Abstract
A novel matrix metalloproteinase-2 (MMP-2) inhibitor JaZ-30, which belongs to the class of C(2)-monosubstituted aziridine – aryl-1,2,3-triazole conjugates, was developed. MTT and crystal violet assays were used to determine cytotoxicity- IC(50) values of compound JaZ-30 on melanoma cell line B16 4A5. Our study proves the anti-cancer properties of JaZ-30 with a wide spectrum of activities. JaZ-30 was revealed as selective inhibitor of matrix metalloproteinase-2. JaZ-30-mediated decrease of Vascular Endothelial Growth Factor (VEGF) secretion results in inhibition of angiogenesis, performed with the human umbilical vein endothelial cell line (HUVEC-2) on Matrigel. A novel inhibitor decreases invasive properties of melanoma cells measured in Matrigel chambers assay. JaZ-30 downregulates phosphorylation of the extracellular signal-regulated kinases 1 and 2 (ERK1/2) in melanoma cells stimulated by phorbol-12-myristate-13-acetate (PMA). Our findings propose a novel MMP-2 inhibitor JaZ-30 as an attractive potential agent for melanoma treatment.
Introduction
Potential targets for the drug development include inhibitors of the matrix metalloproteinases (MMPs), involved in physiological and pathological processes through the cleavage of extracellular matrix, which is crucial for malignant tumor growth, invasion, metastasis and angiogenesis.
The MMPs overexpression in a variety of malignant tumor types is associated with tumor aggressiveness and metastatic potentialCitation1,Citation2. The specific alteration of the MMPs, observed in malignant tissues, their participation in some of the major oncogenic mechanisms, have determined interest in the design and evaluation of matrix metalloproteinases inhibitors (MMPIs) as anticancer agentsCitation3,Citation4.
Among the MMP family members MMP-2 and MMP-9 are generally considered as the cause of malignancy of various tumors which results in a poor prognosis for patientsCitation5,Citation6. Increased expression of MMP-2 was shown to correlate with an invasive phenotype in melanoma, osteosarcoma and other aggressive metastatic cancers.Citation7,Citation8 Recent studies revealed that inhibition of tumor angiogenesis was regulated by suppression of MMP-2, MMP-9 activity and VEGF secretionCitation9,Citation10. Preclinical studies from diverse laboratories have provided overwhelming support for direct relationship between MMP-2 overexpression and tumor metastasis developmentCitation11,Citation12.
Many MMPIs have been synthesized on the basis of a greater understanding of the MMP spatial structure and catalytic center configurationCitation13–15. Most of the MMPIs failed in the early phase clinical trials because of extensive homology between catalytic domains of MMPs. Moreover, most of the synthetic MMPIs were withdrawn during clinical trials due to unanticipated long-term drug intolerance and reduced drug complianceCitation16,Citation17. Thus, the development of effective MMP-2 inhibitors still remains as an actual problem for cancer chemotherapy.
Only a few examples of aziridine-containing MMPIs are known to dateCitation18. In these compounds as well as in other MMPIs the hydroxamic acid moiety is used as well-accepted Zn2+ binding groupCitation19,Citation20. Great attention has been paid to the appropriate hydrophobic interactions between the lipophilic groups of the inhibitor and S1’pocket of MMPsCitation21,Citation22. The latter approach has led to selective MMPIs that do not require interaction with the catalytic Zn2+ cationCitation23,Citation24. On the other hand, Cu(I)-catalyzed azide alkyne dipolar cycloaddition has been extensively used in medicinal chemistryCitation25. Thus, 1,2,3-triazole unit is also present in several MMP inhibitorsCitation26,Citation27.
We report here our findings about C(2)-monosubstituted aziridine – 1,2,3-triazole conjugate, named JaZ-30, which exhibits selective MMP-2 inhibitory activity, suppresses melanoma cells invasion, inhibits secretion of VEGF and angiogenesis, and downregulates ERK1/2 phosphorylation. Therefore it can be regarded as a potential anticancer agent for melanoma treatment.
Materials and methods
Synthesis of 1-(aziridin-2-ylmethyl)-4-(4-butylphenyl)-1H-1,2,3-triazole (compound 2 – Jaz-30) and preparation of its stock solution
A solution of CuSO4 ċ 5H2O (147 mg, 0.59 mM, 0.1 equiv.) in water (8 ml) was added to a stirred solution of 1-butyl-4-ethynylbenzene (1.12 g, 7.08 mM, 1.2 equiv.) and azidomethyl derivative 3 (2.01 g, 5.90 mM, 1.0 equiv.) in acetone (30 ml) at ambient temperature. A solution of sodium ascorbate (234 mg, 1.18 mM, 0.2 equiv.) in water (8 ml) was added to the previous mixture and the resulting reaction mixture was stirred at ambient temperature overnight. The volatiles were evaporated under reduced pressure and the solid residue was redissolved in CH2Cl2 (50 ml). The crude intermediate was purified by silica gel column chromatography (CH2Cl2/acetone) and afforded 4 -(4-butylphenyl)-1 -((1-tritylaziridin-2-yl)methyl)-1H-1,2,3-triazole (2.00 g, 4.0 mM) that was dissolved in a mixture of CH2Cl2 (20 ml) and methanol (20 ml). Trifluoroacetic acid (6.13 ml, 80.0 mM, 20 equiv.) was added to the latter solution at 0 °C. The resulting reaction mixture was stirred at 0 °C for 6 h. Then the crushed ice (25 g) was added and the reaction mixture was evaporated at reduced pressure. The formed precipitate (TrOH + TrOMe) was filtered off and washed on the filter with water. The filtrate was cooled to 0 °C and basified with solid K2CO3 until pH 9–10. The aqueous phase was extracted with CHCl3 (4 × 20 ml), the combined organic layer was washed with brine (10 ml), dried over anhydrous Na2SO4, filtered and evaporated under reduced pressure. The residue was purified by silica gel column chromatography (CH2Cl2/MeOH) and afforded product 4 (0.30 g, 20% in two steps). 1H-NMR (300 MHz, DMSO-D6) δ, ppm: 8.51 (s, 1H, H-C(triazole)), 7.75, 7.26 (2 d, 4H, 3J = 8.1 Hz, H-C(Ar)), 4.41 (dd, 1H, 2J = 14.0 Hz, 3J = 4.1 Hz, Ha-C(1′)), 4.19 (dd, 1H, 2J = 14.0 Hz, 3J = 7.0 Hz, Hb-C(1′)), 2.60 (t, 2H, 3J = 7.5 Hz, H-C(1″)), 2.39–2.31 (bs, 1H, H-N(1)), 1.78–1.72 (bs, 1H, Ha-C(3)), 1.57 (quint., 2H, 3J = 7.5 Hz, H-C(2″)), 1.57–1.43 (bs, 1H, H-C(2)), 1.43–1.37 (bs, 1H, Hb-C(3)), 1.32 (sextet, 2H, 3J = 7.5 Hz, H-C(3″)), 0.90 (t, 3H, 3J = 7.5 Hz, H-C(4’’)). 13C-NMR (75 MHz, DMSO d6) δ, ppm: 146.4, 142.0, 128.8, 128.3, 125.1, 121.1, 53.6, 34.6, 33.1, 28.6, 23.1, 21.8, 13.8. HRMS (ESI): m/z Calcd for C15H20N4+H+: 257.1761. Found 257.1755.
For investigations JaZ-30 was dissolved in DMSO at 10 mM concentration. Depending on the experimental conditions, the stock solution of JaZ-30 was diluted with appropriate solvent.
Cell lines and cultivation
Highly metastatic B16 4A5 melanoma cell line (B16) was obtained from the European collection of Animal cell cultures Collection (ECAC Nr. 94042254). B16 and B16-GFP cell lines were routinely maintained in medium DMEM supplemented with 2 mM l-glutamine and 10% fetal bovine serum. Human umbilical vein endothelial cells-2 (HUVECs) obtained from BD Biosciences were cultured in Vascular Cell Basal medium (ATCC, PCS-100-030) with 2% fetal bovine serum, 10 mM l-glutamine and supplemented with the components of Endothelial Cell Growth kit (ATCC, PCS-100-041). All cell lines were grown in culture incubator at 37 °C with 5% CO2 in the humidified atmosphere.
Stable GFP transfection
GFP (Green Fluorescent Protein)-expressed B16 cells were developed to improve cell visualization during experiment. In brief, B16 cells monolayer in the well of six-well plate was treated with 25 µl DOTAP reagent mixed with the 2 µg of plasmid pEGFP-N1 (Stratagene) and incubated for 4 h in serum free medium with further replacement to complete growth medium. For the selection of B16-GFP cells, 500 µg/ml of G418 was added to cultivation medium in 48 h after transfection.
MMPs inhibitors screening
The specificity of compound JaZ-30 was examined against a panel of 10 matrix metalloproteinase enzymes: MMP-1, -2, -3, -7, -8, -9, -10, -12, -13, -14 using “MMP Inhibitor Profiling kit, fluorometric” (Enzo Life Sciences, Farmingdale, NY). The assay was performed in a 96-well plate with a fluorogenic substrate OmniMMPTM Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 [Mca = (7-methoxycoumarin-4-yl)-acetyl; Dpa = N-3 -(2,4dinitrophenyl)-l-a-b-diaminopropionyl].
Measurement of Relative Fluorescence Units (RFU) at Ex/Em = 328/420 nm was done in the process of fluorogenic substrate cleavage for 15 min. Catalytic activity of MMP without inhibitor was taken as 100% and it served as a control. Inhibitory activity of compound JaZ-30 was tested in a concentration diapason from 0 to 40 µM and expressed as percentage of inhibition compared to the control. The concentration of JaZ-30 compound that resulted in 50% inhibition of control catalytic activity – IC50 was quantified using percentage of inhibition and GraphPad Prism 5.03 software (GraphPad Software Inc., San Diego, CA). All experiments were done in triplicate.
Cytotoxicity assays
Cytotoxic effect of JaZ-30 was evaluated in two colorimetric assays: Crystal Violet (CV)Citation28 and MTTCitation29 staining of living cells. B16-GFP and B16 cells were seeded in the well of 96-well plate at the density 5 × 104/ml in the presence of JaZ-30 (with at least four different concentrations) and in the absence of it (control experiment). Plates were incubated in culture thermostat at 37 °C in 5% CO2 for 72 h for both assays.
Crystal violet staining
Cells were fixed for 15 min with 0.1% glutaraldehyde in PBS, stained for 30 min with a 0.1% solution of CV, rinsed twice with distilled water. The dye was released in 100 µl of 96% ethanol and citrate buffer pH 4.2 diluted 1:1. The absorbance at 540 nm was measured using a microplate reader Anthos HTII.
MTT staining
A total of 20 µl of MTT (5 mg/ml) dye, dissolved in PBS solution was added to each well and the plate was incubated for 4 h in culture thermostat. Medium was replaced in each well with 200 µl of DMSO to dissolve the formed formazan crystals. After 5 min of incubation, the absorbance in each well was measured at 540 nm using microplate reader Anthos HTII.
The cytotoxicity and IC50 were quantified using the absorbance data and GraphPad Prism 5.03 software (GraphPad Software Inc., San Diego, CA). All data are given as the mean of three independent experiments.
Microscopy
Light and fluorescent microscopy was carried out using a NIKON inverted microscope Eclipse TE300 and camera NIKON Coolpix 990 for the photography.
Angiogenesis assay in vitro
To reveal the effect of compounds on tube formation, we performed an angiogenesis assay on Matrigel TM Basement Membrane Matrix (BD Biosciences, Franklin Lakes, NJ) as a substrate for the in vitro study. The Matrigel was thawed overnight at 4 °C and distributed to the 96-well culture plate – 50 µl per well. The plate was incubated for 2 h at 37 °C in cell culture incubator to allow the Matrigel to solidify. HUVECs were seeded 104 cells/well on top of the Matrigel and treated with JaZ-30 compound in the diapason of concentrations from 1.2 to 20 µM or equivalent DMSO per well in control well. Angiogenesis was induced by addition in each well 5 ng/ml of VEGF (Cayman Chemicals, Ann Arbor, MI). Culture plates were incubated for 18 h in cell culture incubator at 37 °C to allow formation of capillary-like structures. At the end of experiment, cells were stained by Calcein AM. Tubular structure of endothelial cells was monitored using an inverted microscope and photographed in four random fields of view per well with the magnification 120×. The level of angiogenesis was expressed in percentage by calculation the numbers of branching points per fields of view in JaZ-30-treated and untreated wells.
HUVECs proliferation assay
HUVECs proliferation was measured using a commercial kit (WST-8, Cayman Chemicals). Briefly, 1 × 104 cells in 100 µl of medium were seeded on a 96-well microplate with/without JaZ-30 compound in the concentration range from 1.2 to 20 µM and 5 ng/ml VEGF. After incubation for 24 h, 10% WST-8 dye was added into each well. After 2 h of incubation at 37 °C, the formation of water-soluble formazan was determined photometrical at 450 nm with a microplate reader Anthos HTII. The cytotoxic effect of JaZ-30 was expressed in percentages of living cells compared to control without JaZ-30.I.
Invasion assay on Matrigel chambers
B16-GFP cell invasion was determined using BD Biocoat Tumor Invasion Assay system (BD Bioscience) according to the instruction of the manufacturer. Briefly, 2.5 × 104 B16-GFP cells were seeded into the upper rehydrated chambers with Matrigel or Control membrane in serum-free medium with JaZ-30 compound or vehicle DMSO. BD BioCoat chambers were placed into the 24-well culture plates and the bottom wells were filled with chemoattractant – complete media with 10% FBS. Plates were incubated for 48 h in a cell culture incubator at 37 °C and 5% CO2. Cells invading through Matrigel and Control membrane were quantified by counting cells in the wells under the insert (n = 3) in inverted fluorescent microscope. GFP-expressed B16 culture allowed counting cells without staining. Percentage of invasion was calculated by a following formula: (number of cells invading through Matrigel/number of cells migrating through Control membrane) × 100.
Estimation of VEGF secretion
Secreted VEGF levels in JaZ-30-treated B16–GFP culture medium were measured using an ELISA kit (Invitrogen Corp., Carlsbad, CA) according to manufacturer instructions.
Assay for detection of ERK phosphorylation
Cell-based ELISA (BioAssay Systems, Hayward, CA) measured dually phosphorylated ERK1/2 in whole B16 cells. In this assay, cells were grown in 96-well plates and treated with JaZ-30 for 4 h and then induced by 0.5 µM PMA for 0–30 min. Cells were then fixed and permeabilized in the wells. ERK1/2 phosphorylated (pERK) and total ERK (ERK) was measured using a double immunoenzymatic labeling procedure. The fluorescence was read at Ex/Em = 535/590 nm for pERK (ΔFpERK) and at Ex/Em = 360/450 nm for ERK (ΔFERK) detection according to manufacturer's protocol.
Estimation of normalized phosphorylated ERK
Normalized pERK was quantified by a formula: (ΔFpERK/ΔFERK)/(ΔFpERK/ΔFERK) 0, where (ΔFpERK/ΔFERK) 0 is the control reference value (e.g. time zero in kinetic studies or untreated wells in drug potency studies).
Statistical analysis
The Student’s t-test was used to analyze the difference between control and JaZ-30-treated Statistical significance was set at p < 0.05. All data are given as the mean of three independent experiments
Results
Synthesis of a novel class of MMP-2 inhibitors
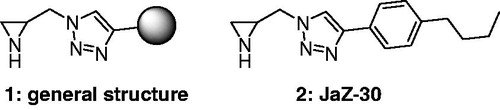
The development of MMP inhibitors remains attractive for creative and innovative research in the field of chemotherapy. We have recently discovered a novel type of selective MMP-2 inhibitorsCitation30 with general structure 1, presented in . Their structures combine an aziridine moiety and a lipophilic side chain via triazole linker. Compound 2 (named JaZ-30), possessing 4-butylphenyl substituent with pronounced lipophilicity at 1,2,3-triazole cycle, has shown the most promising MMP-2 inhibitory activity, therefore was investigated further.
Figure 1. Novel selective MMP-2 inhibitors, where 1 – general structure of aziridine-triazole conjugate; 2 – compound JaZ-30 studied in this work.
Aziridine-triazole conjugate 2 was prepared according to scheme, shown in .
Figure 2. Synthesis of 1-(aziridin-2-ylmethyl)-4-(4-butylphenyl)-1H-1,2,3-triazole (JaZ-30). Details see in Materials and methods section.
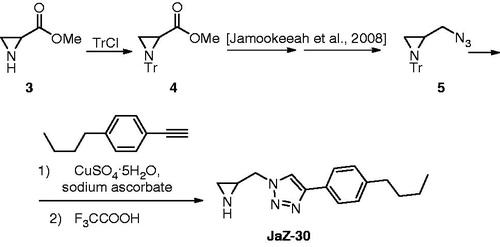
Racemic aziridine carboxylic acid methyl ester (3) was prepared according to previously reported procedureCitation31. Its trityl protected congener 4 was submitted to the synthetic sequence described for enantiomerically pure analogue and resulted azide 5 which is set up for the installation of the side chainCitation32,Citation33. Azide–alkyne click reaction followed by protecting group removal provided target compound JaZ-30.
JaZ-30 inhibits MMP-2 catalytic activity in vitro
Current approach in complex melanoma chemotherapy deals with the search of efficient MMPs-2 inhibitors with anticancer properties.
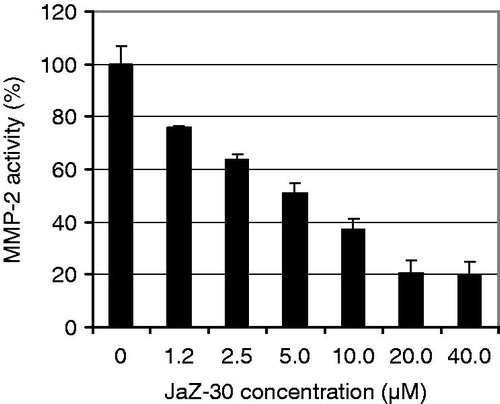
We examined in vitro the specificity of several aziridine-triazole conjugates with general formula 1 in against a panel of 10 MMPs: MMP-1, -2, -3, -7, -8, -9, -10, -12, -13, -14, using recombinant proteins as a MMPs sources. Screening revealed that only JaZ-30 was selective MMP-2 (gelatinase A) inhibitor. The specificity of JaZ-30 inhibitory activity was proven by a dose-dependence in diapason of concentrations between 1.2 and 40 µM that resulted in abrogation of 20.2–75.7% of MMP-2 catalytic activity () with IC50 value 4.6 µM. Our findings showed that JaZ-30 selectively inhibits MMP-2 catalytic activity in vitro.
Figure 3. Dose-dependent inhibitory effect of JaZ-30 on MMP-2 catalytic activity in vitro. Relative Fluorescence Units (RFU) at Ex/Em = 328/420 nm were measured in the process of fluorogenic substrate cleavage. Catalytic activity of MMP without inhibitor was taken as 100% of MMP catalytic activity. Results are presented as mean ± SD of triplicates, p = 0.05, statistically significant.
Previous studies demonstrated that expression of activated MMP-2 correlates with increased malignancy in melanoma in vitro and in vivo and determine its important role of in cell invasion and metastasis developmentCitation34,Citation35, which is especially actual problem for aggressive types of melanoma.
Cytotoxic effect of JaZ-30 compound on melanoma cells
Viability of melanoma cells in the presence of JaZ-30 was detected by MTT and CV assays based on the staining of living cells. Two cultures, B16 and B16-GFP were used in the experiments. A 72-h treatment with JaZ-30 revealed inhibitory effect on melanoma cells proliferation. In the case of B16-GFP cells IC50 values were 27.1 µM (CV) and 34.4 µM (MTT), respectively. The same cytotoxicity was detected on B16 cells. We demonstrated that JaZ-30 is a weak cytotoxic agent and showed negligible proliferation inhibitory property in melanoma cell culture experiments.
JaZ-30 inhibits secretion of VEGF in B16 growth medium
Vascular Endothelial Growth factor (VEGF) is a cell-specific mitogen, released by a variety of tumor cells and is known to play an important role in tumor angiogenesis together with MMP-2 and MMP-9. Cancer cells secrete VEGF in a tumor microenvironment, promoting the growth of endothelial cells and cancer progressCitation36–38.
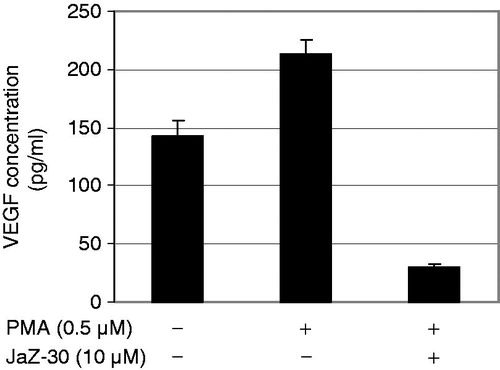
Currently, the most established approach for limiting tumor development is blockade of the VEGF pathway Therefore, we next examined whether JaZ-30 suppresses the secretion of VEGF from melanoma cells. We measured concentration of VEGF in growth medium of B16-GFP cells, stimulated with the 0.5 µM PMA in the presence of sub-cytotoxic concentration of JaZ-30 (10 µM) for 72 h and results are presented in . Our results have shown that JaZ-30 treatment significantly inhibited secreted levels of VEGF in B16-GFP cells. Treatment with 10 µM JaZ-30 diminished VEGF amount in the cultivation medium 7.3-fold, compared to PMA-stimulation alone. Similarly, the same concentration of JaZ-30 inhibited about 60% of MMP-2 activity. Recent studies showed that VEGF released by melanoma cells is an important mediator of neovascularization and this process depends on the level of metalloproteinase-2Citation39. Taken together, our results showed that JaZ-30 inhibited both factors – VEGF and MMP-2, so we could assume the critical role of JaZ-30 in melanoma invasion and angiogenesis.
JaZ-30 inhibits angiogenesis on Matrigel
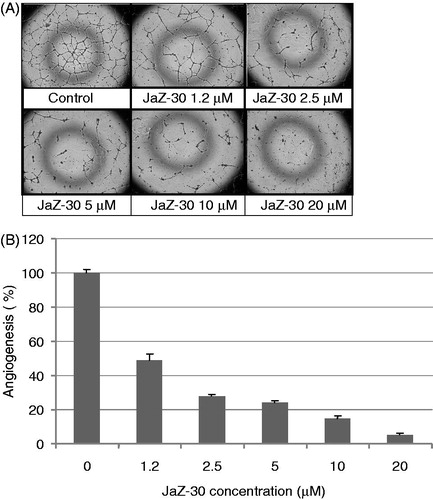
Tumor angiogenesis provides oxygen; nutrients sufficient for tumor growth and progressionCitation40. The complex process of angiogenesis involves the ordered proliferation, assembly and alignment of endothelial cellsCitation41. Since we observed that JaZ-30 inhibits VEGF secretion and MMP-2 activity, we next wanted to see whether JaZ-30 treatment could suppress the capillary-like network formation of endothelial cells on Matrigel matrix, developed as angiogenesis model in vitro. Our experiments with the VEGF-induced formation of the tubular structures on Matrigel-coated wells have shown that JaZ-30 suppressed capillary-like network in a concentration-dependent manner (). The percentage of angiogenesis of JaZ-30-treated and untreated HUVECs is presented in . The cytotoxicity of JaZ-30 on HUVECs growth was evaluated by MTT staining assay to avoid its own effect on tubular structures formation. We revealed that JaZ-30 is not cytotoxic for HUVECs in diapason of concentrations between 1.2 µM and 10 µM. JaZ-30 – mediated inhibition of angiogenesis in this diapason was detected in a dose-dependent manner from 51.2% till 85.3%, respectively. JaZ-30 at 20 µM concentration had a weak cytotoxic effect on HUVECs and inhibited about 95% of angiogenesis. Thus, our findings suggest that non-cytotoxic concentrations of JaZ-30 can suppress the angiogenic properties of VEGF – stimulated endothelial cells with IC50 value 4.3 µM.
Figure 5. Dose-dependent inhibitory effect of JaZ-30 on the capillary-like network formation of human umbilical vein endothelial cells (HUVECs) in vitro. (A) Images of HUVECs the capillary-like network structure, formed on Matrigel-coated wells surface in 24 h. HUVECs were treated with 5 ng/ml VEGF – control, and indicated concentrations of JaZ-30: 1.2 µM; 2.5 µM; 5 µM, 10 µM and 20 µM; magnification 120 ×. (B) The percentage of angiogenesis quantification by calculation the number of branching points in four images, viewed with an inverted microscope. Results are presented as mean ± SD, p < 0.05, statistically significant.
JaZ-30-inhibited invasion of melanoma cells in vitro
Cancer cell migration and invasion play very important role in metastasis. A critical event in metastasis development is the ability of tumor cells to invade through the extracellular matrix, allowing cancer cells to move beyond the area of primary tumorCitation42. To examine whether JaZ-30 inhibits the invasiveness of melanoma cells, Matrigel chamber invasion assay was carried out. B16-GFP cells were non-treated or treated with the different concentrations of JaZ-30. Furthermore, to evaluate the ability of B16-GFP to invade through Matrigel matrix, we counted living cells in the well under the Matrigel camera and calculated percentage of invasion for JaZ-30 – treated and control – untreated cells ().
Figure 6. Inhibitory effect of JaZ-30 on the invasion of B16-GFP cells through Matrigel membrane. Cells, invading through Matrigel and Control membrane for 48 h, were quantified by counting live cells in the wells under the insert in fluorescent microscope. Percentage of invasive cells was calculated by a formula indicated in Materials and methods section. Results are presented as mean ± SD of triplicates, p < 0.05, statistically significant.
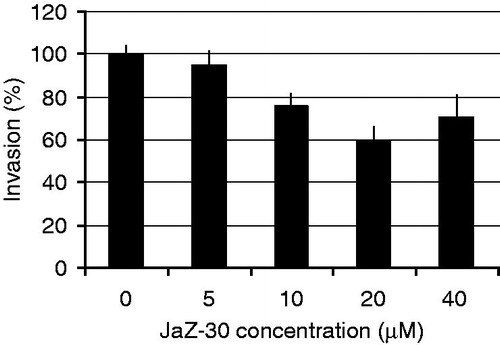
Our results have shown in JaZ-30 inhibited melanoma cell invasion (p < 0.05) through the Matrigel matrix in a dose-dependent manner. Moreover, sub-cytotoxic doses of JaZ-30 (5–20 µM) inhibited invasion from 5 to 40%, while cytotoxic concentration of JaZ-30 – 40 µM had diminished, about 30% of invasion activity (). Thus, we potentiated JaZ-30 anti-metastatic properties. Our findings about inhibitory properties of JaZ-30 on MMP-2 activity are in good agreement with these results. It was frequently verified by different researchers that MMP-2 is the most important mediator of invasion, angiogenesis and tumor development; MMP-2 inhibition resulted in reduction of the carcinogenic processes, both in vitro and in vivoCitation43–45.
JaZ-30 downregulated the phosphorylation of ERK 1/2 in melanoma cells
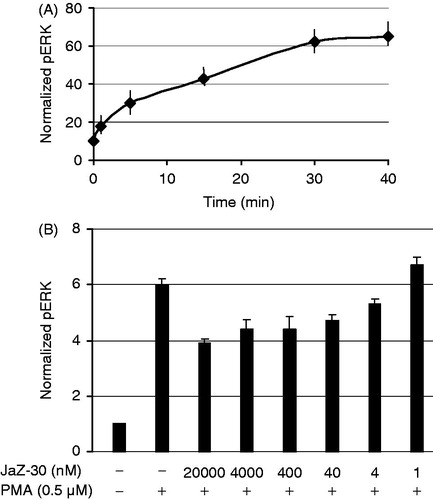
The mitogen-activated protein kinase (MAPK) signaling pathway presents many drug targets for the development of cancer therapies. MAPK pathway plays a key role in cell proliferation, differentiation and invasion. Stimulation by mitogens, such as phorbol-12-myristate-13-acetate (PMA) eventually leads to phosphorylation of extracellular signal-regulated kinases 1 and 2 - ERK1 and ERK2 (ERK1/2). As it was shown, inhibition of the MAPKs pathway prevented angiogenesis, proliferation, invasion and metastasis in a wide range of tumorsCitation46. Summarizing JaZ-30 inhibitory activities, obtained in invasion, angiogenesis and MMPs assays, we arrived at a conclusion that there may be a logical explanation that JaZ-30 mediated ERK1/2 phosphorylation. At first, we examined the kinetics of ERK1/2 phosphorylation (pERK) in B16 cells stimulated with PMA (). Then, B16 cells were additionally treated with the non-toxic concentrations of JaZ-30 in the range between 20 µM and 1 nM. Our studies revealed that there is JaZ-30-mediated reduction of the ERK 1/2 phosphorylation in a dose-dependent manner (p < 0.001) in melanoma cells B16-GFP. JaZ-30 concentration between 20 µM and 4 nM diminished ERK 1/2 phosphorylation 1.53 – 1.13-folds, correspondingly (). These findings suggested JaZ-30-mediated inhibition of extracellular stimuli transduction, thus, resulted in inhibition of such cellular process as cell proliferation and invasion of melanoma cells. It was demonstrated that targeting the ERK/MAP kinase pathway is a promising approach to improve the efficacy of melanoma chemotherapyCitation47.
Figure 7. JaZ-30-mediated phosphorylation of ERK1/2 in B16 cells. (A) Kinetics of ERK1/2 phosphorylation in B16 cells treated with 0.5 µM PMA. (B) Inhibition of ERK1/2 phosphorylation (pERK) by different concentrations of JaZ-30 compound. Cells B16 were treated with JaZ-30 for 3 h and then for 30 min with 0.5 µM PMA. Calculation of normalized pERK (phosphorylated ERK1/2 to the total ERK) presented in the section Materials and methods. Each datum represents mean ± standard deviation of triplicate experiments, p < 0.001, statistically significant.
Discussion
In the present study, we have described cytotoxic and anti-tumor activities of a novel chemical compound JaZ-30. The latter was selected from C(2)-monosubstituted aziridine – 1,2,3-triazole conjugates that represent a new class of MMP-2 inhibitors. JaZ-30 is characterized by 4-butylphenyl side chain which is attached to aziridine cycle via 1,2,3-triazole linker. The synthetic route to JaZ-30 is straightforward and will easily permit to obtain analogs of JaZ-30 and enantiomers thereof, if those will be required for further studies. A distant similarity of title compound structure with thiirane class of gelatinase inhibitors can be imaginedCitation48,Citation49. Nevertheless, aziridines with free NH functionality had never been reported as inhibitors of gelatinases activity. Our novel compound JaZ-30 selectively abrogated catalytic activity of MMP-2 in a dose-dependent manner. The observed MMP-2 selectivity can be explained by the presence of lipophilic side chain that might fit into S1’ pocket of the target enzyme. On the other hand, triazolyl-methyl aziridine moiety can be responsible for coordination to the active site zinc atom. Docking and molecular dynamic experiments will be carried out in the future and will provide more insight into possible mechanism of action of JaZ-30.
The development of novel effective MMPIs constitutes the main focus of current studies in the field of cancer chemotherapy. For successful therapy based on MMP activity inhibition, the new generation of drugs must be developed with the selectivity against validated MMP targets, which provides minimized adverse reactionsCitation50.
MMP-2 play a pivotal role in the process of malignant progression and in that way pharmacologic inhibition of MMP-2 activity could markedly inhibit the invasiveness of primary and metastatic tumors and, therefore, be of therapeutic benefit to patients with cancerCitation51. Moreover, cell adhesion and migration are markedly influenced by local levels of MMP-2 activity. MMP-2 proteolytic degradation of the extracellular matrix being supplemented by stimulation of cell movement by proteolytic fragments, generated by MMP-2 protease activity, lead to creation of a gradient of chemotactic stimuli that promotes and directs cell migration in melanomaCitation52,Citation53.
Recently, Carmeliet and JainCitation40 have shown a pivotal role of MMP-2 in the process of angiogenesis regulation which is a crucial step in tumor progression. Currently, the most established approach for limiting tumor angiogenesis is a blockade of the VEGF pathway. In line with preclinical studies, administration of VEGF inhibitors to patients with various types of cancers has significant therapeutic effect. Nevertheless, VEGF signaling pathway is required for the maintenance of healthy vessels, and its complete blockade lead to side effects resulting from endothelial damage throughout the bodyCitation54. Various in vitro and in vivo studies have uncovered the role of VEGF as a central player in both physiological and pathological angiogenesisCitation55. A number of preclinical studies of angiogenesis inhibition by administration of VEGF blockers have demonstrated significant tumor-suppression effects in various types of cancerCitation36,Citation56. Our findings about JaZ-30-mediated 7.3-fold decrease of VEGF secretion in the cultivation medium of B16 melanoma cells were in a good agreement with these studies. Therefore, there was a logical explanation that JaZ-30 suppressed the capillary-like network formation of VEGF – stimulated HUVECs with IC50 value 4.3 µM, thus revealing anti-angiogenic properties of JaZ-30 compound. Combination with the weak cytotoxic property of JaZ-30 has shown inhibitory effect of HUVECs angiogenesis about 95%.
MMP-2 is also important regulatory protein for tumor invasion of the basement membrane, because (i) it plays a critical role in the degradation of the extracellular matrixCitation57,Citation58 (ii) it is activated in a tumor-specific manner; (iii) its expression correlates with metastatic abilities and poor prognosisCitation59,Citation60.
Previous in vivo studies showed that knockdown of MMP-2 genes rescued the cells from their highly invasive phenotypeCitation61. In the present study, we have shown that nontoxic physiological doses of JaZ-30 reduced at 40% the invasive properties of highly metastatic B16 melanoma cells. Therefore, we suggested JaZ-30 role as a potential invasion inhibitor with anti-MMP-2 and anti-VEGF properties.
MMP gene transcription is induced by a variety of extracellular stimuli, such as cytokines growth factors, and cell–cell or cell–matrix interactions. Binding of these stimulatory ligands to their receptors triggers a cascade of intracellular reactions that are mediated through at least three different classes of MAPKs, stress-activated protein kinase/Jun N-terminal kinases, and p38Citation62,Citation63. VEGF is an important signaling protein involved in stimulation of cellular responses by binding to tyrosine kinase receptors (VEGFRs) on the cell surfaceCitation64. Moreover, inhibition of the MAPKs pathway might prevent angiogenesis, proliferation, invasion and metastasis in a wide range of tumorsCitation65. Previous studies on different cell types has demonstrated that MAPKs like extracellular signal-regulated kinases 1 and 2 (ERK1/2) and p38 seem to play a central role in regulating the expression of urokinase plasminogen activator (uPA) and MMPsCitation66. For example, inhibition of signal transduction transmitted through the MAP kinases markedly inhibits the expression of MMP-2 and the invasive potential of cancer cell linesCitation50. It was shown, that the RAS–RAF–MEK–ERK pathway is deregulated in over 90% of malignant melanomas, and targeting MEK as a central kinase of this pathway is currently tested in clinical trialsCitation67,Citation68. Therefore, the ERK/MAP kinase pathway is a promising approach to improve the efficacy of targeting in melanoma treatment. Due to the importance of JaZ-30 in melanoma cell invasion and angiogenesis, combined with the inhibition of MMP-2 catalytic activity and downregulation of VEGF secretion, we consequently assumed that mechanism of JaZ-30 activities mediated via the RAS–RAF–MEK–ERK signaling pathway. In the present study, we obtained that 20 µM JaZ-30 reduced ERK 1/2 phosphorylation 1.53-fold in melanoma cells, stimulated with PMA. In summary, our data will correspond to the recent studies indicating that mechanism of invasion suppression in cancer cells via the ERK1/2 signaling pathways involved the downregulation of MMP-2, MMP-9 and VEGFCitation9,Citation10 as it was shown by JaZ-30 as well.
Conclusions
We have discovered a novel selective MMP-2 inhibitor JaZ-30 (1-(aziridin-2-ylmethyl)-4-(4-butylphenyl)-1H-1,2,3-triazole) which downregulates VEGF secretion in melanoma cells. It belongs to the class of C(2)-monosubstituted aziridine – aryl-1,2,3-triazole conjugates. We have verified the possibility of proliferation inhibition by JaZ-30 and therefore can propose it as an attractive agent for cancer treatment.
In summary, this report proposes the therapeutic potential of JaZ-30 as inhibitor of invasive ability of melanoma cells through matrix metalloproteinase-2 and downregulation of ERK1/2 phosphorylation. JaZ-30 inhibited angiogenesis which is the key process of the cancer cell development and progress and thus might have broad applications for controlling tumorigenesis. In future, JaZ-30 and its structural analogs have to undergo in vivo experiments to establish whether the title compound and other members from this series have a therapeutic potential.
Declaration of interest
This work was supported by the Latvian Council of Science Grant 10.0030 and ESF grant 2009/0203/1DP/1.1.1.2.0/09/APIA/VIAA/023. The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Kahari VM, Saarialho-Kere U. Matrix metalloproteinases and their inhibitors in tumour growth and invasion. Ann Med 1999;31:34–45. [PubMed: 10219712]
- Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother Pharmacol 1999;43:S42–51. [PubMed: 10357558]
- Denis LJ, Verweij J. Matrix metalloproteinase inhibitors: present achievements and future prospects. Invest New Drugs 1997;15:175–85. [PubMed: 11448904]
- Wojtowicz-Praga SM, Dickson RB, Hawkins MJ. Matrix metalloproteinase inhibitors. Invest New Drugs 1997;15:61–75. [PubMed: 9195290]
- Hanemaaijer R. Verheijen JH, Maguire TM, et al. Increased gelatinase-A and gelatinase-B activitiesin malignant vs. benign breast tumors. Int J Cancer 2000;86:204–7. [PubMed: 10738247]
- Pellikainen JM, Ropponen KM, Kataja VV, et al. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2, and prognosis. Clin Cancer Res 2004;10:7621–28. [PubMed: 15569994]
- Wilson CL, Heppner KJ, Labosky PA, et al. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA 1997;94:1402–7. [PubMed: 9037065]
- Fingleton BM, Heppner Goss KJ, Crawford HC, Matrisian LM. Matrilysin in early stage intestinal tumorigenesis. APMIS 1999;107:102–10. [PubMed: 10190286]
- Zhou L, Wang D-S, Li Q-J, et al. Downregulation of the Notch signaling pathway inhibits hepatocellular carcinoma cell invasion by inactivation of matrix metalloproteinase-2 and -9 and vascular endothelial growth factor. Oncol Rep 2012;28:874–82. [PubMed: 22736202]
- Yarani R, Mansouri K, Mohammadi-Motlagh HR, et al. In vitro inhibition of angiogenesis by hydroalcoholic extract of oak (Quercus infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9 secretion. Pharm Biol 2013;51:361–8. [PubMed: 23137183]
- Brand K, Baker AH, Perez-Canto A, et al. Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases-2 into the liver tissue. Cancer Res 2000;60:5723–30. [PubMed: 11059766]
- Nemeth JA, Yousif R, Herzog M, et al. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst 2002;94:17–25. [PubMed: 11773278]
- Stockman BJ, Waldon DJ, Gates JA, et al. Solution structures of stromelysin complexed to thiadiazole inhibitors. Protein Sci 1998;7:2281–6. [PubMed: 9827994]
- Igarashi N, Kubota T, Otani Y, et al. Preventive effect of matrix metalloproteinase inhibitor, R-94138, in combination with mitomycin C or cisplatin on peritoneal dissemination of human gastric cancer cell line TMK-1 in nude mice. Jpn J Cancer Res 1999;90:116–21. [PubMed: 10076574]
- Kido A, Tsutsumi M, Iki K, et al. Inhibition of spontaneous rat osteosarcoma lung metastasis by 3S-[4-(N-hydroxyamino)-2R-isobutylsuccinyl]amino-1-methoxy-3,4-dihydroc arbostyril, a novel matrix metalloproteinase inhibitor. Jpn J Cancer Res 1999;90:333–41. [PubMed: 10359049]
- Lozonschi L, Sunamura M, Kobari M, et al. Controlling tumor angiogenesis and metastasis of C26 murine colon adenocarcinoma by a new matrix metalloproteinase inhibitor, KB-R7785, in two tumor models. Cancer Res 1999;59:1252–8. [PubMed: 10096556]
- Chen P-S, Shih Y-W, Huang H-C, Cheng H-W. Diosgenin, a steroidal saponin, inhibits migration and invasion of human prostate cancer PC-3 cells by reducing matrix metalloproteinases expression. PLoS One 2011;6:e20164. [PubMed: 21629786]
- Hanessian S, Tremblay M, Kornienko A, Moitessier N. Design, modeling and synthesis of functionalized paromamine analogs. Tetrahedron 2001;57:3255–65
- Zapico JM, Serra P, García-Sanmartín J, et al. Potent “clicked” MMP-2 inhibitors: synthesis, molecular modeling and biological exploration. Org Biomol Chem 2011;9:4587–99. [PubMed: 21552627]
- Serra P, Bruczko M, Zapico JM, et al. MMP-2 selectivity in hydroxamate-type inhibitors. Curr Med Chem 2012;19:1036–64. [PubMed: 22257051]
- Nicolotti O, Miscioscia TF, Leonetti F, et al. Screening of matrix metalloproteinases available from the protein data bank: insights into biological functions, domain organization, and zinc binding groups. J Chem Inf Model 2007;47:2439–48. [PubMed: 17958346]
- Testero SA, Llarrull LI, Fisher JF, et al. Exploring the functional space of thiiranes as gelatinase inhibitors using click chemistry. ARKIVOC 2011;7:221–36
- Amadasi A, Cozzini P, Incerti M, et al. Molecular modeling of bindingbetween amidinobenzisothiazoles, with antidegenerative activity on cartilage, and matrix metalloproteinase-3. Bioorg Med Chem 2007;15:1420–9. [PubMed: 17113299]
- Engel CK, Pirard B, Schimanski S, et al. Structural basis for the highly selective inhibition of MMP-13. Chem Biol 2005;12:181–9. [PubMed: 15734645]
- Tron GC, Pirali T, Billington RA, et al. Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med Chem Rev 2008;28:278–308
- Hayashi R, Jin X, Cook GR. Synthesis and evaluation of novel heterocyclic MMP inhibitors. Bioorg Med Chem Lett 2007;17:6864–70. [PubMed: 7763363]
- Hugenberg V, Breyholz H-J, Riemann B, et al. A new class of highly potent matrix metalloproteinase inhibitors based on triazole-substituted hydroxamates: (radio)synthesis and in vitro and first in vivo evaluation. J Med Chem 2012;55:4714–27. [PubMed: 22540974]
- Saotome K, Morita H, Umeda M. Cytotoxicity test with simplified crystal violet staining method using microtitre plates and its application to injection drugs. Toxicol in vitro 1989;3:317–21. [PubMed: 20702298]
- Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer 1987;56:279–85. [PubMed: 3663476]
- Kreituss I, Rozenberga E, Zemitis J, et al. Discovery of aziridine-triazole conjugates as selective MMP-2 inhibitors. Chem Heterocycl Comp. 2013;8:1191–200. DOI: 10.1039/c2cc36225b
- Trapencieris P, Kalviņš I, Kauliņa L, Kauss V. Unnatural amino acids. 1. A facile synthesis of the methyl ester of aziridine-2-carboxylic acid. Org Process Res Dev 1997;1:259–63
- Jamookeeah CE, Beadle CD, Jackson RFW, Harrity JPA. Investigation of a flexible enantiospecific approach to aziridines. J Org Chem 2008;73:1128–30. [PubMed: 18179238]
- Jamookeeah CE, Beadle CD, Harrity JPA. An enantiospecific approach to triazolylalanine derivatives. Synthesis 2009;1:133–7. DOI: 10.1055/s-0028-1083270
- Hofmann UB, Westphal JR, Waas ET, et al. Matrix metalloproteinases in human melanoma cell lines and xenografts: increased expression of activated matrix metalloproteinase-2 (MMP-2) correlates with melanoma progression. Br J Cancer 1999;81:77–82. [PubMed: 10555745]
- Berube M, Deschambeault A, Boucher M, et al. MMP-2 expression in uveal melanoma: differential activation status dictated by the cellular environment. Mol/Vis 2005;11:1101–11. [PubMed: 16379022]
- Kim KJ, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature 1993;362:841–4. [PubMed: 7683111]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002;2:161–74. [PubMed: 11990853]
- Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 2004;25:581–611. [PubMed: 15294883]
- Löffek S, Zigrino P, Steiger J, et al. Melanoma cell-derived vascular endothelial growth factor induces endothelial tubulogenesis within fibrin gels by a metalloproteinase-mediated mechanism. Eur J Cell Biol 2006;85:1167–77. [PubMed: 16949178]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249–57. [PubMed: 11001068]
- Zhang G, Dass CR, Sumithran E, et al. Effect of deoxyribozymes targeting c-Jun on solid tumor growth and angiogenesis in rodents. J Natl Cancer Inst 2004;96:683–96. [PubMed: 5126605]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010;141:52–67. [PubMed: 20371345]
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997;89:1260–70. [PubMed: 9293916]
- Duffy MJ, Maguire TM, Hill A, et al. Metalloproteinases: role in breast carcinogenesis, invasion and metastasis. Breast Cancer Res 2000;2:252–7. [PubMed: 11250717]
- Yoon S-O, Park S-J, Yun C-H, Chung A-S. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol 2003;36:128–37. [PubMed: 12542983]
- Panek RL, Lu GH, Klutchko SR, et al. In vitro pharmacological characterization of PD 166285, a new nanomolar potent and broadly active protein tyrosine kinase inhibitor. J Pharmacol Exp Ther 1997;283:1433–44. [PubMed: 9400019]
- Ferguson J, Arozarena I, Ehrhardt M, Wellbrock M. combination of MEK and SRC inhibition suppresses melanoma cell growth and invasion. Oncogene 2012;32:86–96. [PubMed: 22310287]
- Skiles JW, Gonnella NC, Jeng AY. The design, structure, and therapeutic application of matrix metalloproteinase inhibitors. Curr Med Chem 2001;8:425–74. [PubMed: 11172697]
- Testero SA, Bouley R, Fisher JF, et al. Exploration of mild copper-mediated coupling of organotrifluoroborates in the synthesis of thiirane-based inhibitors of matrix metalloproteinases. Bioorg Med Chem Lett 2011;21:2675–8. [PubMed: 21256011]
- Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer 2006;94:941–6. [PubMed: 16538215]
- Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst 2001;93:178–93. [PubMed: 11158186]
- Ray JM, Stetler-Stevenson WG. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J 1995;14:90–7. [PubMed: 7534227]
- Giannelli G, Falk-Marzillier J, schiraldi O, et al. Induction of cell migration by matrix metalloproteinase-2 cleavage of laminin-5. Science 1997;277:225–8. [PubMed: 9211848]
- Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J Med 2012;61:47–56. [PubMed: 22760023]
- Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw 2009;20:158–63. [PubMed: 20167554]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature 2005;438:967–74. [PubMed: 16355214]
- Mackay AR, Corbitt RH, Hartzler JL, Thorgeirsson UP. Basement membrane type IV collagen degradation: evidence for the involvement of a proteolytic cascade independent of metalloproteinases. Cancer Res 1990;50:5997–6001. [PubMed: 2144209]
- Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol 2000;18:1135–49. [PubMed: 10694567]
- Liabakk NB, Talbot I, Smith RA, et al. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res 1996;56:190–6. [PubMed: 8548762]
- Johnsen M, Lund LR, Romer J, et al. Cancer invasion and tissue remodeling: common themes in proteolytic matrix degradation. Curr Opin Cell Biol 1998;10:667–71. [PubMed: 9818179]
- Beshir AB, Ren G, Magpusao AN, et al. Raf kinase inhibitor protein suppresses nuclear factor-κB dependent cancer cell invasion through negative regulation of matrix metalloproteinase expression. Cancer Lett 2010;299:137–49. [PubMed: 20855151]
- Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J 1999;13:781–92. [PubMed: 10224222]
- Chen P-N, Hsieh Y-S, Chiou H-L, Chu S-C. Silibinin inhibits cell invasion through inactivationof both PI3K-Akt and MAPK signaling pathways. Chem Biol Interact 2005;156:141–50. [PubMed: 16498067]
- Holmes K, Roberts OL, Thomas AM, Cross MJ. Vascular endothelial growth factor receptor-2: structure, function, intracellular signaling and therapeutic inhibition. Cell Signal 2007;19:2003–12. [PubMed: 17658244]
- Simon C, Juarez J, Nicolson GL, Boyd D. Effect of PD 098059, a specific inhibitor of mitogen-activated protein kinase, on urokinase expression and in vitro invasion. Cancer Res 1996;56:5369–74. [PubMed: 8968087]
- Simon C, Goepfert H, Boyd D. Inhibition of the p38 mitogen-activated protein kinase by SB 203580 blocks PMA-induced Mr 92,000 type IV collagenase secretion and in vitro invasion. Cancer Res 1998;58:1135–9. [PubMed: 9515796]
- Graells J, Vinyals A, Figueras A, et al. Overproduction of VEGF concomitantly expressed with its receptors promotes growth and survival of melanoma cells through MAPK and PI3K signaling. J Invest Dermatol 2004;123:1151–61. [PubMed: 15610528]
- Siveen KS, Kuttan G. Thujone inhibits lung metastasis induced by B16F-10 melanoma cells in C57BL/6 mice. Can J Physiol Pharmacol 2011;89:691–703. [PubMed: 21905822]