Abstract
2-[[5-(2,4-Difluoro/dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio] acetophenone derivatives (3a--s) were designed as human carbonic anhydrase isozymes (hCA-I and hCA-II) inhibitors and synthesized. hCA-I and hCA-II were purified from erythrocyte cells by the affinity chromatography. The inhibitory effects of 18 newly synthesized acetophenones on hydratase activity of these isoenzymes were studied in vitro. The average IC50 values of the new compounds for hydratase activity ranged from 0.033 to 0.14 μM for hCA-I and from 0.030 to 0.11 μM for hCA-II. Among the newly synthesized compounds, 2-[[5-(2,4-dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-bromoacetophenone (3n) can be considered as a promising hCA-II inhibitor owing to its selective and potent inhibitory effect on hCA-II.
Introduction
Carbonic anhydrases (CAs, EC 4.2.1.1) are a superfamily of zinc-containing metalloenzymes, present in prokaryotes and eukaryotes, being encoded by five distinct, evolutionarily unrelated gene families: the α-, β-, γ-, δ-, and ɛ-CAs. 16 different α-CA isoforms were isolated and characterized in mammals, where they play crucial physiological roles. Some of them are cytosolic (CA I, CA II, CA III, CA VII, CA XIII), others are membrane-bound (CA IV, CA IX, CA XII, CA XIV and CA XV), CA VA and CA VB are mitochondrial, and CA VI is secreted in saliva and milk. Three acatalytic forms are also known, the CA related proteins (CARP), CARP VIII, CARP X and CARP XICitation1–4.
CAs, which catalyze the interconversion of carbon dioxide and water to bicarbonate and protons, have attracted a great deal of interest as important targets for drug discovery due to their essential roles in crucial physiological processes connected with respiration and transport of CO2/bicarbonate between metabolizing tissues and lungs, pH and CO2 homeostasis, electrolyte secretion in a variety of tissues/organs, biosynthetic reactions (such as gluconeogenesis, lipogenesis and ureagenesis), bone resorption, calcification, tumorigenicity and many other physiologic or pathologic processesCitation4.
Inhibition of CA isoenzymes is a promising approach for pharmacologic intervention in a variety of disorders such as glaucoma, epilepsy, obesity, and cancer. In the last decades, CA inhibition has also emerged as a major focus of pharmaceutical research for the design of anti-infective agents with a novel mechanism of actionCitation1–9.
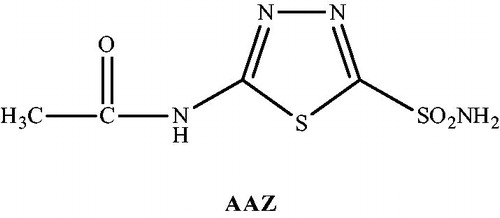
Thiadiazole has received considerable attention as a privileged scaffold due to its significant therapeutic potential. The sulfur atom of thiadiazole ring imparts improved liposolubility and the mesoionic nature of 1,3,4-thiadiazoles also allows these compounds to cross cellular membranes and interact with biological targets with distinct affinitiesCitation10,Citation11. Several derivatives of 5-amino-1,3,4-thiadiazole-2-sulfonamide have been synthesized and used as carbonic anhydrase inhibitors. One of them is acetazolamide (AAZ) (N-(5-sulfamoyl-1,3,4-thiadiazole-2-yl)acetamide), which is used in the treatment of glaucoma ()Citation12–15. Some researchers have reported the potency of the SH moiety as a zinc-binding function in the design of carbonic anhydrase inhibitors and carried out considerable research for the synthesis and evaluation of 5-amino-1,3,4-thiadiazole-2-thiol derivatives as carbonic anhydrase inhibitorsCitation16–18.
On the basis of afore-mentioned findings, herein we report the synthesis and biological evaluation of a series of 2-[[5-(2,4-difluoro/dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]acetophenone derivatives as hCA-I and hCA-II inhibitors.
Methods
Chemistry
All reagents were purchased from commercial suppliers and used without further purification. Melting points (m.p.) were determined on an Electrothermal 9100 melting point apparatus (Weiss-Gallenkamp, Loughborough, UK) and are uncorrected. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded on a Bruker 500 MHz spectrometer (Bruker, Billerica, MA). Chemical shifts were expressed in parts per million (ppm) and tetramethylsilane was used as an internal standard. Mass spectra were recorded on a Agilent LC-MSD-Trap-SL Mass spectrometer (Agilent, Minnesota, MN). Elemental analyses were performed on a Perkin Elmer EAL 240 elemental analyser (Perkin-Elmer, Norwalk, CT).
General procedure for the synthesis of the compounds
4-(2,4-Difluoro/dichlorophenyl)thiosemicarbazide (1a–b)
A mixture of 2,4-difluoro/dichlorophenyl isothiocyanate (0.1 mol) and hydrazine hydrate (0.2 mol) in ethanol (30 ml) was stirred at room temperature for 5 h and then filtered. The residue was crystallized from ethanolCitation19,Citation20.
5-[(2,4-Difluoro/dichlorophenyl)amino]-1,3,4-thiadiazole-2(3H)-thione (2a–b)
4-(2,4-Difluoro/dichlorophenyl)thiosemicarbazide (1a–b) was dissolved in a solution of sodium hydroxide in ethanol. Carbon disulfide was then added while stirring and the reaction mixture was heated under reflux for 10 h. The solution was cooled and acidified to pH 4–5 with hydrochloric acid solution and crystallized from ethanolCitation21.
2-[[5-(2,4-Difluoro/dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]acetophenone derivatives (3a–s)
A mixture of 5-(2,4-difluoro/dichlorophenylamino)-1,3,4-thiadiazole-2(3H)-thione (2a–b) (0.05 mol) and appropriate phenacyl bromide (0.05 mol) in the presence of potassium carbonate (0.05 mol) in acetone was stirred at room temperature for 8 h. The reaction mixture was filtered and crystallized from ethanol.
2-[[5-(2,4-difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]acetophenone (3a)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.96 (s, 2H), 7.07–7.11 (m, 1H), 7.32–7.37 (m, 1H), 7.56–7.59 (m, 2H), 7.69 (t, J = 7.5 Hz, 1H), 8.04–8.06 (m, 2H), 8.24–8.29 (m, 1H), 10.17 (s, 1H).
MS (ESI) (m/z): [M+1]+ 364
Anal. Calcd. for C16H11F2N3OS2: C, 52.88; H, 3.05; N, 11.56. Found: C, 52.90; H, 3.04; N, 11.55.
2-[[5-(2,4-Difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-nitroacetophenone (3b)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 5.00 (s, 2H), 7.06–7.34 (m, 2H), 8.22–8.27 (m, 3H), 8.37 (d, J = 8.5 Hz, 2H), 10.17 (s, 1H).
MS (ESI) (m/z): [M+1]+ 409
Anal. Calcd. for C16H10F2N4O3S2: C, 47.05; H, 2.47; N, 13.72. Found: C, 47.07; H, 2.45; N, 13.70.
2-[[5-(2,4-Difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-fluoroacetophenone (3c)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.94 (s, 2H), 7.07–7.11 (m, 1H), 7.32–7.42 (m, 3H), 8.12–8.15 (m, 2H), 8.24–8.29 (m, 1H), 10.16 (s, 1H).
MS (ESI) (m/z): [M+1]+ 382
Anal. Calcd. for C16H10F3N3OS2: C, 50.39; H, 2.64; N, 11.02. Found: C, 50.41; H, 2.65; N, 11.00.
2-[[5-(2,4-difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-chloroacetophenone (3d)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.94 (s, 2H), 7.08–7.09 (m, 1H), 7.33–7.35 (m, 1H), 7.65 (d, J = 8.5 Hz, 2H), 8.06 (d, J = 8.5 Hz, 2H), 8.25–8.27 (m, 1H), 10.17 (s, 1H).
MS (ESI) (m/z): [M+1]+ 398
Anal. Calcd. for C16H10ClF2N3OS2: C, 48.30; H, 2.53; N, 10.56. Found: C, 48.28; H, 2.54; N, 10.55.
2-[[5-(2,4-difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-bromoacetophenone (3e)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.93 (s, 2H), 7.07–7.10 (m, 1H), 7.31–7.36 (m, 1H), 7.79 (d, J = 8.0 Hz, 2H), 7.97 (d, J = 8.5 Hz, 2H), 8.24-8.29 (m, 1H), 10.16 (s, 1H).
MS (ESI) (m/z): [M+1]+ 443
Anal. Calcd. for C16H10BrF2N3OS2: C, 43.45; H, 2.28; N, 9.50. Found: C, 43.43; H, 2.30; N, 9.49.
2-[[5-(2,4-difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-cyanoacetophenone (3f)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.97 (s, 2H), 7.08–7.09 (m, 1H), 7.33–7.34 (m, 1H), 8.06 (d, J = 8.5 Hz, 2H), 8.19 (d, J = 8.5 Hz, 2H), 8.24–8.25 (m, 1H), 10.17 (s, 1H).
MS (ESI) (m/z): [M+1]+ 389
Anal. Calcd. for C17H10F2N4OS2: C, 52.57; H, 2.60; N, 14.42. Found: C, 52.55; H, 2.59; N, 14.41.
2-[[5-(2,4-Difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-methylacetophenone (3g)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 2.40 (s, 3H), 4.92 (s, 2H), 7.07–7.11 (m, 1H), 7.33–7.39 (m, 3H), 7.94 (d, J = 8.0 Hz, 2H), 8.24–8.29 (m, 1H), 10.06 (s, 1H).
MS (ESI) (m/z): [M+1]+ 378
Anal. Calcd. for C17H13F2N3OS2: C, 54.10; H, 3.47; N, 11.13. Found: C, 54.11; H, 3.45; N, 11.11.
2-[[5-(2,4-Difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-methoxyacetophenone (3h)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.87 (s, 3H), 4.89 (s, 2H), 7.06–7.12 (m, 3H), 7.33–7.37 (m, 1H), 8.01–8.04 (m, 2H), 8.24–8.29 (m, 1H), 10.15 (s, 1H).
MS (ESI) (m/z): [M+1]+ 394
Anal. Calcd. for C17H13F2N3O2S2: C, 51.90; H, 3.33; N, 10.68. Found: C, 51.89; H, 3.31; N, 10.69.
2-[[5-(2,4-Difluorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-methylsulfonylacetophenone (3i)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.32 (s, 3H), 4.99 (s, 2H), 7.08–7.11 (m, 1H), 7.33–7.37 (m, 1H), 8.12 (d, J = 8.5 Hz, 2H), 8.23–8.28 (m, 3H), 10.18 (s, 1H).
MS (ESI) (m/z): [M+1]+ 442
Anal. Calcd. for C17H13F2N3O3S3: C, 46.25; H, 2.97; N, 9.52. Found: C, 46.26; H, 2.95; N, 9.51.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio] acetophenone (3j)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.97 (s, 2H), 7.42 (dd, J = 9.0, 2.5 Hz, 1H), 7.58 (t, J = 7.5 Hz, 2H), 7.64 (d, J = 2.5 Hz, 1H), 7.70 (t, J = 7.5 Hz, 1H), 8.05 (d, J = 7.0 Hz, 2H), 8.33 (d, J = 9.0 Hz, 1H), 9.95 (s, 1H).
MS (ESI) (m/z): [M+1]+ 397
Anal. Calcd. for C16H11Cl2N3OS2: C, 48.49; H, 2.80; N, 10.60. Found: C, 48.51; H, 2.79; N, 10.59.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-nitroacetophenone (3k)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 5.02 (s, 2H), 7.42 (dd, J = 9.0, 2.5 Hz, 1H), 7.64 (d, J = 2.5 Hz, 1H), 8.27 (d, J = 8.5 Hz, 2H), 8.32 (d, J = 8.5 Hz, 1H), 8.39 (d, J = 8.5 Hz, 2H), 9.96 (s, 1H).
MS (ESI) (m/z): [M+1]+ 442
Anal. Calcd. for C16H10Cl2N4O3S2: C, 43.55; H, 2.28; N, 12.70. Found: C, 43.53; H, 2.31; N, 12.69.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-fluoroacetophenone (3l)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.96 (s, 2H), 7.39–7.43 (m, 3H), 7.63 (d, J = 2.5 Hz, 1H), 8.12-8.15 (m, 2H), 8.33 (d, J = 8.5 Hz, 1H), 9.95 (s, 1H).
MS (ESI) (m/z): [M +1]+ 415
Anal. Calcd. for C16H10Cl2FN3OS2: C, 46.38; H, 2.43; N, 10.14. Found: C, 46.40; H, 2.41; N, 10.15.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-chloroacetophenone (3m)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.95 (s, 2H), 7.42 (dd, J = 9.0, 2.5 Hz, 1H), 7.64–7.66 (m, 3H), 8.06 (d, J = 8.5 Hz, 2H), 8.33 (d, J = 9.0 Hz, 1H), 9.95 (s, 1H).
MS (ESI) (m/z): [M+1]+ 431
Anal. Calcd. for C16H10Cl3N3OS2: C, 44.61; H, 2.34; N, 9.75. Found: C, 44.60; H, 2.35; N, 9.72.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-bromoacetophenone (3n)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.94 (s, 2H), 7.42 (dd, J = 9.0, 2.5 Hz, 1H), 7.64 (d, J = 2.5 Hz, 1H), 7.79–7.81 (m, 2H), 7.97-7.99 (m, 2H), 8.32 (d, J = 9.0 Hz, 1H), 9.95 (s, 1H).
MS (ESI) (m/z): [M+1]+ 476
Anal. Calcd. for C16H10BrCl2N3OS2: C, 40.44; H, 2.12; N, 8.84. Found: C, 40.42; H, 2.13; N, 8.85.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-cyanoacetophenone (3o)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 4.99 (s, 2H), 7.42 (dd, J = 9.0, 2.5 Hz, 1H), 7.63 (d, J = 2.5 Hz, 1H), 8.06 (d, J = 8.5 Hz, 2H), 8.19 (d, J = 8.5 Hz, 2H), 8.32 (d, J = 9.0 Hz, 1H), 9.94 (s, 1H).
MS (ESI) (m/z): [M+1]+ 422
Anal. Calcd. for C17H10Cl2N4OS2: C, 48.46; H, 2.39; N, 13.30. Found: C, 48.45; H, 2.41; N, 13.29.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-methylacetophenone (3p)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 2.41 (s, 3H), 4.93 (s, 2H), 7.38 (d, J = 8.0 Hz, 2H), 7.42 (dd, J = 9.0, 2.5 Hz, 1H), 7.64 (d, J = 2.0 Hz, 1H), 7.95 (d, J = 8.5 Hz, 2H), 8.33 (d, J = 9.0 Hz, 1H), 9.94 (s, 1H).
MS (ESI) (m/z): [M+1]+ 411
Anal. Calcd. for C17H13Cl2N3OS2: C, 49.76; H, 3.19; N, 10.24. Found: C, 49.75; H, 3.20; N, 10.25.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-methoxyacetophenone (3r)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.86 (s, 3H), 4.90 (s, 2H), 7.07–7.12 (m, 2H), 7.40–7.45 (m, 1H), 7.62-7.65 (m, 1H), 8.03 (d, J = 8.5 Hz, 2H), 8.33 (d, J = 8.5 Hz, 1H), 9.94 (s, 1H).
MS (ESI) (m/z): [M+1]+ 427
Anal. Calcd. for C17H13Cl2N3O2S2: C, 47.89; H, 3.07; N, 9.86. Found: C, 47.90; H, 3.05; N, 9.85.
2-[[5-(2,4-Dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-methylsulfonylacetophenone (3s)
1H-NMR (500 MHz, DMSO-d6) δ (ppm): 3.32 (s, 3H), 5.01 (s, 2H), 7.42 (dd, J = 9.0, 2.5 Hz, 1H), 7.65 (d, J = 2.5 Hz, 1H), 8.12 (d, J = 8.0 Hz, 2H), 8.27 (d, J = 8.5 Hz, 2H), 8.32 (d, J = 9.0 Hz, 1H), 9.96 (s, 1H).
MS (ESI) (m/z): [M+1]+ 475
Anal. Calcd. for C17H13Cl2N3O3S3: C, 43.04; H, 2.76; N, 8.86. Found: C, 43.03; H, 2.74; N, 8.85.
Biochemistry
Sepharose-4B, sulfanilamide, l-tyrosine, Tris, Na2SO4, protein assay reagents, and chemicals for electrophoresis were purchased from Sigma-Aldrich Co. (St. Louis, MO). All other chemicals were of analytical grade and obtained from Merck (Darmstadt, Germany).
Purification of carbonic anhydrase isozymes (hCA-I and hCA-II) from human erythrocytes by affinity chromatography
Fresh human blood was obtained from the blood center, Ataturk University, it was stored at 4 °C used within 2–3 d at most. The blood samples were centrifuged to separate erythrocytes at 2500 rpm for 15 min plasma and white blood cells which is the upper part the layers carefully removed and discarded. Then, underlying erythrocytes were washed with 0.9% NaCl solution twice and upper portions were also discarded. The erythrocytes were hemolyzed with distilled water at 0 °C, it was stirred for half an hour at 4 °C. The hemolysate was centrifuged at 20 000rpm for 30 min and cell membranes were separated. pH was adjusted to 8.7 with solid Tris. So, the hemolysate was recovered to be applied to the columnCitation22,Citation23.
The affinity gel was prepared on Sepharose-4B matrix. After Sepharose-4B was activated with CNBr, l-tyrosine was covalently fitted. Then sulfanilamide was coupled to tyrosine with diazotization reaction as a ligand. The hemolysate was applied to the prepared Sepharose-4B-l-tyrosine-sulfanilamide affinity column equilibrated with 25 mM Tris-HCl/0.1 M Na2SO4 (pH 8.7). The affinity gel was washed with 25 mM Tris-HCl/22 mM Na2SO4 (pH 8.7). The human carbonic anhydrase (hCA I and hCA II) isozymes were eluted with 1 M NaCl/25 mM Na2HPO4 (pH 6.3) and 0.1 M CH3COONa/0.5 M NaClO4 (pH 5.6), respectively. All procedures were performed at 4 °CCitation22,Citation24.
Hydratase activity assay
Carbonic anhydrase activity was determined using the Wilbur–Anderson Method which was modified by Rickli and SlyCitation25,Citation26. This method, as a result hydration of CO2 is released H+ ions and the pH changes were determined by means of bromine thymol blue indicator, based on measuring the elapsed time. Enzyme Unit (EU) were calculated using the equation (to-tc/tc) to and tc are the times for pH change of the nonenzymatic and the enzymatic reactions, respectively.
Inhibition assays
It was studied with compounds 3a–s to calculate values of IC50 of hCA-I and hCA-II enzymes on the hydratase activity at different concentrations while maintaining constant the substrate concentration. Activities of enzymes in the medium without inhibitors were used as 100% activity. The activity % values of enzymes were calculated by measuring hydratase activity in the presence of different concentrations of inhibitors. The IC50 value was calculated by utilizing graphs of % activity-[I] for each inhibitorCitation27,Citation28.
Protein determination
Protein assay is performed according to the Bradford method for all purification steps. This method bases on the principle which Coomassie brilliant blue G-250 was binding with proteins in medium of phosphoric acid. Formed complex shows a maximum absorbance at 595 nm. The sensitivity of this method is between 1 and 100 μgCitation29.
SDS polyacrylamide gel electrophoresis
Then purifying hCA-I and-II isoenzymes from erythrocytes, discontinuous 3-10% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis was performed in accordance Laemmli and the purity of enzymes were checkedCitation30.
Results and discussion
The synthesis of compounds 3a–s followed the general pathway outlined in Scheme 1. Initially, 4-(2,4-difluoro/dichlorophenyl)thiosemicarbazides (1a–b) were obtained by the reaction of 2,4-difluoro/dichlorophenylisothiocyanates with hydrazine hydrate. 5-[(2,4-Difluoro/dichlorophenyl)amino]-1,3,4-thiadiazole-2(3H)-thiones (2a–b) were synthesized via the ring closure reaction of 4-(2,4-difluoro/dichlorophenyl)thiosemicarbazides (1a–b) with carbon disulfide in the presence of sodium hydroxide. Finally, the nucleophilic substitution reaction of 5-[(2,4-difluoro/dichlorophenyl)amino]-1,3,4-thiadiazole-2(3H)-thiones (2a–b) with appropriate phenacyl bromides afforded 2-[[5-(2,4-difluoro/dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]acetophenone derivatives (3a–s). Some physicochemical properties of the compounds were given in .
Scheme 1. The synthetic route for the preparation of the thiadiazole derivatives (3a–s). Reagents and conditions: (i) NH2NH2 ċ H2O, ethanol, rt, 5 h; (ii) (1) CS2/NaOH, ethanol, reflux, 10 h; (2) HCl, pH 4–5; (iii) PhCOCH2Br, K2CO3, acetone, rt, 8 h.
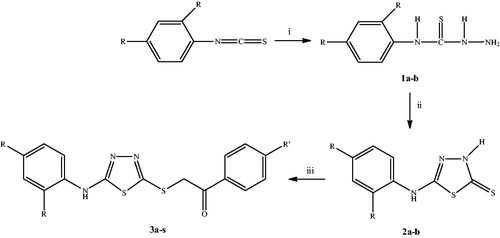
Table 1. Physicochemical properties of the compounds (3a–s).
The structures of compounds 3a–s were confirmed by 1H-NMR, mass spectral data, and elemental analysis. In the 1H-NMR spectra of compounds 3a–s, the signal due to the S–CH2 protons was observed in the region 4.89–5.02 ppm as a singlet peak. The signal due to the N–H proton attached to the thiadiazole ring occurred in the region 9.94–10.18 ppm as a singlet. The other aromatic and aliphatic protons were observed at expected regions.
Mass spectral data and elemental analysis results were in full accordance with their depicted structures. hCA-I and hCA-II isoenzymes were purified from human blood using Sepharose-4B-l-tyrosine-sulphanylamide affinity chromatography. hCA-I was purified with a specific activity of 689.0 EU/mg and a yield of 52.9% and hCA-II was purified with a specific activity of 1391.1 EU/mg and a yield of 43.9%. The overall purification was approximately 2460.7-fold for hCA-I and 4968.2-fold for hCA-II (). The newly synthesized compounds (3a–s) were investigated for their in vitro inhibitory effects on hCA-I and hCA-II and IC50 values were calculated for all derivatives ().
Table 2. Summary of purification for hCA-I and hCA-II from human erythrocyte.
Table 3. IC50 values for the in vitro inhibition of hCA-I and hCA-II with compounds 3a–s.
Generally, compounds bearing 2,4-dichlorophenyl group except compounds 3j and 3k were more effective than compounds bearing 2,4-difluorophenyl group. The inhibitory effects of eight derivatives (3a, 3b, 3g, 3h, 3i, 3j, 3l, 3r) were more significant on hCA-II than hCA-I, whereas the inhibitory effects of four derivatives (3k, 3m, 3o, 3p) were more significant on hCA-I than hCA-II.
The inhibitory effects of compounds 3c, 3d, 3e and 3n on hCA-I and hCA-II were fairly close. Compound 3f stands out as the only derivative exhibiting the same inhibitory effect on hCA-I and hCA-II. log P and MR values of the compounds were calculated and compared with the inhibitory effects on hCA-I and hCA-II, but no correlation was found. Eleven compounds (3o ≈ 3p > 3r ≈ 3m ≈ 3k > 3c >3d = 3n > 3l > 3f > 3e) were more effective on hCA-I than other compounds. According to IC50 values, seven derivatives (3r > 3l > 3o > 3a = 3c ≈ 3d = 3p) exhibited more inhibitory effect on hCA-II.
Conclusion
In this article, we synthesized a series of 2-[[5-(2,4-difluoro/dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]acetophenone derivatives (3a–s) and evaluated their ability to inhibit carbonic anhydrase isozymes (hCA-I and hCA-II). Although compounds 3a–s do not carry a sulfonamide group, an important pharmacophore for hCA inhibitory activity, it is a remarkable finding that compounds 3a–r exhibited significant inhibitory effects on hCA-I and hCA-II. Among the newly synthesized compounds, 2-[[5-(2,4-dichlorophenylamino)-1,3,4-thiadiazol-2-yl]thio]-4′-bromoacetophenone (3n) can be evaluated as a potential hCA-II inhibitor due to its selective and potent inhibitory effect on hCA-II.
Declaration of interest
The authors report no conflicts of interest.
References
- Supuran CT. Structure-based drug discovery of carbonic anhydrase inhibitors. J Enzyme Inhib Med Chem 2012;27:759–72
- Supuran CT. Carbonic anhydrases – an overview. Curr Pharm Des 2008;14:603–14
- Supuran CT. Carbonic anhydrase inhibitors. Bioorg Med Chem Lett 2010;20:3467–74
- Supuran CT, Scozzafava A. Carbonic anhydrases as targets for medicinal chemistry. Bioorg Med Chem 2007;15:4336–50
- Şentürk M, Çavdar H, Talaz O, Supuran CT. Carbonic anhydrase ınhibitors and activators: small organic molecules as drugs and prodrugs. In: Ekinci D, ed. Medicinal chemistry and drug design. Rijeka: InTech Croatia; 2012:315–28
- Supuran CT. Carbonic anhydrase inhibitors and activators for novel therapeutic applications. Future Med Chem 2011;3:1165–80
- Alterio V, Di Fiore A, D'Ambrosio K, et al. Multiple binding modes of inhibitors to carbonic anhydrases: how to design specific drugs targeting 15 different isoforms? Chem Rev 2012;112:4421–68
- Supuran CT, Vullo D, Manole G, et al. Designing of novel carbonic anhydrase ınhibitors and activators. Curr Med Chem – Cardiovasc Hematol Agents 2004;2:49–68
- Supuran CT. Carbonic anhydrase ınhibition/activation: trip of a scientist around the world in the search of novel chemotypes and drug targets. Curr Pharm Des 2010;16:3233–45
- Jain AK, Sharma S, Vaidya A, et al. 1,3,4-Thiadiazole and its derivatives: a review on recent progress in biological activities. Chem Biol Drug Des 2013;81:557–76
- Li Y, Geng J, Liu Y, et al. Thiadiazole-a promising structure in medicinal chemistry. Chem Med Chem 2013;8:27–41
- Kasımoğulları R, Bülbül M, Seçkin Arslan B, Gökçe B. Synthesis, characterization and antiglaucoma activity of some novel pyrazole derivatives of 5-amino-1,3,4-thiadiazole-2-sulfonamide. Eur J Med Chem 2010;45:4769–73
- Supuran CT, Briganti F, Tilli S, et al. Carbonic anhydrase inhibitors: sulfonamides as antitumor agents? Bioorg Med Chem 2001;9:703–14
- Kasımoğulları R, Bülbül M, Gunhan H, Guleryuz H. Effects of new 5-amino-1,3,4-thiadiazole-2-sulfonamide derivatives on human carbonic anhydrase isozymes. Bioorg Med Chem 2009;17:3295–301
- Kasımoğulları R, Bülbül M, Mert S, Güleryüz H. Synthesis of 5-amino-1,3,4-thiadiazole-2-sulphonamide derivatives and their inhibition effects on human carbonic anhydrase isozymes. J Enzyme Inhib Med Chem 2011;26:231–7
- Abdel-Hamid MK, Abdel-Hafez AA, El-Koussi NA, et al. Design, synthesis, and docking studies of new 1,3,4-thiadiazole-2-thione derivatives with carbonic anhydrase inhibitory activity. Bioorg Med Chem 2007;15:6975–84
- Almajan GL, Innocenti A, Puccetti L, et al. Carbonic anhydrase inhibitors. Inhibition of the cytosolic and tumor-associated carbonic anhydrase isozymes I, II, and IX with a series of 1,3,4-thiadiazole- and 1,2,4-triazole-thiols. Bioorg Med Chem Lett 2005;15:2347–52
- Abdel-Hamid MK, Abdel-Hafez AA, El-Koussi NA, Mahfouz NM. Quantitative structure-activity relationship (QSAR) studies on a series of 1,3,4-thiadiazole-2-thione derivatives as tumor-associated carbonic anhydrase IX inhibitors. J Enzyme Inhib Med Chem 2009;24:722–9
- Ho GD, Yang S-W, Smith EM, et al. Preparation of substituted pyrazoloquinolines and derivatives thereof. PCT Int Appl 2010, WO 2010062559 A1 20100603
- Calıs U, Septioglu E, Dilsiz Aytemir M. Synthesis and anticonvulsant evaluation of some novel (thio)semicarbazone derivatives of arylalkylimidazole. Arzneimittel Forschung 2011;61:327–34
- Wahab A. Thiadiazole derivatives. IV. Synthesis of some 5-mercapto-1,3,4-thiadiazoles and 5-ethylthio-2-arylamino-1,3,4-thiadiazoles. Boll Chim Farm 1979;118:391–6
- Arslan O, Nalbantoğlu B, Demir N, et al. A new method for the purification of carbonic anhydrase isoenzymes by affinity chromatography. Turk J Med Sci 1996;26:163–6
- Demir Y, Demir N, Nadaroğlu H, Bakan E. Purification and characterization of carbonic anhydrase from bovine erythrocyte plasma membrane. Prep Biochem Biothech 2000;30:49–59
- Demir Y, Demir N, Yıldırım S, et al. The activities of carbonic anhydrase and alkaline phosphatase ın ancient human bones. purification and characterization of outer peripheral, cytosolic, ınner peripheral, and ıntegral CA. Prep Biochem Biotech 2001;31:291–304
- Rickli EE, Ghazantar SAS, Gibbons BH, Edsall JT. Carbonic anhydrase from human erythrocytes. J Biol Chem 1964;239:1065–78
- Sly WS, Hu PY. Human carbonic anhydrase and carbonic anhydrase deficiencies. Annu Rev Biochem 1995;64:375–401
- Demir Y, Nadaroğlu H, Demir N. Effects of omeprazole, farmotidine, and ranitidine on the enzyme activities of carbonic anhydrase from bovine stomach in vitro and rat erytrocytes in vivo. Biol Pharm Bull 2004;27:1730–4
- Demir Y, Nadaroğlu H, Demir N. Purification and characterization of carbonic anhydrase from bovine stomach and ınhibitory effects of some chemical substances on enzyme activities. J Enzyme Inhib Med Chem 2005;20:75–80
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantites of protein utilizing the principle of protein-dye binding. Annal Biochem 1976;72:248–54
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970;227:680–5