Australian plant Leionema ellipticum, was the first compound shown to possess significant CA inhibitory activityCitation1. By means of X-ray crystallography of it’s adduct with the human (h) isoform hCA II, it was evidenced the presence of a substituted 2-hydroxy-cinnamic acid in the enzyme active site, which is the hydrolysis product (mediated by the CA) of the original coumarinCitation1. The same situation has been thereafter observed for the simple, unsubstituted coumarinCitation2. The 2-hydroxy-cinnamic acids formed from the original coumarins occluded the entrance to the enzyme active site, a mechanism never evidenced before for CA inhibitionCitation2,Citation11. This region of the CA active site is on the other hand the most variable one among the 16 CA isoforms present in mammals, and this explains the observations that many coumarin derivatives show a high selectivity ratio for inhibiting various CA isoforms, many of which have pharmacologic applicationsCitation1–3.
In fact recently, some glycosyl-substituted coumarins with potent inhibitory activity and selectivity for the tumor-associated isozyme CA IX and XII, were shown to strongly inhibit the growth of primary tumors and metastases overexpressing these enzymes, in an animal model of breast cancerCitation3. For this reason, exploring novel coumarin derivatives as CAIs, or compounds known in the literature but not yet investigated for this activity, is of interest in the design of isoform-selective pharmacological agents of this type. Indeed, CAIs have a range of applications as antiglaucoma, antiobesity, antiepileptic or diuretic agentsCitation12–26. Furthermore, inhibition of CAs from various bacteria or fungi may also lead to the development of antiinfectives based on CAIsCitation27,Citation28. Here, we report the synthesis of several coumarin/benzocoumarin derivatives and their inhibition against medicinally important CA isoforms, such as CA I, II, IX and XII.
Materials and methods
Chemistry
The Pechmann condensation is the most widely used method for coumarin synthesis, as it uses simple starting materials and gives good yields for variously substituted coumarinsCitation29,Citation30. The Pechmann condensation allows the synthesis of coumarins by reaction of phenols with β-keto esters. The reaction was carried out by addition of TiCl4 to the mixture of the reagents, with stirring as exemplified below for the preparation of coumarins/benzocoumarins 1–5.
Synthesis of 7-hydroxy-4-methyl-2H-2-one 1
Resorcinol (10 mmol, 1.10 g) and ethyl acetoacetate (20 mmol, 2.5 ml) were mixed thoroughly in round bottom flask and titanium (IV) chloride (10 mmol) was added to the mixture with constant stirring at room temperature. Small amount of ethanol was added, to facilitate the stirring of mixture. The reaction was completed in 5–6 h. The thin layer chromatography was performed to check the completion of reactions. On the completion of reactions as indicated by TLC, solvent was evaporated and reaction mixture was poured onto crushed ice and the resulting crude product was filtered off and recrystallized from ethanol–water mixture (1:1) and then, purified by column chromatography. Yield 78%; Rf: 0.428 (ethyl acetate/hexane 3:7); m.p.: 188–190 °C; 1HNMR (400 MHz, DMSO): δ ppm 2.257 (s, 3H, Me), 6.001(s, 1H, C=CH), 6.626–7.439 (m, 3H, ArH), 10.463 (s, 1H, ArOH); IR (KBr) 3491, 3123, 2949, 1672, 1672, 1601, 1272 cm−1. EIMS: m/z: 176 (M+).
Synthesis of 5,7-dihydroxy-4-methyl-2H-chromen-2-one 2
Phloroglucinol (10 mmol, 1.26 g) and ethyl acetoacetate (20 mmol, 2.5 ml) were mixed thoroughly in round bottom flask and titanium (IV) chloride (10 mmol) was added to the mixture with constant stirring at room temperature. Small amount of ethanol was added, to facilitate the stirring of mixture. The reaction was completed in 18–20 h. The thin layer chromatography was performed to check the completion of reactions. On the completion of reactions as indicated by TLC, solvent was evaporated and reaction mixture was poured onto crushed ice and the resulting crude product was filtered off and recystallized from ethanol–water mixture (1:1) and purified by column chromatography. Yield 70%; Rf: 0.269 (ethyl acetate/hexane 3:7); m.p.: 260–262 °C; 1HNMR (400 MHz, DMSO): δ ppm 2.503 (s, 3H, Me), 5.848(s, 1H, C=CH), 6.162–6.255 (d, 2H ArH), 10.297 (s, 1H, ArOH), 10.523 (S, 1H, ArOH); IR (KBr) 3421, 3124, 2922, 1663, 1618, 1296 cm−1; EIMS: m/z: 192 (M+).
Synthesis of 7,8-dihydroxy-4-methyl-2H-chromen-2-one 3
Pyrogallol (benzene-1,2,3-triol, 10 mmol) and ethyl acetoacetate (20 mmol) were mixed thoroughly in round bottom flask and titanium (IV) chloride (10 mmol) was added to the mixture with constant stirring at room temperature. Small amount of ethanol was added, to facilitate the stirring of mixture. The reaction was completed in 8–9 h. The thin layer chromatography was performed to check the completion of reactions. On the completion of reactions as indicated by TLC, solvent was evaporated and reaction mixture was poured onto crushed ice and the resulting crude product was filtered off and recystallized from ethanol–water mixture (1:1) and purified by column chromatography. Yield 72%; Rf: 0.283 (ethyl acetate/hexane 3:7); m.p.: 260–262 °C; 1HNMR (400 MHz, DMSO): δ ppm 2.339 (s, 3H, Me), 6.108 (s, C=CH), 6.783–7.090 (m, 2H, ArH), 9.644 (s, 1H, ArOH); IR (KBr) 3412, 3233, 2865, 1663, 1609, 1597, 1236 cm−1; EIMS: m/z: 192 (M+).
Synthesis of 4-methyl-2H-benzo[h]chromen-2-one 4
α-Naphthol (10 mmol) and ethyl acetoacetate (20 mmol) were mixed thoroughly in round bottom flask and titanium (IV) chloride (10 mmol) was added to the mixture with constant stirring at room temperature. Small amount of ethanol was added, to facilitate the stirring of mixture. The reaction was completed in 3–4 h. The thin layer chromatography was performed to check the completion of reactions. On the completion of reactions as indicated by TLC, solvent was evaporated and reaction mixture was poured onto crushed ice and the resulting crude product was filtered off and recystallized from ethanol–water mixture (1:1) and purified by column chromatography. Yield 60%; Rf: 0.352 (ethyl acetate/hexane 3:7); m.p.: 154–156 °C; 1HNMR (400 MHz, DMSO): δ ppm 1.827 (s, 3H, Me), 6.464 (s, 1H, C=CH), 7.694–8.326 (m, 6H, ArH)); IR (KBr) 3059, 2920, 1707, 1609, 1273 cm−1.; EIMS: m/z: 210 (M+).
Synthesis of 4-methyl-2H-benzo[g]chromen-2-one 5
β-Naphthol (10 mmol) and ethyl acetoacetate (20 mmol) were mixed thoroughly in round bottom flask and titanium (IV) chloride (10 mmol) was added to the mixture with constant stirring at room temperature. Small amount of ethanol was added, to facilitate the stirring of mixture. The reaction was completed in 3–4 h. The thin layer chromatography was performed to check the completion of reactions. On the completion of reactions as indicated by TLC, solvent was evaporated and reaction mixture was poured onto crushed ice and the resulting crude product was filtered off and recystallized from ethanol–water mixture (1:1) and purified by column chromatography. Yield 81%; Rf: 0.403 (ethyl acetate/hexane 3:7); m.p.: 150–150 °C; 1HNMR (400 MHz, DMSO): δ ppm 1.689 (s, 3H, Me), 7.052–7.749 (m, 6H, ArH); IR (KBr) 3273, 2866, 1674, 1630, 1277 cm−1.; EIMS: m/z: 210 (M+).
CA inhibition
An applied photophysics stopped-flow instrument has been used for assaying the CA catalyzed CO2 hydration activity. Phenol red (at a concentration of 0.2 mM) has been used as indicator, working at the absorbance maximum of 557 nm, with 20 mM Hepes (pH 7.5) as buffer, and 20 mM Na2SO4 (for maintaining constant the ionic strength), following the initial rates of the CA-catalyzed CO2 hydration reaction for a period of 10–100 s. The CO2 concentrations ranged from 1.7 to 17 mM for the determination of the kinetic parameters and inhibition constantsCitation31. For each inhibitor at least six traces of the initial 5–10% of the reaction have been used for determining the initial velocity. The uncatalyzed rates were determined in the same manner and subtracted from the total observed rates. Stock solutions of inhibitor (0.1 mM) were prepared in distilled-deionized water and dilutions up to 0.01 nM were done thereafter with distilled-deionized water. Inhibitor and enzyme solutions were preincubated together for 15 min to 72 h at room temperature (15 min) or 4 °C (all other incubation times) prior to assay, in order to allow for the formation of the E-I complex or for the eventual active site mediated hydrolysis of the inhibitor. Data reported in show the inhibition after 6 h incubation, which led to the completion of the in situ hydrolysis of the coumarin and formation of the 2-hydroxy-cinnamic acids. The inhibition constants were obtained by non-linear least-squares methods using PRISM 3, as reported earlierCitation1–3, and represent the mean from at least three different determinations. CA isoforms were recombinant ones obtained in house as reported earlierCitation1–3.
Table 1. hCA I, II, IX and XII inhibition data with coumarins 1–5 by a stopped flow, CO2 hydrase assayCitation31.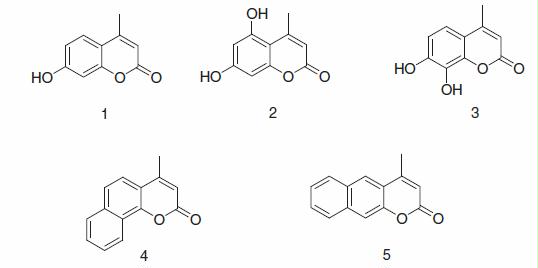
Discussion and conclusions
In previous contributions from our laboratory, we showed that even simple coumarins derivatives may lead to interesting inhibition profiles against various CA isoforms, with the discovery of a wide range of isoform-selective CAIsCitation1,Citation2,Citation29,Citation30. Thus, we investigated here the synthesis of new coumarins and inhibition of several pharmacologically important CA isoforms (e.g. CA I, II, IX and XII) with a small series of such compounds, i.e. coumarins (1–3) and benzocoumarins (4 and 5) ().
Scheme 1. Preparation of the coumarins/benzocoumarins 1–5 from phenols A, C and D and ethyl acetoacetate B.
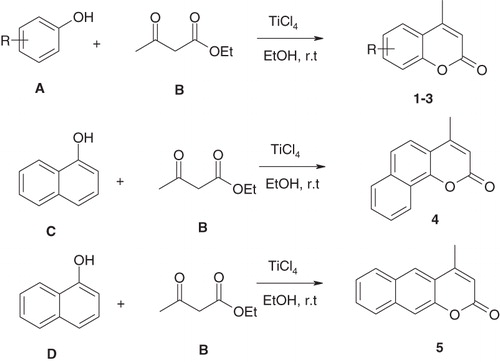
Compounds 1–5 were prepared by literature procedures, by the Pechmann condensation of phenols A or naphthols C and D, with ethyl acetoacetate B, in the presence of Ti(IV) chloride as catalystCitation29,Citation30. Compounds 1–5 have been characterized by standard physico–chemical procedures which confirmed their structures (“Materials and methods” section for details).
Coumarins 1–5 were investigated as inhibitorsCitation31 of four catalytically active hCA isoforms, hCA I, hCA II (cytosolic isoforms) as well as hCA IX and hCA XII (transmembrane, tumor-associated enzymes)Citation32 (). The following structure–activity relationship (SAR) can be evidenced from the data of :
The slow cytosolic isoform hCA I was moderately inhibited by coumarins 1–5, with inhibition constants in the range of 4.8–9.0 µM. The bets inhibitor was 4-methyl-7-hydroxy-coumarin 1 (KI of 4.8 µM), whereas the remaining 4 derivatives showed a flat, less potent inhibition (KIs around 9 µM, ).
As for other simple coumarins investigated earlierCitation29,Citation30, also coumarins 1–3 and benzocoumarins 4 and 5 were devoid of inhibitory activity against the dominant isoform hCA II, which is a very interesting feature of this class of CAIs. Indeed, the house-keeping, and quite abundant isoform hCA II showed KIs > 200 µM with all these compounds, which constitutes a highly attractive feature for inhibitors that should target isoforms involved in tumorigenesis (CA IX and XII)Citation32 or in other processes, such as for example obesity (CA VA and VB)Citation33,Citation34.
The tumor-associated hCA IX was well-inhibited by compounds 1–5, with KIs in the submicromolar range, i.e. of 0.19–0.45 µM (). The best inhibitors were the linear benzocoumarin 5 (KI of 0.20 µM) and the dihydroxy-4-methyl-coumarin 2 (KI of 0.19 µM), but the remaining derivatives were only slightly less effective CAIs than 2 and 5.
The second tumor-associated isoform, hCA XII, was also inhibited by these compounds but less efficiently compared to hCA IX. Indeed, the coumarins/benzocoumarins 1–5 showed inhibition constants in the range of 3.3–6.6 µM. Again thus, a quite compact behavior, with little SAR, which may be explained by the rather similar structures of the investigated compounds.
In conclusion, a small series of coumarins/benzocoumarins incorporating methyl and hydroxyl moieties in the heterocyclic ring were investigated as CAIs. These coumarins were very weak or ineffective as inhibitors of the house-keeping, offtarget isoforms CA I and II, but showed effective, submicromolar inhibition of the transmembrane, tumor-associated isoforms CA IX and XII. The nature and position of the groups substituting the coumarin ring influenced CA inhibitory properties. 5,7-Dihydroydroxycoumarin showed KIs > 200 µM against CA II, of 0.19 µM against CA IX and of 6.4 µM against CA XII, being thus an isoform-selective, efficient inhibitor for the tumor-associated over cytosolic CA isoforms. These compounds are interesting leads for designing isoform-selective enzyme inhibitors.
Declaration of interest
The authors report no conflict of interest. This work was supported by an EU FP7 research grant (Metoxia project).
Acknowledgements
We are grateful to Dr Alfonso Maresca and Dr Daniela Vullo for technical support.
References
- Maresca A, Temperini C, Vu H, et al. Non-zinc mediated inhibition of carbonic anhydrases: coumarins are a new class of suicide inhibitors. J Am Chem Soc 2009;131:3057–62
- Maresca A, Temperini C, Pochet L, et al. Deciphering the mechanism of carbonic anhydrase inhibition with coumarins and thiocoumarins. J Med Chem 2010;53:335–44
- Touisni N, Maresca A, McDonald PC, et al. Glycosyl coumarin carbonic anhydrase IX and XII inhibitors strongly attenuate the growth of primary breast tumors. J Med Chem 2011;54:8271–7
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nature Rev Drug Discov 2008;7:168–81
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nature Rev Drug Discov 2011;10:767–77
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74
- Pastorekova S, Parkkila S, Pastorek J, Supuran CT. Carbonic anhydrases: current state of the art, therapeutic applications and future prospects. J Enzyme Inhib Med Chem 2004;19:199–229
- Supuran CT. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med Chem 2011;3:1165–80
- Supuran CT. Bacterial carbonic anhydrases as drug targets: towards novel antibiotics? Front Pharmacol 2011;2:34
- Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev 2003;23:146–89
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72
- Liu F, Martin-Mingot A, Lecornué F, et al. Carbonic Anhydrases inhibitory effects of new benzenesulfonamides synthesized by using superacid chemistry. J Enzyme Inhib Med Chem 2012;27:886–91
- Alp C, Ozsoy S, Alp NA, et al. Sulfapyridine-like benzenesulfonamide derivatives as inhibitors of carbonic anhydrase isoenzymes I, II and VI. J Enzyme Inhib Med Chem 2012;27:818–24
- Kolayli S, Karahalil F, Sahin H, et al. Characterization and inhibition studies of an α-carbonic anhydrase from the endangered sturgeon species Acipenser gueldenstaedti. J Enzyme Inhib Med Chem 2011;26:895–900
- Kazancıoğlu EA, Güney M, Sentürk M, Supuran CT. Simple methanesulfonates are hydrolyzed by the sulfatase carbonic anhydrase activity. J Enzyme Inhib Med Chem 2012;27:880–5
- Cavdar H, Ekinci D, Talaz O, et al. α-Carbonic anhydrases are sulfatases with cyclic diol monosulfate esters. J Enzyme Inhib Med Chem 2012;27:148–54
- Ekinci D, Kurbanoglu NI, Salamcı E, et al. Carbonic anhydrase inhibitors: inhibition of human and bovine isoenzymes by benzenesulphonamides, cyclitols and phenolic compounds. J Enzyme Inhib Med Chem 2012;27:845–8
- Ekinci D, Al-Rashida M, Abbas G, et al. Chromone containing sulfonamides as potent carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:744–7
- Singh S, Supuran CT. QSARs on human carbonic anhydrase VA and VB inhibitors of some new not yet synthesized, substituted aromatic/heterocyclic sulphonamides as anti-obesity agent. J Enzyme Inhib Med Chem 2012;27:666–72
- Fabrizi F, Mincione F, Somma T, et al. A new approach to antiglaucoma drugs: carbonic anhydrase inhibitors with or without NO donating moieties. Mechanism of action and preliminary pharmacology. J Enzyme Inhib Med Chem 2012;27:138–47
- Sahin H, Can Z, Yildiz O, et al. Inhibition of carbonic anhydrase isozymes I and II with natural products extracted from plants, mushrooms and honey. J Enzyme Inhib Med Chem 2012;27:395–402
- Sentürk M, Ekinci D, Göksu S, Supuran CT. Effects of dopaminergic compounds on carbonic anhydrase isozymes I, II, and VI. J Enzyme Inhib Med Chem 2012;27:365–9
- Bootorabi F, Jänis J, Hytönen VP, et al. Acetaldehyde-derived modifications on cytosolic human carbonic anhydrases. J Enzyme Inhib Med Chem 2011;26:862–70
- Chohan ZH, Shad HA, Supuran CT. Synthesis, characterization and biological studies of sulfonamide Schiff's bases and some of their metal derivatives. J Enzyme Inhib Med Chem 2012;27:58–68
- Ozensoy O, Arslan M, Supuran CT. Carbonic anhydrase inhibitors: purification and inhibition studies of pigeon (Columba livia var. domestica) red blood cell carbonic anhydrase with sulfonamides. J Enzyme Inhib Med Chem 2011;26:749–53
- Sahin H, Aliyazicioglu R, Yildiz O, et al. Honey, pollen, and propolis extracts show potent inhibitory activity against the zinc metalloenzyme carbonic anhydrase. J Enzyme Inhib Med Chem 2011;26:440–4
- Del Prete S, De Luca V, Scozzafava A, et al. Biochemical properties of a new α-carbonic anhydrase from the human pathogenic bacterium Vibrio cholerae. J Enzyme Inhib Med Chem 2013. [Epub ahead of print]
- Capasso C, De Luca V, Carginale V, et al. Biochemical properties of a novel and highly thermostable bacterial α-carbonic anhydrase from Sulfurihydrogenibium yellowstonense YO3AOP1. J Enzyme Inhib Med Chem 2012;27:892–7
- Maresca A, Supuran CT. Coumarins incorporating hydroxy- and chloro-moieties selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II. Bioorg Med Chem Lett 2010;20:4511–14
- Maresca A, Scozzafava A, Supuran CT. 7,8-Disubstituted- but not 6,7-disubstituted coumarins selectively inhibit the transmembrane, tumor-associated carbonic anhydrase isoforms IX and XII over the cytosolic ones I and II in the low nanomolar/subnanomolar range. Bioorg Med Chem Lett 2010;20:7255–8
- Khalifah RG. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J Biol Chem 1971;246:2561–73
- Ebbesen P, Pettersen EO, Gorr TA, et al. Taking advantage of tumor cell adaptations to hypoxia for developing new tumor markers and treatment strategies. J Enzyme Inhib Med Chem 2009;24:1–39
- Supuran CT. Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin Emerg Drugs 2012;17:11–15
- Alterio V, Di Fiore A, D’Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68

