Abstract
A simple and efficient method for the synthesis of highly diverse pyrano[2,3-c]pyridazines was achieved by a one pot multicomponent reaction using piperidine as the organocatalyst. The synthesis of a series of heterocyclic derivatives with varying functionality (e.g. thiazine, tetrazole and pyrimidine) incorporating the pyrano[2,3-c]pyridazine moiety were achieved via reaction of 2a–e with different reagents. The structures of the synthesized derivatives were elucidated by FTIR, MS, 1H and 13C NMR spectroscopy. A number of the newly synthesized targeted compounds 2b–e, 3a–c and 4a–c were evaluated for their in vitro antibacterial activity and were compared with chloramphenicol and nystatin as broad spectrum reference standard antibiotics. Tests were carried out against Staphylococcus aureus (MTCC3160) and Enterococcusi fecalis as Gram-positive bacteria, and Escherichia coli (MTCC1652) and Klebsiella pneumonia as Gram-negative bacteria. Antifungal potential against Candida albicans, and Aspergillus albicans strains were also evaluated. The results revealed that compounds 3a and 3c showed strong significant activity relative to the reference against these bacterial and fungal strains.
Introduction
The synthesis of heterocyclic compounds with potential biological activity is one of the main challenges in chemical biology and medicinal chemistry. Pyridazine compounds have been reported to possess varied biological activities such as anti-HIVCitation1, anti-TMVCitation2, antidepressantCitation3, antihypertensiveCitation4, antithromboticCitation5, anticonvulsantCitation6, cardiotonicCitation7, anti-bacterialCitation8, diureticCitation9 and anticancerCitation10. Antibacterial, antifungal, antituberculosis, antiviral, anti-inflammatory, anticancer and effectiveness against cardiovascular disorders have also been reportedCitation11,Citation12.
Compounds having the pyran structural motif exhibit a wide range of biological activities, such as diuretic, analgesic, myorelaxantCitation13, anticancerCitation14 and anti-HIVCitation15,Citation16. In addition, they are also useful for the treatment of neurodegenerative disorders including Alzheimer’s disease, amyotrophic lateral sclerosis, Huntington’s disease and Parkinson’s diseaseCitation17. Due to the important biological activities of pyridazines and pyrans, we hypothesized that the synthesis of new heterocycles containing both pyridazine and pyran moieties might result in the design of new drug candidates. Therefore, in continuation of our efforts on the design and efficient synthesis of new biologically active compounds and in particular pyridazinesCitation18–27, herein we report a new and simple method for the synthesis of polyfunctionalized pyrano-fused pyridazine systems.
Materials and methods
Chemistry
All the melting points were uncorrected and determined on a Gallenkamp melting point apparatus Model MFB 595-0366. Infrared spectra were measured on Perkin-Elmer spectrophotometer Model 1430 using potassium bromide pellets and frequencies are reported in cm−1. The 1H-NMR and 13C-NMR spectra were measured in DMSO-d6 on Varian Genini-300 MHz spectrometer and chemical shifts, δ are in ppm. The mass spectral (m/z) values were measured on an HP model GC MS-QPL000EX (Shimadzu) at 70-eV mass spectrometer. Spectroscopic analyses and antimicrobial activity evaluations were carried out at the Main Laboratories of The War Chemical War Department, Ministry of Defense ARE.
Synthesis of 7-amino-5-(aryl)-3-(aryl)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile (2a,d,e)
A mixture of compound 1a,b (0.01 mol), and an appropriate amount of arylidene malononitrile (0.01 mol) and piperidine (0.01 mol) in ethanol (10 ml) was refluxed for 3 h. The resulting crude product obtained after cooling was filtered and crystallized from ethanol to give 2a,d,e.
7-Amino-5-(3,4,5-trimethoxyphenyl)-3-(4-tolyl)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile (2a)
Yellow needles, yield 78%; m.p. 122–124 °C, ir (KBr pellet): 3368–3214 two NH spikes for (NH2), 2220 for (C≡N), 1658 for (C=C) and 1620 for (C=N) cm−1, 1H NMR: δ 10.80 (s, 2H, NH2), 7.97–7.11 (m, 6H, 2ArH), 6.58 (s, 2H, 2CH hetero), 3.84–3.80 (s, 9H, 3OCH3) and 3.21 (s, 3H, CH3-Ar) ppm. Anal. calcd. for C24H22N4O4 (430.47):C, 66.97, H, 5.15, N, 13.02. Found C, 66.89, H, 5.25, N, 13.27. M/z 438 (M+ + 8).
7 Amino-5-phenyl-3-(4-tolyl)-5H-pyrano[2,3a–c] pyridazine-6-carbonitrile (2d)
Yellow needles, yield 90%; m.p. 140–142 °C, ir (KBr pellet): 3316–3219 two NH spikes for (NH2), 2220 for (C≡N), 1659 for (C=C) and 1606 for (C=N) cm−1. 1H NMR: δ 9.80 (s, 2H, NH2), 7.90–7.21 (m, 9H, 2ArH), 6.58 (s, 2H, 2CH hetero) and 3.21 (s, 3H, CH3) ppm. Anal. calcd. for C21H16N4O (340.38): C, 74.10, 4.74, N, 16.46. Found C, 3.89, H, 4.65, N, 16.27. M/z 340(M+).
7-Amino–5-phenyl-3-(2-methoxyphenyl)-5H-pyrano[2,3-c]pyridazine-6-carbonitrile (2e)
Pale yellow needles, yield 88%; m.p. 120–122 °C, ir (KBr pellet): 3316–32198 two NH spikes for (NH2), 2219 for (C≡N), 1659 for (C=C) and 1616 for (C=N) cm−1. 1H NMR δ 9.79 (s, 2H, NH2), 7.96–7.26 (m, 9H, 2ArH), 6.28 (s, 2H, 2CH hetero) and 3.74–3.70 (s, 3H, OCH3) ppm. Anal. calcd. for C21H16N4O2 (356.38): C, 70.77, H, 4.53, N, 15.72. Found C, 70.89, H, 4.25, N, 16.00. M/z 358 (M+ + 2).
Synthesis of 7-amino-5(2,4,6-trimethoxyphenyl)-3-(4-tolyl)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile (2b), and 6-amino-5-(2,4,6-trimethoxyphenyl)-3-(4-tolyl)-5H-pyrano[2,3--c] pyridazine-7-carbontrile (2c)
A mixture of 1a (0.01 mol), 2,4,6-trimethoxybenzylidene malonitrile (0.01 mol) and piperidine (0.01 mol) in ethanol (10 ml) was refluxed for 3 h. The resulting crude product was obtained as a mixture in solution. The mixture was separated by adding petroleum ether (40–60). The solid that precipitated was filtered off and crystallized from ethanol to give (2c). The filtered solution was evaporated, and the resulting crude product was crystallized from benzene to give (2b). The purity of both compounds was monitored by TLC.
7-Amino-5-(2,4,6-trimethoxypheyl)-3-(4-methylphenyl)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile (2b)
Golden yellow needles, yield 84%; m.p. 118–120 °C, ir (KBr pellet): 3310–3212 two NH spikes for (NH2), 2222 for (C≡N), 1658 for (C=C) and 1620 for (C=N) cm−1. 1H NMR: δ 10.86 (s, 2H, NH2), 8.66–7.21 (m, 6H, 2ArH), 6.72 (s, 2H, 2CH hetero), 3.86–3.84 (s, 9H, 3OCH3) and 3.25 (s, 3H CH3) ppm. 13C NMR: δ, 209.86, 166.92, 161.21, 160.74, 152.84, 143.50, 133.19, 129.14, 128.93, 126.35, 125.54, 125.44, 113.63, 108.45, 56.20, 55.89, 25.97 and 20.64 ppm. M/z 436 (M+ + 6).
6-Amino-5-(2,4,6-trimethoxyphenyl)-3-(4-tolyl)-5H-pyrano[2,3-c] pyridazine-7-carbontrile (2c)
Yellow needles, yield 12%; m.p. 142–143 °C, ir (KBr pellet): 3315–3217 two NH spikes for (NH2), 2189 for (C≡N), 1658 for (C=C) and 1610 for (C=N) cm−1, 1H NMR: δ 10.85–10.80 (d, 2H, NH2), 7.71–6.55 (s, 6H, 2ArH), 6.52–6.41(s, 2H, 2CH hetero), 3.92–3.80 (s, 9H, 3OCH3) and 2.92 (s, 3H, CH3-Ar) ppm. M/z 430 (M+).
Synthesis of 4-amino-5-(aryl)-7-(aryl)pyridazino [4′,3′:5,6] pyrano[2,3-c][1,2]-thiazine-3(5H)-thione (3a–c)
A mixture of aminonitrile 2b,d,e (0.01 mol), carbon disulphide (0.01 mol) in ethanol (20 ml) and a few drops of piperidine was refluxed for 3 h. The resulting crude product was obtained after cooling and crystallized from ethanol to give 3a–c.
4-Amino-5-(2,4,6-trimethoxyphenyl)-7-(4-tolyl)pyridazino[4′,3′:5,6]pyrano[2,3-c][1,2]-thiazine-3(5H)-thione (3a)
Yellow precipitate, yield 29%; m.p. 139–140 °C, ir: 3311–3207 for two NH spikes (NH2), 1658 (C=C), 1621 for (C=N) and 1296 for (C=S) cm−1. 1H NMR: δ 10.83 (s, 2H, NH2), 8.65–7.22 (m, 6H, 2ArH), 6.80–6.73 (s, 2H, 2CH hetero), 3.84–3.82 (s, 9H, 3OCH3) and 3.73 (s, 3H CH3Ar) ppm. 13C NMR: δ 166.92, 161.30, 153.12, 149.34, 138.81, 133.18, 130.17, 129.21, 128.93, 125.54, 121.73, 105.67, 105.47, 104.16, 92.62, 56.03, 55.79, 25.97 and 20.69. Anal. calcd. for C25H22N4O4S2 (506.60): C, 59.27, H, 4.38, N, 11.06, S, 12.66, Found C, 58.91, H, 4.45, N, 11.41, S, 12.83. M/z 507 (M+).
Synthesis of 4-amino-5-(phenyl)-7-(4-tolyl)pyridazino[4′,3′:5,6]pyrano[2,3-c][1,2]-thiazine-3(5H)-thione (3b)
Greenish yellow precipitate, yield 42%; m.p. 130–132 °C, ir: 3320–3217 for two NH spikes (NH2), 1660 (C=C), 1620 for (C=N) and 1294 for (C=S) cm−1. 1H NMR: δ 10.00 (s, 2H, NH2), 8.10–7.32 (m, 9H, 2ArH), 6.73–6.69 (s, 2H, 2CH hetero) and 3.00 (s, 3H, CH3) ppm. Anal. calcd. for C22H16N4OS2 (416.53): C, 63.44, H, 3.87, N, 13.45, S, 15.40. Found C, 63.79, H, 3.75, N, 13.41, S, 15.73. M/z 417 (M+).
Synthesis of 4-amino-5-(phenyl)-7-(2-methoxyphenyl) pyridazino[4′,3′:5,6] pyrano[2,3-c][1,2]-thiazine-3(5H)-thione (3c)
Reddish precipitate, yield 59%; m.p. 158–159 °C, ir: 3321–3217 for two NH spikes (NH2), 1658 (C=C), 1621 for (C=N) and 1297 for (C=S) cm−1. 1H NMR: δ 9.83 (s, 2H, NH2), 8.00–6.92 (m, 9H, 2ArH), 6.64–6.63 (s, 2H, 2CH hetero) and 3.84–3.82 (s, 3H OCH3) ppm. Anal. calcd. for C22H16N4O2S2 (432.52): C, 61.09, H, 3.73, N, 12.95, S, 14.83. Found C, 60.91, H, 3.66, N, 13.23, S, 14.83. M/z 432 (M+).
Synthesis of 3-(aryl)-5-(aryl)-[2,3-c] pyridazine-5H-pyrano [2,3-d] pyrimidine-2,4-dithiol (4a--c)
A mixture of aminonitrile 2b,d,e (0.2 mol) and carbon disulphide (0.02 mol) in (20 ml) ethanol was refluxed for 4 h. The resulting crude product was obtained after cooling and crystallized from ethanol to give 4a–c.
3-(4-Tolyl)-5-(2,4,6-trimethoxyphenyl)-[2,3-c]pyridazine-5H-pyrano[2,3-d] pyrimidine-2,4-dithiol (4a)
Golden yellow crystals, yield 20%; m.p. 190–192 °C, ir: 2527 for (SH), 1653 (C=C) and 1621 for (C=N) cm−1. 1H NMR: δ 8.65 (s, 2H, 2SH), 8.23–7.22 (m, 6H, 2ArH), 6.87–6.64 (s, 2H, 2CH hetero), 3.84–3.82 (s, 9H, 3OCH3) and 3.77–3.73 (s, 3H, CH3Ar) ppm.13C NMR: δ: 161.33, 160.94, 159.13, 155.20, 140.21, 130.13, 129.22, 128.42, 126.26, 124.58, 111.28, 105.71, 105.47, 60.27, 59.97, 56.07, 55.76, 25.32 and 20.45. Anal. calcd. for C25H22N4O4S2 (506.60): C, 59.27, H, 4.38, N, 11.06, S, 12.66. Found C, 58.86, H, 4.12, N, 11.37, S, 12.75. M/z 508 (M+ + 1).
3-(4-Tolyl)-5-(phenyl)-[2,3-c]pyridazine-5H-pyrano[2,3-d]pyrimidine-2,4-dithiol (4b)
Pale green crystals, yield 40%; m.p. 120–122 °C, ir: 2525 for (SH), 1660 (C=C) and 1621 for (C=N) cm−1. 1H NMR: δ 8.91 (s, 2H, 2SH), 8.23–7.22 (m, 9H, 2ArH), 6.87–6.64 (s, 2H, 2CH hetero) and 3.77--3.73 (s, 3H, CH3) ppm. Anal. calcd. for C22H18N4OS2 (416.52): C, 63.44, H, 3.87, N, 13.45, S, 15.40. Found C, 63.29, H, 4.02, N, 13.30, S, 15.75. M/z 415 (M+-2).
3-(2-Methoxyphenyl)-5-(phenyl)-[2,3-c] pyridazine-5H-pyrano [2,3-d]pyrimidine-2,4-dithiol (4c)
Orange precipitate, yield 47%; m.p. 138–140 °C, ir: 2518 for (SH), 1651 (C=C) and 1619 for (C=N) cm−1. 1H NMR: δ 9.00 (s, 2H, 2SH), 7.78–6.89 (m, 9H, 2ArH), 6.77–6.43 (s, 2H, 2CH hetero) and 3.72–3.70 (s, 3H, OCH3) ppm. Anal. calcd. for C22H16N4O2S2 (432.52): C, 61.09, H, 3.73, N, 12.95, S, 14.83. Found C, 61.36, H, 3.62, N, 13.07, S, 14.75. M/z 432 (M+).
Synthesis of 3-(4-tolyl)-5-(3,4,5-trimethoxyphenyl)-6-(1H-tetrazol-5-yl)-5H-pyrano[2,3-c] pyridazine-7-amino (5)
A mixture of aminonitrile 2a (0.01 mol), sodium azide (0.4 g, 6 mmol) and ammonium chloride (0.31 g, 6 mmol) in ethanol (15 ml) was refluxed for 5 h. The resulting crude product obtained after cooling was crystallized from ethanol to give 5.
Yellowish brown precipitate, yield 50% m.p. 138–140 °C, ir (KBr pellet): 3309–3206 for NH and two NH spikes of (NH2), 1657 (C=C) and 1620 (C=N) cm−1. 1H NMR: δ 13.09 (s, 1H, NH), 10.82 (s, 2H, NH2), 8.01–7.21 (m, 6H, 2ArH), 6.98–6.94 (s, 2H, 2CH hetero), 3.79–3.77 (s, 9H, 3OCH3) and 3.30 (s, 3H, CH3) ppm. 13C NMR: δ: 166.91, 160.71, 159.72, 156.90, 149.32, 138.80, 133.18, 130.21, 129.11, 128.93, 127.42, 125.53, 104.11, 92.67, 88.80, 56.23, 25.97 and 20.69. Anal. calcd. for C24H23N7O4 (473.48): C, 60.88, H, 4.90, N, 20.71, Found C, 60.86, H, 4.72, N, 20.37. M/z 473 (M+).
Synthesis of compound (6)
A mixture tetrazolo derivative 5 (0.01 mol), benzaldehyde (0.01 mol) and few drops of piperidine in (15 ml) ethanol was refluxed for 2 h. The resulting crude product was obtained after cooling, and then crystallized from ethanol to give 6.
Off white precipitate, yield 40%; m.p. 159–160 °C, ir: 3309 for (NH), 1659 for (C=C) and 1620 for (C=N) cm−1, 1H NMR: δ 10.85 (s, 1H, NH), 8.01–7.05 (m, 11H, 2ArH), 6.98–6.95 (s, 3H, 3CH, hetero) 3.79–3.77 (s, 9H, 3OCH3) and 3.32(s, 3H, CH3) ppm. 13C NMR: δ: 166.91, 161.70, 159.70, 149.32, 143.51, 138.80, 133.19, 131.83, 130.24, 129.55, 129.14, 129.06, 128.91, 126.57, 125.59, 125.43, 106.00, 104.12, 99.49, 74.30, 56.23, 25.98 and 20.63. Anal. calcd. for C31H27N7O4 (561.59): C, 66.30, H, 4.85, N, 17.46. Found C, 66.50, H, 4.72, N, 17.78. M/z 563 (M+ + 1).
Synthesis of 3-(4-tolyl)-5-(2,4,6-trimethoxyphenyl)-5,7-dihydro[5′,4′:5,6]pyrano[2,3-c] pyridazine-6-one (7)
A solution of aminonitrile 2b (0.01 mol) in formic acid (10 ml) was refluxed for 12 h. The resulting crude product was obtained after cooling then crystallized from acetic acid to give 7.
Pale brown precipitate, yield 27%; m.p. 74–76 °C, ir: 3419 for (NH), 1700 for (C=O), 1651 for (C=C) and 1620 for (C=N) cm−1, 1H NMR: δ 8.88 (d, 1H, NH), 7.98–7.89 (m, 6H, 2ArH), 6.65–6.54 (d, 1H, CH hetero), 6.22 (s, 2H, 2CH hetero) 3.81–3.74 (s, 9H, 3OCH3) and 3.63 (s, 3H, CH3) ppm. Anal. calcd. for C25H22N4O5 (458.47): C, 65.49, H, 4.84, N, 12.22. Found C, 65.19, H, 4.92, N, 12.58. M/z 452 (M+-6).
Synthesis of 3-(4-tolyl)-5-(2,4,6-trimethoxyphenyl)-8-methyl-5,7-diydro-6H-pyrimido[5′,4′:5,6]pyrano[2,3-c] pyridazine-6-one (8)
A solution of aminonitrile 2b (0.1 mol) and acetic anhydride (10 ml) was refluxed for 12 h. The resulting crude product was obtained after cooling and crystallized from benzene to give (8).
Orange crystals, yield 70%; m.p. 130–131 °C, ir: 3407 for (NH), 1717 for (C=O) 1656 for (C=C) and 1620 for (C=N) cm−1. 1H NMR: δ 8.67 (s, 1H, NH), 7.77–7.27 (m, 6H, 2ArH), 6.22 (s, 2H, 2CH hetero) 3.25–3.07(s, 9H, 3OCH3) and 3.02 (s, 6H, 2CH3) ppm. 13C NMR: δ: 216.70, 206.11, 178.97, 169.87, 167.16, 153.62, 140.11, 132.36, 129.23, 128.08, 126.43, 126.27, 121.70, 104.12, 102.52, 56.23, 28.93, 25.92 and 22.12. Anal. calcd. for C26H24N4O5. (472.17): C, 66.09, H, 5.12, N, 11.86. Found C, 66.00, H, 5.10, N, 11.58. M/z 472(M+).
Synthesis of 3-(4-tolyl)-5-(2,4,6-trimethoxyphenyl)-6,7-dihydro-5H-pyrimido[5′,4′:5,6] pyrano[2,3-c]pyridazine-6-amine (9)
A mixture of aminonitrile 2b (0.01 mol) and formamide (1 ml, 0.01 mol) in ethanol (10 ml) was refluxed for 4 h. The resulting crude product was obtained after cooling and crystallized from ethanol to give 9.
Red precipitate, yield 32%; m.p. 275–277 °C, ir: 3311–3208 two NH, spikes for (NH2), 1658 for (C=C) and 1620 for (C=N) cm−1. 1H NMR: δ 10.83 (s, 2H, NH2), 7.94–7.16 (m, 6H, 2ArH), 6.98 (s, 3H, 3CH hetero), 3.74–3.62 (s, 9H, 3OCH3) 3.31 (s, 3H, CH3) ppm. Anal. calcd. for C25H23N5O4 (457.18): C, 65.63, H, 5.07, N, 15.31. Found C, 66.00, H, 4.92, N, 15.58. M/z 452 (M+-5).
Synthesis of 5-(2,4,6-trimethoxyphenyl)-7-(4-tolyl)-5H-pyrimido[4′,3′:5,6]pyrano[2,3-d] [1,2,3] triazin-4-amine (10)
A mixture of aminonitrile 2b (0.01 mol) and hydrazine hydrate (0.01 mol) in ethanol (10 mol) was refluxed for 3 h. The resulting crude product was obtained after cooling and crystallized from ethanol to give 10. Silver white crystals, yield 50%; m.p. 138–139 °C, ir: 3311–3209 for two NH spikes of (NH2), 1658 for (C=C) and 1620 for (C=N) cm−1. 1H NMR: δ 10.86 (s, 2H, NH2), 7.71–7.21 (m, 6H, 2ArH), 6.98 (s, 2H, 2CH hetero), 3.78–3.42 (s, 9H, 3OCH3) and 3.35 (s, 3H, CH3) ppm. 13C NMR: δ: 166.91, 163.50, 159.78, 159.70, 159.10, 149.32, 138.80, 133.19, 129.19, 129.12, 128.92, 125.54, 106.60, 101.29, 99.50, 56.23, 25.97 and 21.82. Anal. calcd. for C24H22N6O4 (458.17): C, 62.87, H, 4.84, N, 18.33. Found C, 63.00, H, 4.92, N, 18.48. M/z 459 (M+).
Synthesis of 6,8 diamino-3-(4-tolyl)-5-(2,4,6-trimethoxyphenyl)-5H-pyrido[3′,2′:5,6] pyrano[2,3-c] pyridazine-7-carbonitrile (11)
A mixture of aminonitrile 2b (0.01 mol) and malononitrile (0.01 mol) in the presence of few drops of piperidine in ethanol was refluxed for 10 h. The resulting crude product was obtained after cooling and crystallized from dioxane to give 11.
White crystals, yield 42%; m.p. 139–140 °C, ir: 3300–3208 two NH, spikes for (NH2), 2261 for (C≡N) and 1658 for (C=C) and 1621 for (C=N). 1H NMR: δ 10.79 (s, 4H, 2NH2), 7.65–7.21 (s, 6H, 2ArH), 6.70 (s, 2H, 2CH hetero) 3.26–3.14 (s, 9H, 3OCH3) and 2.95 (s, 3H, CH3) ppm. 13C NMR: δ: 169.99, 166.91, 163.50, 160.75, 159.78, 159.70, 152.83, 149.32, 138.80, 133.19, 129.19, 129.10, 128.92, 127.17, 125.53, 113.67, 106.40, 92.54, 59.85, 55.90 and 25.97. Anal. calcd. for C27H24N6O4 (496.52): C, 65.31, H, 4.87, N, 16.93. Found C, 65.11, H, 5.00, N, 16.78. M/z 497 (M+).
Biological evaluation
In vitro antimicrobial measurement
The antimicrobial activities of the new compounds in addition to the reference drugs chloramphenicol and nystatin were evaluated against four bacterial and two fungal strains by the agar well diffusion methodCitation28 modified by OlurinolaCitation29. All microbial strains were kindly provided by Main Laboratories of The War Chemical, Chemical War Department, Ministry of Defense (ARE). These strains are common contaminants in the Egypt environment and some of which are involved in human and animal diseases (A. albicans, C. albicans) or frequently reported from contaminated soil, water and food substances (Klebsiella pneumonia, Escherichia coli, Enterococcusi fecalis and Staphylococcus aureus).
Determination of MIC of the tested compounds
Compounds inhibiting the growth of one or more of the above microorganisms were further tested for their minimum inhibitory concentration (MIC) and were determined by broth dilution techniqueCitation30. The nutrient broth and the yeast extract broth media, which contained 1 ml of different concentrations of the tested compounds (2.5, 5, 10, 15, 20, 25 μg/ml) were inoculated with the microbial strains. The bacterial cultures were incubated for 24 h at 37 °C, whereas the fungal ones were incubated at 30 °C for 48 h. The growth was monitored spectrophotometrically. The lowest concentration required to arrest the microbial growth was regarded as MICs (μg/ml).
Results and discussion
Chemistry
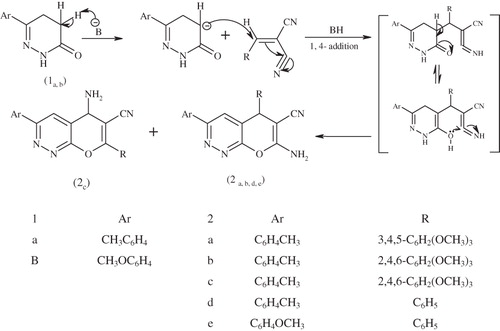
The active methylene group in pyridazine derivatives 1a,b were exploited to synthesis new fused pyranopyridazine derivatives through their reaction with some electrophiles. Thus, 7-amino-3-(aryl)-5-(aryl)-5H-pyrano[2,3-c] pyridazine-6-carbonitriles 2a,d,e were synthesized in good yield. They were readily formed on treatment of compounds 1a,b with 2-arylidene malononitrile under reflux in absolute ethanol and in the presence of catalytic amounts of piperidine. Compounds 2a,d,e were characterized by elemental and spectroscopic analysis and were assumed to be formed via Michael addition of the active methylene group in pyridazine derivatives (1a,b) to the activated double bond in cyanoarylidine to form the not isolated intermediate, followed by the intermolecular cyclization to furnish 7-amino-3-(aryl)-5-(aryl)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile 2a,d,e. The proposed mechanism for this reaction is outlined in Scheme 1. In a similar manner the reaction of compound 1a with 2-(2,4,6-trimethoxybenzylidene)malononitrile under reflux in absolute ethanol and in presence of catalytic amounts of piperidine, interestingly afforded not only 7-amino-3-(4-tolyl)-5-(2,4,6-trimethoxybenzylidene)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile 2b, but also 6-amino-3-(4-tolyl)-5-(2,4,6-trimethoxybenzylidene)-5H-pyrano[2,3-c] pyridazine-7-carbonitrile 2c. The unexpected isomeric product, 6-amino-3-(4-tolyl)-5-(2,4,6-trimethoxybenzylidene)-5H-pyrano[2,3-c] pyridazine-7-carbonitrile 2c may well have been formed due to the presence of two carbons attacking centers at 2-(2,4,6-trimethoxybenzylidene) malononitrile. The ir spectra of compounds 2a–e exhibit strong absorption bands at 3310–3212 and at 2220–2189 cm−1 for amino and cyano functional groups, respectively. In addition, the 1H NMR spectra of compounds 2a–e revealed the absence of proton bands of the methylene moiety. The existence of compound 2c was supported by its 1H NMR: δ 10.85–10.80 (d, 2H, NH2), 7.71--6.55 (s, 6H, 2ArH), 6.52--6.41 (s, 2H, 2CH hetero), 3.92--3.80 (s, 9H, 3OCH3) and 2.92 (s, 3H, CH3-Ar) ppm.
Scheme 1. Mechanism for the synthesis of 2a–e. Reagents and conditions: arylbenzylidine, piperidine, EtOH, reflux 3 h.
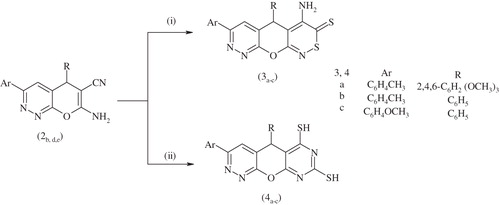
The reactivity of 7-amino-3-(aryl)-5-(aryl)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile 2b,d,e toward carbon disulphide under different reaction conditions was investigated. Under basic conditions, 4-amino-5-(aryl)-7-(aryl)pyridazino[4′,3′:5,6]pyrano[2,3-c] [1,2]-thiazine-3(5H)-thione 3a–c were obtained by reaction of 7-amino-3-(aryl)-5-(aryl)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile 2b,d,e with carbon disulphide under reflux in absolute ethanol. The structures of compounds 3a–c were supported by their spectral data. The ir spectra revealed no bands for the cyano group. However 6-amino-3-(aryl)-5-(aryl)pyrimido[5′,4′:5,6]pyrano[2,3-c] pyridazine-8-thiol 4a–c were obtained by reaction of 7-amino-3-(aryl)-5-(aryl)-5H-pyrano[2,3-c] pyridazine-6-carbonitrile 2b,d,e with carbon disulphide in refluxing absolute ethanol (Scheme 2). The ir spectrum of compound 4a revealed none of the bands of cyano group, and the presence of band at 2527 cm−1 for (SH) group.
Scheme 2. Synthesis of compounds 3a–c, 4a–c. Reagents and conditions: (i) CS2, piperidine, EtOH, reflux 4 h and (ii) CS2, EtOH, reflux 4 h.
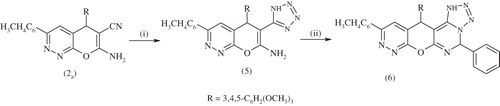
Treatment of the fused pyranopyridazine 2a with sodium azide and ammonium chloride in refluxing dimethylformamide yielded 3-(4-tolyl)-5-(3,4,5-trimethoxyphenyl)-6-(1H-tetrazol-5-yl)-5-H-pyrano[2,3-c] pyridazin-7-amine 5. The IR spectrum of compound 5 revealed no bands of cyano group, and 1H NMR spectrum showed bands at δ 13.09 (s, 1H, NH), 10.82 (s, 2H, NH2), 8.01–7.21 (m, 6H, 2ArH), 6.98–6.94 (s, 2CH hetero), 3.79–3.77 (s, 9H, 3OCH3) and 3.30 (s, 3H, CH3) ppm. The fused tetrazolo compound was obtained from refluxing compound 5 with benzaldehyde in absolute ethanol containing few drops of piperidine to afford 3-(4-tolyl)-5-(3,4,5-timethoxyphenyl)-5H-pyrano-[2,3-e]tetrazolo-9-phenyl-[1,5-c]pyrimidine-[2,3-c] pyridazine 6 (Scheme 3). The IR spectrum of compound 6 revealed no bands of cyano groups. The 1H NMR spectrum showed bands at δ 10.85 (s, 1H, NH), 8.01--7.05 (m, 11H, 2ArH), 6.98--6.96 (s, 3H, 3CH hetero), 3.79--3.77 (s, 9H, 3OCH3) and 3.32 (s, 3H, CH3) ppm.
Scheme 3. Synthesis of compounds 5,6. Reagents and conditions: (i) NaN3, NH4Cl, DMF, reflux 5 h and (ii) C6H5CHO, EtOH, piperidine, reflux 2 h.
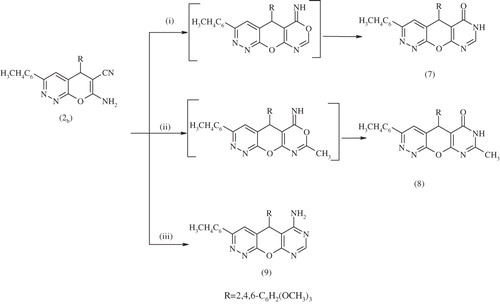
The behavior of compound 2b towards formic acid or acetic anhydride was also investigated. Thus, heating compound 2b with formic acid or acetic anhydride achieved a cyclization reaction to give the corresponding pyrimidopyranopyridazine derivatives 3-(4-tolyl)-5-(2,4,6-trimethoxyphenyl)-5,7-dihydro[5′,4′:5,6]pyrano[2,3-c] pyridazine-6-one 7 and 3-(4-tolyl)-5-(2,4,6-trimethoxyphenyl)-8-methyl-5,7-dihydro-6H-pyrimido[5′,4′:5,6]pyrano [2,3-c] pyridazin-6-one 8, respectively, with a new fused ring system. The ir spectrum of 7 showed bands at 3419 for (NH), 1700 for (C=O), 1651 for (C=C) and 1620 for (C=N) cm−1.
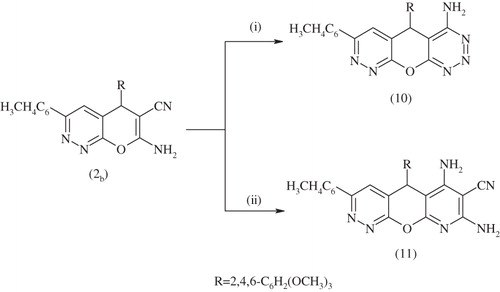
Compounds 7 and 8 were formed via the Dimruth rearrangement which is illustrated in Scheme 4. Intermolecular cyclization occurred via reaction of key material 2b with an equimolar amount of formamide under reflux in ethanol to afford 3-(4-tolyl)-5-(2,4,6-trimethyoxyphenyl)-6,7-dihydro-5H-pyrimido[5′,4′:5,6] pyrano[2,3-c] pyridazine-6-amine 9. The 1H NMR spectrum of compound 9 showed bands at δ 10.83 (s, 2H, NH2), 8.90--7.16 (m, 6H, 2ArH), 6.98 (s, 3H, 3CH hetero), 3.74–3.62 (s, 9H, 3OCH3), 3.31(s, 3H, CH3) ppm. The hydrazinolysis of compound 2b with hydrazine hydrate furnished 5-(2,4,6-trimethoxyphenyl)-7-(4-tolyl)-5H-pyridazino[4′,3′:5,6]pyrano[2,3-d][1,2,3] triazin-4-amine (10). The 13C NMR of compound 10 showed bands at δ: 166.91, 163.50, 159.78, 159.70, 159.10, 149.32, 138.80, 133.19, 129.19, 129.12, 128.92, 125.54, 106.60, 101.29, 99.50, 56.23, 25.97 and 21.82. The interaction of fused pyranopyridazine 2b with malononitrile in the presence of piperidine as the organocatalyst, afforded 6,8-diamino-3-(4-tolyl)5-(2,4,6-trimethoxyphenyl)-5H-pyrido[3′,2′:5,6]pyrano[2,3-c] pyridazine-7-carbonitrile 11 (Scheme 5). The existence of compound 11 was confirmed by its mass spectrum which revealed a molecular ion peak at M/z 497 (M+).
In vitro antimicrobial evaluation
The antimicrobial activities of 10 selected new compounds were evaluated against 4 bacterial and 2 fungal strains by the agar well diffusion methodCitation28, modified by OlurinolaCitation29, using broad spectrum antibiotics Chloramphenicol and Nystatin as references.
The new compounds 2b–e, 3a–c and 4a–c were screened against S. aureus, (MTCC3160), and Enterococcusi fecalis as Gram-positive bacteria, and Escherichia coli (MTCC1652) and K. pneumonia as Gram-negative bacteria. Screening against two fungal strains; Aspergillus flavus (MTCC2456) and C. albicans was also carried out. The microbial studies were assessed by the MIC using the agar well diffusion techniqueCitation30. The results of MIC (µ/ml) and inhibition zone diameters (mm) values are depicted in and and . From the results in the 5H-pyrano[2,3-c] pyridazine-6-carbonitrile compounds 2b–e showed no significant activity against these neither bacterial nor fungal strains. Pyrano [2,3-c] [1,2]-thiazine-3(5H)-thione compounds 3a,c showed strong, significant activity relative to the reference antibiotics against these bacterial and fungal strains but compound 3b showed strong significant activity to the reference antibiotic against the bacterial strains only. Pyridazine-5H-pyrano[2,3-d] pyrimidine-2,4-dithiol compounds 4a–c showed no significant activity against the bacterial strains, however 4a and 4c, showed significant activity against the fungal strains. On the other hand, compound 4b showed no significant activity against the tested microbial and fungal strains.
Figure 1. Inhibition zones (mm) and MIC (µ/ml) of compounds 3a–c and chloramphenicol against Klebsiella pneumonia, Escherichia coli, Enterococcusi fecalis and S. aureus strains.
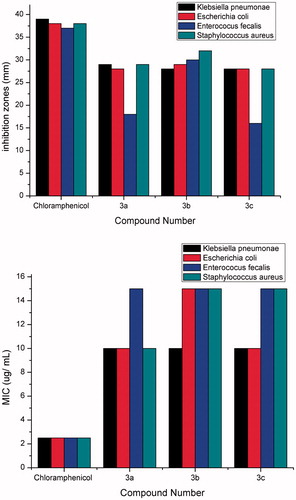
Figure 2. Inhibition zones (mm) and MIC (µ/ml) of compounds 3a,c and 4a,c and Nystatin against Aspergillus flavus and Candida albicans strains.
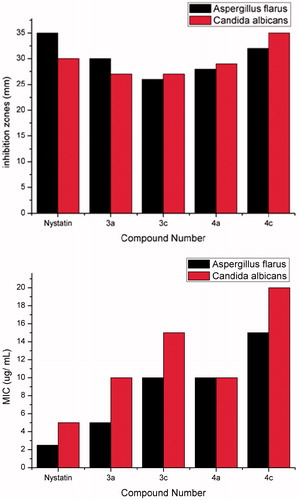
Table 1. Minimal inhibitory concentrations (MIC, µg/ml) and zones of inhibition (mm) of compounds (2b–e, 3a–c and 4a–c).
Conclusion
In this present work, we report convenient and simple methods for the synthesis of a new series of heterocyclic derivatives (e.g. thiazine, tetrazole and pyrimidine) incorporating the pyrano[2,3-c] pyridazine moiety with the aim of preparing new antimicrobial heterocyclic compounds. The data of MICs of the in vitro antimicrobial evaluation of some new compounds against several pathogenic bacterial strains revealed that compounds 3a–c showed significant activity against the bacterial species (Escherichia coli, K. pneumonia, S. aureus and Enterococcusi fecalis). The results indicated that compounds 3a,c and 4a,c showed excellent significant activity against the fungal strains C. albicans, and A. flavus which may be due to the presence of the methoxy groups in their chemical structures.
Declaration of interest
The authors report no conflict of interest.
References
- Sweeney ZK, Dunn JP, Li Y, et al. Discovery and optimization of pyridazinone non-nucleoside inhibitors of HIV-1 reverse transcriptase. Bioog Med Chem Lett 2008;18:4352–4
- Zou XJ, Jin GY. Hydrazine derivatives of 1-aryl-1,4-dihydro-3-carboxy-6-methyl-4-pyridazone. Synthesis and biological activity. Chin J Org Chem 2003;23:62–5
- Coelho A, Sotelo E, Ravina E. Pyridazine derivatives. Part 33: sonogashira approaches in the synthesis of 5-substituted-6-phenyl-3(2H)-pyridazinones. Tetrahedron 2003;59:2477–84
- Demirayak S, Karaburun AC, Beis R. Some pyrrole substituted aryl pyridazinone and phthalazinone derivatives and their antihypertensive activities. Eur J Med Chem 2004;39:1089–95
- Monge A, Parrado P, Font M, Alvarez EF. Selective thromboxane synthetase inhibitors and antihypertensive agents. New derivatives of 4-hydrazino-5H-pyridazino[4,5-b]indole, 4-hydrazinotriazino[4,5-a]indole, and related compounds. J Med Chem 1987;30:1029–35
- Rubat C, Coudert P, Refouvelet B, et al. Anticonvulsant activity of 3-oxo-5-substituted benzylidene-6-methyl-(4H)-2-pyridazinylacetamides and 2-pyridazinyl acetylhydrazides. Chem Pharm Bull 1990;38:3009–13
- Sircar I, Weishaar RE, Kobylarz D, et al. Cardiotonic agents. Inhibition of separated forms of cyclic nucleotide phosphodiesterase from guinea pig cardiac muscle by 4,5-dihydro-6-[4-(1H-imidazol-1-yl) phenyl]-3(2H)-pyridazinones and related compounds. Structure–activity relationships and correlation within vivopositive inotropic activity. J Med Chem 1987;30:1955–62
- Longo JG, Verde I, Castro ME. Pyridazine derivatives XI: antihypertensive activity of 3-hydrazinocycloheptyl [1,2-c]pyridazine and its hydrazone derivatives. J Pharm Sci 1993;82:286–90
- Akahane A, Katayama H, Mitsunaga T. Discovery of 6-oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-1(6H)-pyridazine butanoic acid (FK 838): a novel non-xanthine adenosine A1 receptor antagonist with potent diuretic activity. J Med Chem 1999;42:779–83
- Jiang JK, Boxer MB, Vander Heiden MG, et al. Evaluation of thieno[3,2-b]pyrrole[3,2-d]pyridazinones as activators of the tumor cell specific M2 isoform of pyruvate kinase. Bioorg Med Chem Lett 2010;20:3387–93
- Mangalagiu II. Recent achievements in the chemistry of 1,2-diazines. Curr Org Chem 2011;15:730–52
- Wermuth CG. Are pyridazines privileged structures. Med Chem Commun 2011;2:935–41
- Bonsignore L, Loy G, Secci D, Calignano A. Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivative. Eur J Med Chem 1993;28:517–20
- Wu JYC, Fong WF, Zhang JX, et al. Emodin induces apoptosis of human cervical cancer cells through poly(ADP-ribose) polymerase cleavage and activation of caspase-9. Eur J Pharmacol 2003;473:117–25
- Kashman Y, Gustafson KR, Fuller RW, et al. HIV inhibitory natural products. Part 7. The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J Med Chem 1992;35:2735–43
- Patil AD, Freyer AJ, Eggleston DS, et al. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J Med Chem 1993;36:4131--8
- Andreani LL, Lapi E. Aspects and orientations of modern pharmacognosy. Bull Chim Farm 1960;99:583–6
- Mohamed MI, Zaky HT, Kandile NG. Novel synthesis and antibacterial activity of some pyridazine derivatives. J Chinese Chem Soc 2004;51:963–8
- Mohamed MI, Zaky HT, Mohamed HM, Kandile NG. Novel heterocyclic systems of pyridazines. Afinidad 2005;62:48–56
- Kandile NG, Mohamed MI, Zaky HT, Mohamed HM. Novel pyridazine derivatives: synthesis and antimicrobial activity evaluation. Eur J Med Chem 2009;44:1989–96
- Kandile NG, Mohamed MI, Zaky HT, Mohamed HM. Silver nanoparticles effect on antimicrobial and antifungal activity of new heterocycles. Bull Korean Chem Soc 2010;31:3530–8
- Kandile NG, Zaky HT, Saleh YG, Ahmed NA. Synthesis of a new class of antimicrobial agents incorporating the indolin-2-one moiety. J Enz Inhib Med Chem 2013;28:853–62
- Kandile NG, Mohamed MI, Ismaeel HM. Antiproliferative effects of metal complexes of new isatin hydrazones against HCT116, MCF7 and HELA tumor cell lines. J Enz Inhib Med Chem 2012;27:330–8
- Zaky HT. New oxazolones as synthons for antimicrobial compounds. J Bulg Chem Commun 2007;39:134–41
- Zaky HT. 3,1-Benzothiazine, benzimidazole, amide and quinazoline derivatives via 2(3H) furanones. J Bulg Chem Commun 2006;38:248–54
- Kandile NG, Zaky HT, Mohamed MI, et al. Synthesis, characterization and in vitro antimicrobial evaluation of new compounds incorporating oxindole nucleus. J Enz Inhib Med Chem. 2012;27:599–608
- Mohamed MI, Zaky HT, Kandile NG. Microwave-assisted synthesis of antimicrobial agents based on pyridazine moiety. J Enz Inhib Med Chem 2013;28:1307–15
- Murray PR, Baron EJ, Pfaller MA, et al. Manual of clinical microbiology. 6th ed. Washington DC: ASM Press; 1995:15–18
- Olurinola PF. A laboratory manual of pharmaceutical microbiology. Abuja, Nigeria: Idu; 1996
- Tadeg H, Mohammed E, Asres K, Gebre-Mariam T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J Ethnopharmacol 2005;100:168–75