Abstract
Carbonic anhydrase was purified and characterized from erythrocytes of the Turkish native chicken, Gerze, for the first time. The enzyme was purified 57.65-fold with a yield of 52%, and a specific activity of 954.08 U/mg proteins having optimum pH at 8.0; optimum temperature at 30 °C; optimum ionic strength at 10 mM and stable pH at 8.0. The purified enzyme had apparent KM and Vmax values of 0.73 mM and 0.236 μmol × min−1, respectively. Al+3, Hg+2, Cu+2, Pb+2, and Cd+2 showed inhibitory effects on the enzyme. Pb+2 exhibited the strongest inhibitory action. Cd+2 and Hg+2 were moderate inhibitor, whereas Al+3 and Cu+2 showed weaker actions. All tested metals inhibited the enzyme in competitive manner. Our findings indicate that these metals inhibit the chicken enzyme in a similar manner to other α-CAs from mammals investigated earlier, but susceptibility to various metals differ between the native chicken and other mammalian enzymes.
Introduction
Carbonic anhydrases (EC 4.2.1.1, CAs) are widespread zinc containing metalloenzyme family. The enzyme catalyzes the reversible inter-conversion of carbon dioxide and bicarbonate. This reaction is the main role of CA enzymes in physiological conditionsCitation1–5. Sixteen isozymes have been described up to now in mammals, the most active ones as catalysts for carbon dioxide hydration being CA II and CA IXCitation1,Citation2. The 16 isozymes differ in their subcellular localization, catalytic activity and susceptibility to different classes of inhibitors. Some of them are cytosolic (CA I, CA II, CA III, CA VII and CA XIII), others are membrane bound (CA IV, CA IX, CA XII and CA XIV), two are mitochondrial (CA VA and CA VB), and one is secreted in saliva (CA VI). It has been reported that CA XV isoform is not expressed in humans or in other primates, but it is abundant in rodents and other vertebratesCitation1,Citation3–7. CAs are produced in a variety of tissues where they participate in several important biological processes such as acid–base balance, respiration, carbon dioxide and ion transport, bone resorption, ureagenesis, gluconeogenesis, lipogenesis and body fluid generationCitation1,Citation4–6. Up to now, CA has been purified from many different tissues including human erythrocytesCitation8–10, fish liverCitation5, fish erythrocytesCitation6, rainbow trout gill and liverCitation6,Citation11, similarly some chemicals and metal ions inhibition effects on human and rainbow trout enzymes are investigatedCitation4–14.
Gerze chicken, also known as Hacı Kadı, is the one of two endemic chicken breeds of Turkey. It has been rearing in Sinop province of Northern part of Turkey, but unfortunately, nowadays Gerze chicken is threatened with extinction because of its disadvantages on economic productivity according to commercial hybrids. Today, a limited number of Gerze chicken enthusiasts and governmental institutes as Lalahan Livestock Central Research Institution and Agricultural District Directorate of Gerze affiliated to the Turkish Ministry of Food, Agriculture and Animal Husbandry, conserve this genetic resourceCitation15,Citation16. Consequently, there is a deep need to define existing chicken populations and to investigate their biochemical features so as to benefit people living in rural areas and scientists interested in this areaCitation17.
In the present study, we purified and characterized carbonic anyhdrase from gerze chicken erythrocytes for the first time, and investigated inhibitory effects of some metal ions on enzyme activity.
Methods and materials
Chemicals
Al(NO3)3, Cu(NO3)2, Pb(NO3)2, Cd(NO3)2, Hg(NO3)2, Sepharose 4B, protein assay reagents, 4-nitrophenylacetate were obtained from Sigma-Aldrich Co., Munich, Germany. All other chemicals were of analytical grade and obtained from Merck, Darmstadt, Germany.
Preparation of the hemolysate
Blood was sampled from the caudal vein using a 10 ml plastic heparinized syringe (5 IU/ml) and transferred to tubes. Blood sample were centrifuged at 2500 × g for 15 min. The plasma was removed by drip. After the package of red cells was washed with KC1 solution (0.16 M) three times, the samples were centrifuged at 2500 × g each time and supernatants were removed. The erythrocytes were hemolyzed with five volumes of ice-cold water and centrifuged (+4 °C, 10 000× g) for 30 min to remove the ghosts and intact cellsCitation6.
Purification of carbonic anhydrase from chicken erythrocytes by affinity chromatography
The pH of the hemolysate was adjusted to 7.5 with solid Tris. The homogenate was applied to the prepared Sepharose 4BL-tyrosine-sulfanilamide affinity column equilibrated with 10 mM Tris-HCl/0.1 M Na2SO4 (pH 8.0). The affinity gel was washed with 10 mM Tris-HCl/22 mM Na2SO4 (pH 8.0). The gerze chicken erythrocytes carbonic anhydrase enzyme was eluted with 1.2 M NaCl/25 mM Na2HPO4 (pH 6.3). All procedures were performed at 4 °C.
Esterase activity assay
Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a period of 3 min at 25 °C using a spectrophotometer according to the method described by Verpoorte et al.Citation18. The enzymatic reaction, in a total volume of 3.0 ml, contained 1.4 ml 0.05 M Tris-SO4 buffer (pH 7.4), 1 ml 3 mM 4-nitrophenylacetate, 0.5 ml H2O and 0.1 ml enzyme solutionCitation18. A reference measurement was obtained by preparing the same cuvette without enzyme solution. The inhibitory effects of Al+3, Cu+2, Cd+2, Pb+2 and Hg+2 were examined. All compounds were tested in triplicate at each concentration used. Different inhibitor concentrations were used. Control cuvette activity in the absence of inhibitor was considered as 100%. For each inhibitor, an Activity (%)−[Inhibitor] graph was drawn. To determine Ki values, three different inhibitor concentrations were tested. In these experiments, 4-nitrophenylacetate was used as substrate at five different concentrations (0.10–0.60 mM). The Lineweaver–Burk curves were drawnCitation19.
Protein determination
Protein during the purification steps was determined spectrophotometrically at 595 nm according to the Bradford method, using bovine serum albumin as the standardCitation20.
SDS polyacrylamide gel electrophoresis
SDS polyacrylamide gel electrophoresis was performed after purification of the enzymes. It was carried out in 10% and 3% acrylamide for the running and the stacking gel, respectively, containing 0.1% SDS according to Laemmli procedure. A 20 μg sample was applied to the electrophoresis medium. Gels were stained for 1.5 h in 0.1% Coommassie Brilliant Blue R-250 in 50% methanol and 10% acetic acid, then destained with several changes of the same solvent without the dyeCitation21.
Stable pH determination
For this aim, equal volumes of the buffers K-phosphate at pH of 6.0, 6.5, 7.0, 7.5 and 8.0, Tris-HCl at pH of 7.5, 8.0, 8.5 and 9.0, and purified enzyme were mixed and kept in a refrigerator (+4 °C). The enzyme activity was assayed at 5-day intervalsCitation22.
Optimum pH determination
In order to determine the optimum pH, Tris-HCl and K-phosphate buffers were used in the pH range of 5.5–8.0 and 7.5–9.0, respectivelyCitation22.
Optimum temperature determination
For determination of the optimum temperature, enzyme activity was assayed at different temperatures in the range from 5 °C to 70 °C. The desired temperature was provided by using a Grant bathCitation22.
Optimum ıonic strength determination
For determination of optimum ionic strength, enzyme activity was determined using different concentrations of K-phosphate buffer, pH: 7.5, in the range from 10 mM to 1000 mMCitation22.
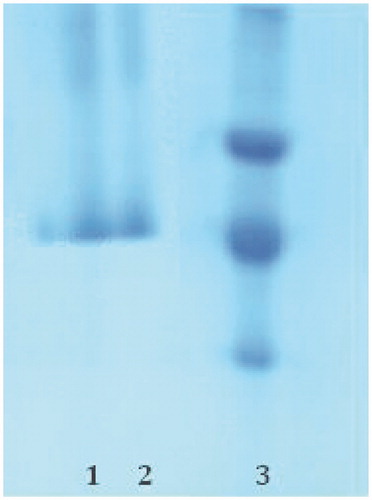
Molecular weight determination with SDS-PAGE
The subunit of the enzyme was determined by SDS-PAGE (). E. coli β-galactosidase (116 kDa), Rabbit phosphorylase B (97.4 kDa), bovine albumin (66 kDa), chicken ovalbumin (45 kDa) and bovin carbonic anhdrase (29 kDa) were used as standards (Sigma MW-SDS-200).
In vitro effects of metal ıons
In order to determine the effects of the metal ions on chicken erythrocytes CA, different concentrations of metal ions were added to the reaction medium. The enzyme activity was measured and an experiment in the absence of inhibitor was used as control (100% activity). The IC50 values were obtained from activity (%) versus metal ion concentration plots.
In order to determine Ki constants in the media with inhibitor, the substrate (NPA) concentrations were 0.01, 0.02, 0.035, 0.05, and 0.07 mM. Inhibitor solutions (metal salts) were added to the reaction medium, resulting in three different fixed concentrations of inhibitors in 1 ml of total reaction volume. Lineweaver–Burk graphs were drawn by using 1/V versus 1/[S] values and Ki constant were calculated from these graphsCitation19. Regression analysis graphs were drawn for IC50 using inhibition % values by a statistical package (SPSS-for windows; version 10.0) on a computer (student t test; n = 3).
Results and discussion
Here, we isolated the CA enzyme from the Turkish native Gerze chicken erythrocytes for the first time and characterized. We achieved to purify the enzyme in a single step using affinity chromatography on Sepharose 4B tyrosine-sulfanilamide. The enzyme was purified 57.65-fold with a recovery ratio of 52.82% compared to the homogenate (). After the sample had completely passed through, the column was washed with 10 mM Tris-HCl/0.1 M Na2SO4 buffer whose pH was 8.0. During washing, absorbencies of fractions were spectrophotometerically measured at 280 nm and 348 nm. These values of the absorbance showed that some proteins, bound to the affinity material, have been removed from the column by the washing solutions. Then, the enzyme was eluted with 1 M NaCl/25 mM Na2HPO4 pH 6.3. At the end of the last step, a highly purified enzyme was obtained exhibiting a single band on SDS-PAGE (). We used only one chromatographic technique, Sepharose 4B tyrosine-sulfanilamide affinity chromatography by modification of washing and elution conditions.
Table 1. Summary of purification procedure for gerze chicken erythrocytes carbonic anhydrase enzyme by a Sepharose-4B-tyrosine-sulfanilamide affinity column chromatography.
The optimum pH, optimum temperature, optimum ionic strength and stable pH were determined to be 8.0, 30 °C, 10 mM and 8.0 for the enzyme, respectively. The stable pH profile of the enzyme was determined at four different pHs in 10 mM Tris-HCl and five different pHs in 10 mM K-phosphate buffer. The enzyme was able to protect 92% of maximum activity at the end of 14 days in 10 mM Tris-HCl buffer (pH: 8.0). The optimum pH was found to be 8.0. The result is similar to CAs obtained from human erythrocytesCitation23,Citation24. The enzyme was seen to exhibit the highest activity at 25 °C in a study of temperatures between 5 and 70 °C. The optimum ionic strength of the enzyme was estimated to be 10 mM Tris-HCl buffer. However, about 85% of the maximum activity was present in the broad range from 10 mM to 1000 mM.
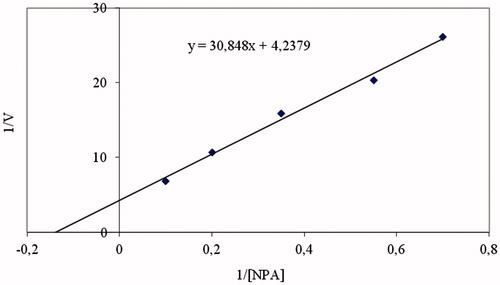
The molecular weight was determined to be 30 kDa. Similar results have been observed for the enzyme from different sources. For example, human erythrocyte CA is 29 kDa, bovine erythrocyte CA is 29 kDa and European seabass liver CA is 30.2 kDaCitation3–6. In addition to establishment of molecular weight of the enzyme, Rf values were calculated for the enzyme and standard proteins, and Rf-LogMW graph was obtained according to Laemmli’ method. The molecular weight of the enzyme was determined to be 30 kDa. KM and Vmax values were calculated for NPA by Lineweaver–Burk graph (). KM constants were calculated as 0.73 mM, Vmax values as 0.236 μmol × min−1 for NPA.
Figure 2. Lineweaver–Burk curves for p-NPA hydrolysis catalyzed by the gcCA enzyme, at five different concentrations of substrate.
Moreover, effects of substrates on enzyme activity were studied. Measurements showed that, as NPA concentration became higher, the activity became less and less. This result shows that aggregation occurs and activity decreases similarly at high NPA concentrations.
In addition to purification and characterization of the enzyme, Al +3, Cu+2, Cd+2, Pb+2 and Hg+2 were chosen to investigate their inhibitory effects on gerze chicken erythrocytes CA (gcCA) and Ki parameters of these metals were determined. Metal ions inhibited the enzyme activity at low concentrations. It is clear that Pb2+ and Hg2+ are the most potent inhibitors for gcCA enzyme (). According to values, the best inhibitor for gcCA is lead. The mechanism of toxicological effects of the metals is probably due to the interactions between the histidines and the metals or the interaction of the metals with other aminoacids around the active site.
Table 2. Ki values for the inhibition of hCA I, hCA II, sCA IV, dCA and gcCA (gerze chicken CA) with metal ions (mM).
Conclusions
Consequently, we purified carbonic anhydrase from Turkish native chicken, Gerze for the first time, and analyzed characteristic features. Our results are in good agreement with others reported in literature. Also, inhibitory effects of metals on enzyme activity were reported. This study is supportive for medicinal chemists who are interested in enzyme inhibitors. It is also useful for paultry researchers because economically efficient hybrids have begun to replace the indigenous breeds with lower potential due to spread of intensive production. This endemic Gerze chicken is under the risk of extinction and therefore, the studies about its specific physiological responses to environmental conditions, especially chemical toxicity has great importance.
Declaration of interest
The authors report no conflicts of interests. The authors alone are responsible for the content and writing of this article.
References
- Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 2008;7:168–81
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74
- Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov 2011;10:767–77
- Ekinci D, Beydemir S, Kufrevioglu OI. In vitro inhibitory effects of some heavy metals on human erythrocyte carbonic anhydrases. J Enzyme Inhib Med Chem 2007;22:745–50
- Ceyhun SB, Senturk M, Yerlikaya E, et al. Purification and characterization of carbonic anhydrase from the teleost fish Dicentrarchus labrax (European seabass) liver and toxicological effects of metals on enzyme activity. Environ Toxicol Pharmacol 2011;32:69–74
- Demirdag R, Yerlikaya E, Senturk M, et al. Heavy metal ion inhibition studies of human, sheep and fish α-carbonic anhydrases. J Enzyme Inhib Med Chem 2013;28:278–82
- Ekinci D, Beydemir S. Evaluation of the impacts of antibiotic drugs on PON 1; a major bioscavenger against cardiovascular diseases. Eur J Pharmacol 2009;617:84–9
- Balaydin HT, Durdagi S, Ekinci D, et al. Inhibition of human carbonic anhydrase isozymes I, II and VI with a series of bisphenol, methoxy and bromophenol compounds. J Enzyme Inhib Med Chem 2012;27:467–75
- Balaydin HT, Soyut H, Ekinci D, et al. Synthesis and carbonic anhydrase inhibitory properties of novel bromophenols including natural products. J Enzyme Inhib Med Chem 2012;27:43–50
- Cavdar H, Ekinci D, Talaz O, et al. α-Carbonic anhydrases are sulfatases with cyclic diol monosulfate esters. J Enzyme Inhib Med Chem 2012;27:144–54
- Ekinci D, Beydemir S. Purification of PON1 from human serum and assessment of enzyme kinetics against metal toxicity. Biol Trace Elem Res 2010;135:112–20
- Aksakal E, Ceyhun SB, Erdoğan O, Ekinci D. Acute and long-term genotoxicity of deltamethrin to insulin-like growth factors and growth hormone in rainbow trout. Comp Biochem Physiol C Toxicol Pharmacol 2010;152:451–5
- Gulcin I, Beydemir S, Coban A, Ekinci D. The inhibitory effect of dantrolene sodium and propofol on 6-phosphogluconate dehydrogenase from rat erythrocyte. Fresen Environ Bull 2008;17:1283–7
- Cakmak R, Durdagi S, Ekinci D, et al. Design, synthesis and biological evaluation of novel nitroaromatic compounds as potent glutathione reductase inhibitors. Bioorg Med Chem Lett 2011;21:5398–402
- Ekinci D, Ceyhun SB, Aksakal E, Erdogan O. IGF and GH mRNA levels are suppressed upon exposure to micromolar concentrations of cobalt and zinc in rainbow trout white muscle. Comp Biochem Physiol C Toxicol Pharmacol 2011;153:336–41
- Mercan, L. Analysis of genetic dissimilarity between native and commercial chicken genotypes by SSR (Simple sequence repeats) method [PhD thesis]. Samsun: Ondokuz Mayis University Natural Science Institute; 2010
- Mercan L, Okumus A, Senturk M, Ekinci D. In vitro enzymatic response of Turkish native chicken “Gerze” to heavy metal exposure. J Enzyme Inhib Med Chem 2013;28:52–7
- Verpoorte JA, Mehta S, Edsall JT. Esterase activities of human carbonic anhydrases B and C. J Biol Chem 1967;242:4221–9
- Lineweaver H, Burk D. The determination of enzyme dissocation constants. J Am Chem Soc 1934;56:658–66
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72:248–54
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5
- Ekinci D, Ceyhun SB, Şentürk M, et al. Characterization and anions inhibition studies of an α-carbonic anhydrase from the teleost fish Dicentrarchus labrax. Bioorg Med Chem 2011;19:744–8
- Ekinci D, Cavdar H, Talaz O, et al. NO-releasing esters show carbonic anhydrase inhibitory action against human isoforms I and II. Bioorg Med Chem 2010;18:3559–63
- Alp C, Ekinci D, Gultekin MS, et al. A novel and one-pot synthesis of new 1-tosyl pyrrol-2-one derivatives and analysis for carbonic anhydrase inhibitory potencies. Bioorg Med Chem 2010;18:4468–74