Abstract
Pancreatic lipase (PL) is considered as one of the safest target for diet-induced anti-obesity drug development. Orlistat is the only PL inhibitor approved for anti-obesity treatment till date. In the process of exploration of new PL inhibitors, we have screened culture filtrates of 70 endophytic fungi of medicinal plants using qualitative as well as quantitative in-vitro PL assays. The qualitative assays indicated potential PL inhibition in only three isolates, namely #57 TBBALM, #33 TBBALM and #1 CSSTOT. Only ethyl acetate extracts of the culture filtrates of these isolates exhibited the PL inhibition. #57 TBBLAM ethyl acetate extract of culture filtrate exhibited potential PL inhibition with an IC50 of 3.69 µg/ml which was comparable to the positive control, i.e. Orlistat exhibiting IC50 value of 2.73 µg/ml. Further molecular phylogenetic tools and morphological studies were used to identify the isolate #57 TBBALM as Penicillium species.
Introduction
Obesity is a disease resulting from improper balance between energy intake and expenditure and is increasingly becoming a major cause of preventable mortality. In the present millennium, it has become a major global health threat with more than 1 billion overweight adults of which nearly 300 million have been designated as clinically obeseCitation1,Citation2. The primary cause of obesity is the sedentary lifestyle, stress and high-calorie food intake. Obesity is also a precursor to variety of serious diseases like hypertension, hyperlipidemia, atherosclerosis and type II diabetesCitation3. Obesity is being recognized as a distinct medical problem hence obesity therapies are going to be an integral part of medicine and healthcare industry. Thus, targeting novel metabolic pathways for obesity treatment is a major research focus in pharmaceutical and biopharmaceutical industry.
One of the key enzymes in lipid metabolism is pancreatic lipase (PL). PL hydrolyzes approximately 50–70% of total triglycerides present in food into glycerol and fatty acids which are eventually absorbed in the small intestine. Hence, PL appears to be a valuable therapeutic target for the treatment of diet induced obesity in humans. Xenical (Orlistat, Roche, Basel, Switzerland), a PL inhibitor isolated from the actinobacterium Streptomyces toxytricini has become one of the best selling drugs for treatment of diet-induced obesity. It reduces approximately 30–40% of fat absorption from the mealsCitation4. However it possesses side effects like oily stools, flatulence, faecal urgency, abdominal cramps, leaking of oil from the rectum leading to oily spotting. The success of orlistat has stimulated research for identifying new enzyme inhibitors from natural sources which may lack some unpleasant side effects of orlistat.
The discovery of Penicillin from the fungi Penicillium notatum marked the golden era of antibiotics and since then fungi have been exploited extensively in development of some remarkable drugs like Cyclosporine (an immunosuppressant) and Penicillin’s, Cephalosporin’s (β-lactams antibiotics) and Griseofulvin (antifungal antibiotic). Fungi like Monascus ruber and Aspergillus terreus have also produced potent 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors like Mevastatin and Lovastatin, which are extensively being used as commercial cholesterol lowering drugs. Fungi also exist inside the plants without any indication of their existence. These are known as endophytic fungi. Endophytic fungi have been reported to be lucrative sources of bioactive compounds possessing antibacterial, anti-cancer, cytotoxic, neuroprotective and anti-oxidant activities some of which are under clinical trialsCitation5.
Bio-prospecting of endophytic fungi for production of PL inhibitors is a nascent area with very scanty preliminary data. Methanol and dichloromethane extracts of some of the wood damaging mushrooms and macrofungi possess PL inhibitory activityCitation6. However, endophytic fungi have not been exploited for search of PL inhibitors. We carried out an in vitro screening program of PL inhibition by cell-free culture filtrate of 70 endophytic fungi of plants inhabiting in the Western Ghats and north-east regions of India.
Here, we report the potential of a lead extract from the culture filtrate of endophytic fungal isolate #57 TBBALM which induced complete inhibition of PL activity and identification of the fungi using molecular and morphological approaches for its exploitation in the isolation of the PL inhibitor and its evaluation as a pharmacophore for treatment of obesity.
Materials and methods
Isolation of endophytic fungi
The plant material (bark) was collected from conserved forest area of Western Ghats and North eastern Himalayas. Each collected sample was properly labelled and placed in a sterile bag and stored at 4 °C till further use. Isolation was done following a modified procedure described byCitation7. Briefly, the plant samples were washed in running tap water for 5 min and then sequentially surface sterilized with 1% sodium hypochlorite for 3 min followed by 70% ethanol for 1 min and then 30% ethanol for 30 s. The surface sterilized plant subsequently allowed to surface dry aseptically and then cut into 1–3 mm segments with the help of sterile blade. These segments were then placed over Potato Dextrose Agar (PDA) with ventral side facing the medium and incubated at 26 ± 1 °C for 10 d with 12 h light/dark cycles. The germinating fungal hyphae were picked from the fine tipped sterile needle and sub-cultured over PDA. The isolates were maintained as pure cultures over PDA slants supplemented with 10% glycerol.
Production of culture filtrates
Liquid cultures of endophytic fungi were prepared by the procedure of Raviraja et al.Citation8. Concisely, the method comprised of inoculation of 100 ml pre-sterilized Richard’s Broth (composition: Sucrose – 5 g; Potassium Nitrate – 1 g; Potassium dihydrogen phosphate – 800 mg, Magnesium Sulphate – 250 mg; Ferric chloride – 2 mg; pH 4.5) aseptically with 5 mm mycelial disc of 7-d-old culture of endophytic fungus followed by incubation at 26 ± 1 °C, 120 rpm for 15 d. After the culmination of incubation period, the mycelial mass was separated using Whatmann filter paper no.4 followed by centrifugation at 1000 rpm for 10 min at room temperature. The supernatant was then passed through 0.22 µm nitrocellulose membrane making it cell free. The cell-free broths were stored at −20 °C until subjected to qualitative screening for PL inhibition.
Qualitative screening for PL inhibitors
Two chromogenic plate assays were used for screening the lipase inhibitory activity where the PL inhibition was indicated by decrease in halo (Rhodamine-olive oil plate assay) and change in color (Phenol-red olive oil plate assay).
Rhodamine olive oil plate assay
The assay was used for the preliminary screening of the PL inhibitory activity using the cell-free culture broth of the endophytic fungi. The assay was carried as per the method ofCitation9which involved preparation of 4 mm thick olive-agarose plate consisting of 2.5% olive oil, 1.3% agarose and 0.3% rhodamine as indicator. After solidification, 5 mm wells were punched using a sterile cork borer. Subsequently, 35 µl of the master-mix containing pre-incubated 15 µl of porcine PL (Stock = 40 U/ml) and 20 µl of culture filtrates was dispensed into 5 mm wells and incubated at 37 °C for 24 h. The control comprised of 15 µl of porcine PL and 20 µl of sterile saline. Appearance of orange-colored halo under ultra violet (UV) rays indicated the PL activity in the control while reduction the diameter of halo as compared to control indicated PL inhibition. All the tests were performed in triplicates and their mean and SD was calculated. Orlistat was used as a positive control for lipase inhibitory activity.
Phenol red olive oil plate assay
The assay assessed the change in color due to pH indicator as a result of fatty acid formation by the PL activity by hydrolysis of olive oilCitation10. Briefly, the assay plates were prepared using olive oil as substrate (2.5%) and phenol red (0.01%) as pH indicator dye with 2% agar. Wells measuring 5 mm were punched aseptically and 35 µl of master mix having the same composition as mentioned in the rhodamine assay was dispensed and plates were incubated at 37 °C for 24 h. The control comprised of 15 µl of porcine PL (Stock = 40 U/ml) and 20 µl of sterile saline. The change in color from red to orange infers lipase production and decrease in the halo formation as compared to the test indicates lipase inhibitory potential of the culture filtrates/solvent extracts. All the tests were performed in triplicates and their mean and SD was calculated. Orlistat was used as positive control.
Liquid–liquid extraction
Liquid–liquid extraction procedure was adopted for extracting bioactive metabolites from the culture filtrate of endophytic fungi. The cell-free culture filtrate was extracted thrice with ethyl acetate in the ratio 2:1. The organic layer so obtained was pooled and then dehydrated using anhydrous sodium sulphate. The remaining aqueous layer was then extracted with chloroform and hexane following the same procedure. The solvent was evaporated using nitrogen blowout to obtain ethyl acetate, chloroform and hexane extract residue at room temperature. The residue so obtained was weighed and a stock solution was prepared using methanol and stored at −20 °C till further use.
Quantitative screening of PL inhibition
The culture filtrate and ethyl acetate residue of selected endophytic fungi exhibiting potential inhibition in the in vitro plate assays were further evaluated quantitatively using a spectrophotometric assay. Briefly, the assay comprises of using PNPL (p-nitrophenyl laurate) as substrate for PL activity. Hydrolysis of the substrate releases p-nitrophenyl which is a chromogen of yellow color measured spectrophotmetrically at 410 nm. The reduction in intensity of yellow color is indicative of PL inhibition. Cell-free culture filtrates of endophytic fungi were pre-incubated with PL (prepared in potassium phosphate buffer, pH 7.4) at 37 °C for 1 h prior to assaying the activity. The reaction was started by adding 100 µl of PNPL (2 mM) as a substrate and 20 µl enzyme (40U) and rest of the volume is made up to 250 µl using buffer. After incubation at 37 °C for 3 h, the amount of p-nitrophenol released in the reaction was measured at 410 nm using a Bioteck Powerwave 340 plate reader. The control comprised of the enzyme and substrate without cell-free culture filtrate and orlistat was used as a positive control in the assay. All the tests were performed in triplicates. The percentage inhibition (I) was given by the following formula;
where “A” is the activity without inhibitor; “a” is the negative control without inhibitor; “B” is the activity with inhibitor; and “b” is the negative control with inhibitorCitation11.
Identification of PL inhibiting endophytic fungi
The potential isolate was identified using microscopic as well as molecular methods.
Microscopic identification of the bioactive fungi
The bioactive endophytic fungus was grown over PDA and incubated at 26 °C for 5 d. The morphological structures like the colony size, texture, color and microscopic characters like the hyphae, conidia, stipe, phialides, were observed and recorded. The microscopic characters were studied using a polarising optical microscope (Olympus BX-51 P, Tokyo, Japan) coupled with CCD camera and measurements carried out using Image J software. At least 30 observations were made per structureCitation12,Citation13.
ITS-based molecular taxonomy and phylogenetic analysis
For the genomic DNA isolation, about 0.1–0.2 g of cultured mycelia was scrapped off from the 3-to-4-d-old culture with sterile inoculation loop and crushed to very fine powder in pestle and mortar using liquid nitrogen. Further DNA extraction was done by using the Wizard® Genomic DNA purification kit (Promega, Madison, WI) as per the manufacturer instructions.
The ITS1, 5.8S, ITS2 rDNA sequence was amplified using Bio Rad thermocycler. PCR reaction was carried out by using universal ITS primersCitation14. Amplification was performed in 25 µl reaction mixture containing 1 µl of extracted genomic DNA, 10 µM of each primer, 2.5 mM of dNTP, 1.5U of Taq DNA Polymerase in 10 X Taq buffer containing 25 mM MgCl2. The PCR cycling conditions consisted of initial denaturation at 96 °C for 5 min followed by 39 cycles of 95 °C for 45 s, 60 °C for 45 s, 72 °C for 45 s followed by final extension at 72 °C for 5 min. PCR products (500–600 bp) were sent for sequencing to Chromus Biotech Labs (Bangalore, India). The accession number of the sequences in NCBI Genbank is KF537624.
Sequence assembly, alignment and phylogenetic analysis
Sequence similarity search for the obtained sequences of #57 TBBALM was performed using the BLAST algorithm against the non-redundant database maintained by the National Center for Biotechnology Information (NCBI). The phylogenetic analysis of #57 TBBALM involved 17 sequences which comprised of 1 sequence under study, 15 sequences from BLAST search which are representative sequences of Penicillium species and 1 Muscodor species chosen as outgroup. All ambiguous positions were removed from each sequence pair. These sequences were then aligned using Clustal W option in MEGA 5 (Tempe, AZ)Citation15. The aligned sequences were then trimmed using the primer sequences so as to make the alignment uniform. The aligned files of the sequences were exported to FASTA as well as MEGA format. The matrix was analyzed by the Neighbor-Joining methodCitation16 using the Kimura-2-parameter modelCitation17 to calculate the evolutionary distances, and 1000 bootstrap replicates were taken into account to infer the consensus tree for the representation of evolutionary history.
Results
Qualitative screening
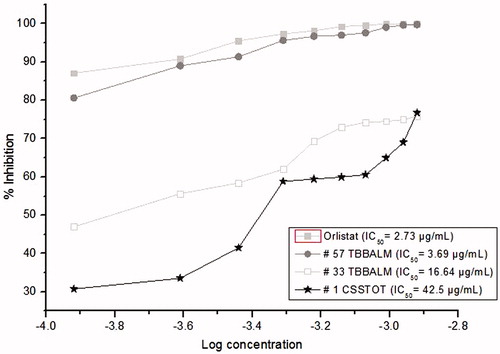
In the present study, 70 endophytic fungal isolates were screened for the PL Inhibitory potential. Approximately, 66% cultures were isolated from bark of Taxus baccata while 34% cultures were isolated from different parts. Cinnamomum zeylanicum, Camellia sinensis, Piper nigrum, Rauwolfia serpentina, Tabernaemontana divaricata and Nerium oleander. Dominant species among the endophytic isolates were of Pestalotiopsis, Alternaria, Fusarium and Penicillium. Three sterile fungi were also reported during the isolation procedure (). In the rhodamine as well as phenol red plate assay it was found that #57 TBBALM exhibited the maximum enzyme inhibition ( and ). The other common organisms selected for further quantitative evaluation of the inhibitory potential of the PL were #33 TBBALM and #1 CSSTOT.
Table 1. Endophytic fungi from different medicinal plants used during the study with their corresponding inhibition activities in two chromogenic in vitro qualitative assays.
Table 2. In vitro porcine pancreatic lipase inhibitory activity of the culture filtrates in phenol red plate assay.
Table 3. Inhibitory activity of the endophytic culture filtrates against Pl in rhodamine plate assay.
Quantitative screening
Further spectrophotometric assay ascertained the quantitative reduction in PL activity by the crude solvent extracts of culture filtrates of #57 TBBALM, #33 TBBALM and #1 CSSTOT which were compared to the positive control, Orlistat. The IC50 values of Orlistat and ethyl acetate fraction of #57 TBBALM culture filtrate was 2.73 µg/ml and 3.69 µg/ml, respectively (). The inhibition pattern of ethyl acetate fraction of #57 TBBALM culture filtrate was quite similar to that of orlistat. Further the IC50 values of ethyl acetate fraction of #33 TBBALM and #1 CSSTOT were found to be 16.64 µg/ml and 42.5 µg/ml, respectively. Chloroform and hexane extracts of #57 TBBALM, #33 TBBALM and #1 CSSTOT did not exhibit any PL inhibitory activity. These results suggest that the ethyl acetate fraction of #57 TBBALM possess a bioactive compound which has potential PL inhibitory activity.
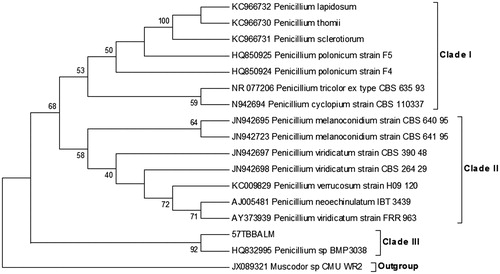
ITS based molecular taxonomy and phylogenetic analysis ()
The phylogenetic tree based upon the ITS1-5.8S-ITS2 region sequence of #57 TBBALM using MEGA5 comprised of three clades. Clade I consisted of Penicillium lapidosum, P. thomii, P. sclerotiorum, type strains, namely P. tricolor, P. cyclopium and two strains of P. polonicum The clade II clustered the two strains of P. melanoconidium, three strains of P. viridicatum, and one strain each of P. verrucosum and P. neoechinulatum. On the other hand, #57 TBBALM along with Penicillium sp BMP3038 diverged as a sister clade from clade I and clade II with significantly high bootstrap support value thereby confirming its placement in the genus Penicillium.
Morphotaxonomy
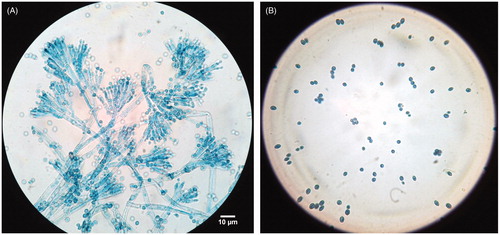
Over PDA medium the fungus forms flat, velutinous, fast growing (18–20 mm) colonies which are initially white later turning to green color on incubation at 26 ± 2 °C for 5 d. The colony is pale colored from reverse. It forms septate, hyaline hyphae (1.51)−2.59 ± 0.56−(3.69) µm. Long broad stipe (2.22)–3.64 ± 0.87−(5.47) µm arises from the hyphae either singly or synmeta. Metulae (8.75)−10.73 ± 1.58−(13.92) µm × (1.76)−2.79 ± 0.52−(4.23) µm arises from the stipe which carries flask shaped phialides. Metulae are 3–4 in number. Each metulae gives rise to four phialides (5.51)−7.75 ± 1.61−(10.82) µm × (1.15)−2.13 ± 0.45−(2.79) µm which forms brush like clusters known as penicilli. The conidia (2.19)−2.95 ± 0.51−(4.09) µm are darkly stained, unicellular, globose shaped arranged in basipital chain. ( and B).
Discussion and conclusion
Our present findings implicate that the ethyl acetate extract of culture filtrate of #57 TBBALM exhibits a promising porcine PL inhibitory activity when compared to plant extracts, namely grape seed extractCitation18 and Nelumbo nucifera extractCitation19. Further, in terms of IC50 values, the ethyl acetate extract of #57 TBBALM exhibited a potential lipase inhibitory activity when compared to Carnosic acidCitation20 and HersperidinCitation21isolated from Salvia officinalis and citrus fruits, respectively.
It is expected further purification of the ethyl acetate fraction would improve the inhibition kinetics of porcine PL which would be helpful in arriving to the exact IC50 of the pure compound. Microbial compounds like VibralactoneCitation22 and PercyquininCitation23 have exhibited better IC50 values as compared to their crude extracts.
Thus, endophytic fungi also offer to be a promising underexplored resource for screening potential PL inhibitors. Till date, there exists no report on endophytic fungi producing PL inhibitor. The present study is a pioneer work wherein we have explored the potential PL inhibitory activity of endophytic fungi which were isolated from different medicinal plants. Based on the qualitative and quantitative in vitro assays, it was found that an isolate from Taxus baccata, #57 TBBALM possess potential porcine PL inhibitory activity. Hence, this activity of the ethyl acetate extract of the culture filtrate of the endophytic fungus #57 TBBALM identified as Penicillium species can be further exploited. Further purification and characterization of this compound is underway for possible development into a drug for obesity management.
Declaration of interest
The authors declare no conflicts of interests. The authors alone are responsible for the content and writing of this article. Mahiti Gupta thanks the University Grants Commission (UGC), New Delhi for providing UGC-BSR fellowship.
References
- Arbeeny CM. Addressing the unmet medical for safe and effective weight loss therapies. Obes Res 2004;12:1191–6
- Harrold J. Neuroendocrine targets for the treatment of obesity: physiological roles and unrealized opportunities. Curr Med Chem 2003;3:141–55
- Birari R, Bhutani K. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov Today 2007;12:19–20
- Franson K, Rossner M. Fat intake and food choices during weight reduction with diet, behavioural modification and a lipase inhibitor. J Intern Med 2000;247:607–14
- Kaul S, Gupta S, Ahmed M, Dhar MK. Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites. Phytochem Rev 2012;11:487–505
- Slanc P, Doljak B, Mlinaric A, Strukelj B. Screening of wood damaging fungi and macrofungi for inhibitors of pancreatic lipase. Phytother Res 2004;18:758–62
- Schulz B, Wanke U, Drager S, Aust HJ. Endophytes from herbaceous plants and shrubs: effectiveness of surface sterilization methods. Mycol Res 1993;97:1447–50
- Raviraja NS, Maria GL, Sridhar KR. Antimicrobial evaluation of endophytic fungi inhabiting medicinal plants of the western ghats of India. Eng Life Sci 2006;6:515--20
- Sheikh Abdul HN, Zen HB, Tein QB, et al. Screening and identification of extracellular lipase-producing thermophilic bacteria from a Malaysian hot spring. World J Microb Biot 2003;19:961–8
- Singh R, Gupta N, Goswami V, Gupta R. A simple activity staining protocol for lipases and esterases. Appl Microbiol Biotechnol 2006;6:679–82
- Kim YS, Lee YM, Kim H, et al. Anti-obesity effect of Morus bombycis root extract: anti-lipase activity and lipolytic effect. J Ethnopharmacol 2010;130:621–4
- Barnett HL, Hunter BB. Illustrated genera of imperfect fungi. 4th ed. NewYork (NY): Macmillan; 1998
- Frisvad JC, Samson RA. Polyphasic taxonomy of Penicillium subgenus Penicillium: a guide to identification of food and air-borne terverticillate penicillia and their mycotoxins. Stud Mycol 2004;49:1–173
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: White BA, ed. PCR protocols: a guide to methods and applications. New York (NY): Academic Press; 1990:315–22
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 2011;28:2731–9
- Saitou N, Nei M. The Neighbor-Joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 1987;4:406–25
- Kimura M. Rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 1980;16:111–20
- Moreno DA, Ilic N, Poulev A, et al. Inhibitory effects of grape seed extract on lipases. Nutrition 2003;19:876–9
- Ono Y, Hattori E, Fukaya Y, et al. Anti-obesity effect of Nelumbo nucifera leaves extract in mice and rats. J Ethnopharmacol 2006;106:238–44
- Ninomiya K, Matsuda H, Shimoda H, et al. Carnosic acid: a new class of lipid absorption inhibitor from sage. Bioorg Med Chem Lett 2004;14:1943–6
- Kawaguchi K, Mizuno T, Aida K, Uchino K. Hesperidin as an inhibitor of lipases from porcine pancreas and Pseudomonas. Biosci Biotechnol Biochem 1997;61:102–4
- Liu D, Wang F, Liao T, et al. Vibralactone: a lipase inhibitor with an unusual fused beta-lactone produced by cultures of the basidiomycete Boreostereum vibrans. Org Lett 2006;8:5749–52
- Hopmann C, Kurz M, Mueller G, Toti L. Percyquinnin, a process for its production and its use as a pharmaceutical. European Patent no. EP 1274698, 2003 Assignee: Aventis Pharma GmBH